Oxygen Consumption Response to Gradual Hypoxia in Spider Crab, Maja brachydactyla: Critical and Lethal Oxygen Saturations and Recovery Ability
Abstract
Spider crab, Maja brachydactyla, is a crustacean with interesting biological characteristics for intensive aquaculture. However, physiological studies are needed to establish the optimal conditions for its culture and maintenance. We analyzed the oxygen consumption response of spider crab to gradual hypoxia in relation to animal weight (W: 0.42–1.62 kg) and water temperature (T: 17.8–26.3 C), along with its ability to recover. Spider crab behaved as an oxygen regulator, maintaining its oxygen consumption constant until it reached critical oxygen saturation. This value varied between 24.1 and 53.3% oxygen saturation (42.4 ± 7.4% Sat.; 2.95 ± 0.43 mgO2/L), and significantly depended on the temperature (P < 0.01). The lethal oxygen saturation varied from 6.8 to 19.3% Sat. (12.57 ± 4.25% Sat.; 0.88 ± 0.29 mgO2/L) depending on temperature and body weight (P < 0.05). When oxygen levels were re-established after exposure to acute hypoxia, the animals recovered, oxygen consumption remaining above the routine value for up to 15–20 h. We suggest survival oxygen saturation levels for spider crab of between 100 and 65% in the experimental conditions of this study.
Spider crab, Maja brachydactyla (Balss, 1922), is a decapod crustacean that exhibits promising biological characteristics for intensive aquaculture. M. brachydactyla shows high reproduction rates, accompanied by rapid larval development and growth, and it also has a high commercial value (González-Gurriarán et al. 1995; Iglesias et al. 2002; Sampedro et al. 2003). At present, several groups are studying its possible reproduction in controlled intensive conditions (Iglesias et al. 2002; Andrés et al. 2007).
Among water quality parameters, the level of dissolved oxygen is of special interest because this gas plays an essential role in respiration and metabolic processes, in the degradation of waste products and in the economic viability of intensive culture conditions. Most studies have shown that above a critical level of oxygen (critical oxygen saturation –Scrit), crustaceans are oxygen regulator and are capable of maintaining their oxygen consumption within a wide range of concentrations (Childress 1975; Bridges and Brand 1980a; Villarreal and Ocampo 1993; Schmitt and Uglow 1998). The ability of crustaceans to tolerate hypoxia is because of the existence of adaptation mechanisms, including increased ventilation volume and hemolymph flow through the gills, along with increased cardiac output and oxygen transport capacity (McMahon 2001).
When the concentration of oxygen falls below Scrit, anaerobic energy production is stimulated, lactic acid is accumulated and pH decreases, preventing oxygen saturation of hemolymph (Taylor 1981), although a great diversity of responses have been described (Bouchet and Truchot 1985; Lallier et al. 1987). Subsequently, although oxygen levels may recover, oxygen consumption remains above routine values for several hours (the so-called oxygen debt) due to gluconeogenesis and the need to eliminate lactic acid (Bridges and Brand 1980b; Hill et al. 1991; Hervant et al. 1997). Hypoxia may also affect the immune system of crustaceans (Verghese et al. 2007), making them more susceptible to disease and lack of appetite (Legeay and Massabuau 2000), and curtailing growth (Das and Stickle 1993). Therefore, the determination of Scrit is of special interest as a water quality parameter for the cultivation of new species for aquaculture.
Furthermore, both water temperature and the body weight of specimens may affect the value of Scrit and the lethal concentration of oxygen. An increase in water temperature provokes greater oxygen consumption in crustaceans (Thomas et al. 2000; Whiteley et al. 2001; Re et al. 2004), a diminution in the affinity of hemocyanin for oxygen (Taylor 1981) and a decrease in oxygen solubility (Weiss 1970). Therefore, higher levels of dissolved oxygen seem necessary to offset any increase in water temperature. In addition, body weight is important because specific oxygen consumption is higher in smaller animals (Bridges and Brand 1980a; Carvalho and Phan 1997; Perera et al. 2007), which may therefore be more susceptible to low dissolved oxygen concentrations.
The objective of this study was to determine the oxygen consumption response to gradually imposed hypoxia and the recovery capacity of M. brachydactyla, and to estimate the critical and lethal oxygen levels for different weights and temperatures. Knowledge of these parameters will be of great help in establishing safe oxygen levels for maintaining this species.
Materials and Methods
Experimental Animals
A total of 17 specimens of spider crab, M. brachydactyla, from Galician beds (NW Spain) weighing between 0.42 and 1.62 kg were used. The animals were transported from the place of origin to the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA) research center at San Pedro del Pinatar (Murcia, SE Spain) using an express refrigerated transport service in expanded polystyrene boxes lined with wet cloth. Upon arrival at IMIDA, the animals were placed in circular 480-L tanks equipped with recirculating seawater with controlled temperature, ultraviolet lamps, and mechanical and biological filtration systems (temperature 17–20 C; oxygen saturation 80–100%; salinity 37 g/L; pH 7.5–8.0; total ammonia nitrogen [TAN] <0.2 mg/L). During the acclimatization period, photoperiod was the natural for the area (37°50′N, 0°46′W). The animals were fed daily to satiety with fresh or defrosted mussels, Mytilus edulis.
Respirometry
Measurements were made between February 2008 and July 2008, when the animals were transferred individually to 170 L respirometers. There, they were allowed to acclimatize to the new experimental temperature, allowing 1 week for each two degrees of difference from the initial temperature, whilst they were fed regularly. Those with damaged legs, with eggs in the abdomen, that had recently moulted or which had been seen to feed irregularly were rejected. One day before the respiration measurements the animals fasted (24 h were enough to bring oxygen consumption to baseline levels according to Cerezo Valverde et al. 2009), were weighed and sexed (observation of the abdomen).
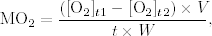
Two measurements were made: (a) oxygen consumption between levels close to 100% saturation and lethal values (specimens N° 1–14) and (b) as above but restoring the oxygen supply before lethal levels were reached to analyze the oxygen consumption response and recovery capacity after submitting the animals to acute hypoxia (specimens N° 15–17). The experimental conditions for measurements are shown in Table 1.
| Specimen N° | SEX | Weight (kg) | Temperature (C) | S crit (%) | LS (%) |
|---|---|---|---|---|---|
| 1 | Male | 0.53 | 18.74 ± 0.75 | 36.63 | 8.89 |
| 2 | Female | 1.39 | 17.79 ± 1.00 | 38.95 | 6.82 |
| 3 | Male | 0.54 | 18.57 ± 0.82 | 36.43 | 7.83 |
| 4 | Male | 1.08 | 19.29 ± 0.65 | 34.85 | 11.46 |
| 5 | Male | 0.60 | 18.67 ± 0.35 | 24.10 | 9.08 |
| 6 | Male | 0.45 | 18.75 ± 0.43 | 36.08 | 12.64 |
| 7 | Male | 0.52 | 22.14 ± 0.82 | 53.26 | 19.12 |
| 8 | Male | 1.25 | 21.83 ± 0.69 | 42.81 | 12.70 |
| 9 | Male | 0.42 | 23.06 ± 0.88 | 44.36 | 19.33 |
| 10 | Male | 0.47 | 22.61 ± 0.56 | 50.71 | 17.07 |
| 11 | Male | 0.54 | 25.95 ± 0.69 | 45.58 | 12.68 |
| 12 | Male | 0.59 | 26.33 ± 0.82 | 49.43 | 16.00 |
| 13 | Female | 1.62 | 25.28 ± 0.35 | 47.89 | 7.43 |
| 14 | Male | 0.56 | 25.66 ± 0.61 | 40.12 | 14.93 |
| 15 | Male | 0.44 | 25.20 ± 0.95 | 43.50 | n.d. |
| 16 | Male | 0.85 | 24.11 ± 1.06 | 50.02 | n.d. |
| 17 | Male | 1.08 | 24.20 ± 1.06 | 46.11 | n.d. |
| Mean ± SD | 0.75 ± 0.37 | 22.24 ± 3.01 | 42.40 ± 7.38 | 12.57 ± 4.25 |
- n.d., not determined.
With this in mind, the water supply to the respirometer was interrupted and the volume of water therein adjusted to 100 L for the smallest specimens (<0.5 kg) or 170 L for the largest (>0.5 kg). The respirometers were then covered with a floating plastic film to prevent interchange with atmospheric oxygen. The possible transfer of oxygen across the plastic film and the biological demand for oxygen were measured in a control respirometer without animals. However, these were not taken into account in subsequent calculations because of the low values detected (<0.02 mgO2/L/h). Homogeneous conditions were ensured in each respirometer by a water pump and chemical filter of zeolites to avoid increases in water TAN. Notwithstanding, pH and TAN were recorded every 24 h to check the water quality with a pH probe (Multiparameter probe YSI® 556 MPS, YSI, Yellow Springs, OH, USA) and a portable colorimeter (Thermo Orion AQUAfast® IV Beverly, MA, USA), respectively. Each determination was carried out at one fixed temperature controlled by a thermostat and an air conditioner into laboratory. A continuously operated oxygen and temperature probe (Luminescent Oxygen Detector, LDO, Hach Lange, Dusseldorf, Germany) was installed in each respirometer and connected to a data logger (Display SC1000, Hach Lange). Later, the oxygen and temperature data were sent to a PC for data treatment. Therefore, the mechanism selected for determining oxygen consumption response reflects the effects of acute hypoxia, a situation that may arise because of an interruption in the oxygen supply.
With reference to recovery period, to restore the oxygen level in the respirometers a discontinuous flow of water was applied and oxygen consumption was measured from the fall in oxygen concentration during the periods the flow was stopped. Water inflow was controlled by an electrovalve (Cepex, Valpes-Valve Control System, Model L 20, Type J2, Cepex, La Garriga, Barcelona, Spain) programmed to allow water to enter for 15 min every 2 h. In this way, the oxygen level never dropped below 70% saturation and temperature was controlled by a heat pump (Air-Energy, Heat Pumps Inc., Model 400-Ti, Coral Springs, FL, USA). Subsequently, the water inflow was stopped and 15 min was allowed for the conditions to settle, while the remaining 90 min of the 2-h cycle was used to measure the diminution in oxygen concentration (45 min for each measurement).
Parameters Calculated
Oxygen consumption was graphically represented as a function of oxygen saturation for each crab, and the following parameters were calculated (Fig. 1): Scrit was calculated as the intersection of the average MO2 in the range of oxygen saturation where the animals were “regulators” and a regression line fitted to the observed MO2 values in the range of metabolic oxygen conformity (P for slope <0.05); lethal oxygen saturation (LS) was calculated using the interpolation of the regression line fitted to the observed MO2 values in the range of metabolic oxygen conformity with the oxygen saturation x-axis.
Oxygen consumption (MO 2 ) response as a function of oxygen saturation in one specimen of Maja brachydactyla. Lethal and critical oxygen saturations are represented (LS and Scrit, respectively). The dots correspond to the constant MO2values and the crosses to the MO2values modified at low oxygen levels.
Values obtained as mgO2/L were converted to percentage of oxygen saturation and vice versa assuming a relative humidity of 100% and atmospheric pressure of 760 mmHg according to Weiss (1970). Finally, for the recovery experiments, we represent the oxygen consumption values as a function of time before and after the re-establishment of the inflow of water to the respirometer.
Statistical Analyses
The mean values and standard deviation of each of the parameters, together with the partial correlation coefficients between these and the factors weight and temperature, were calculated. A simple or multiple regression analysis was carried out between the pairs of variables that showed significant partial correlation coefficients, fitting the data to the linear equation (Y = a + b× Temperature + c× Weight). Analysis of variance was used to test the significance of the equation and the Student's t test for the coefficient significance. The fit was evaluated from the simple or adjusted coefficient of determination (R2 or R2-adjusted, respectively) and standard error of the estimation. A significance level of P < 0.05 was employed.
Results
The experiments lasted between 26 and 150 h, depending on the size of the animals, water temperature, and the volume of water in the respirometers. The TAN values during the measurements ranged from 0.02 to 0.92 mg/L (0.21 ± 0.26 mg/L), the highest values corresponding to the largest animals. The pH ranged from 7.3 to 8 (7.59 ± 0.25) with the lowest values being recorded at the end of the experiments.
Figure 1 shows how M. brachydactyla behaved as an oxygen regulator organism, maintaining its oxygen consumption over a wide range of oxygen saturation until Scrit was reached. Frequently some specimens would increase their oxygen consumption before Scrit was reached. Scrit ranged from 24.10 to 53.26% Sat. (42.40 ± 7.38% Sat.; 2.95 ± 0.43 mgO2/ L), and was positively and significantly correlated with water temperature but not with body weight (P < 0.01; Table 2). The regression analysis to explain the values of Scrit as a function of temperature explained 46% of the variance and significantly fitted a linear equation (Fig. 2):
- * P < 0.05.
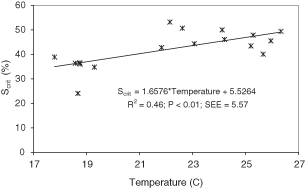
Effect of temperature on critical oxygen saturation (Scrit) in Maja brachydactyla.
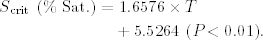
LS ranged between 6.82 and 19.33% Sat. (12.57 ± 4.25% Sat.; 0.88 ± 0.29 mgO2/L), and was positively and significantly correlated with water temperature (P < 0.05) and negatively with weight. The regression analysis to explain LS as a function of temperature and weight explained 44% of the variance and significantly fitted the following equation (Fig. 3):
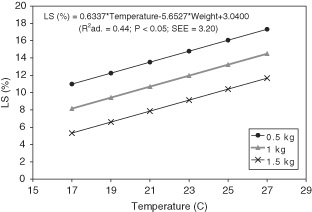
Estimated lethal oxygen saturation (LS) as a function of temperature and body weight in Maja brachydactyla.
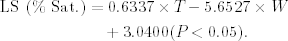
Figure 4 shows the oxygen consumption response of M. brachydactyla after re-establishing the oxygen levels following exposure to hypoxia. It can be observed that after the flow was re-established oxygen consumption remained above initial levels up to about 15–20 h, after which it gradually fall to the same levels. These results were similar in specimens N° 15–17 and no mortality was observed on subsequent days.
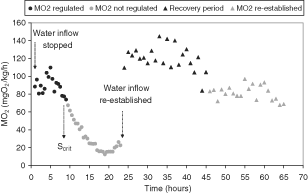
Oxygen consumption (MO 2 ) response as a function of time in a specimen of Maja brachydactyla (N°16:0.85 kg, 24.1 C, female) before and after re-establishing the inflow of water into the respirometer. Oxygen saturation at which MO2is modified or critical value is represented (Scrit).
Discussion
In the conditions prevailing in this study, M. brachydactyla behaved as an oxygen regulator, maintaining its oxygen consumption constant until a critical oxygen saturation of 24–54% was reached, this value depending on water temperature but not on body weight. This is similar to the pattern observed in most freshwater and seawater crustaceans (Taylor 1981; Villarreal 1990; Villarreal and Ocampo 1993; Schmitt and Uglow 1998). A conformist response has only been observed occasionally in species that live in constant oxygen concentrations and which have not developed response mechanisms to hypoxia (Belman 1975), although such a response has also been recorded as a result of inadequate experimental protocols. Allowing sufficient time for adaptation to imposed experimental conditions and eliminating waste metabolites from the respirometers, both essential aspects in this kind of research, has led to defining species as oxygen regulators when they had previously been defined as conformist (see revision of McMahon 2001). In the case of TAN, crustaceans show a higher tolerance than fish (Regnault 1987). Therefore, TAN values recorded in our experimental conditions should not have implied changes in the metabolic response of M. brachydactyla, although confirmation of this should form part of future research. TAN values below 2.3 mg/L are considered safe for fish such as Sparus aurata and Dicentrachus labrax (Person Le-Ruyet et al. 1995). In the case of peneid crustaceans, these values may vary between 1.8 and 17.2 mg/L at pH values of 8 and 7, respectively (Wickins 1976), with more restrictive safe values of 0.5 mg/L in freshwater crustaceans like Macrobrachium rosenbergii (Naqvi et al. 2007).
Table 3 presents a summary of the Scrit values recorded for different crustacean species at different temperatures. Values as low as 2.0% Sat. have been recorded in nektonic marine crustaceans like Boreomysis californica (Childress 1975), and up to 53.1% in freshwater crustaceans like Cherax tenuimanus (Villarreal 1990). In decapod crustaceans like Carcinus maenas values of 38.5% Sat. have been recorded at 18 C (Taylor 1981), similar to those estimated for M. brachydactyla in this study (35.4% Sat.). It has been suggested that the usual partial oxygen pressures in the medium where the species live play an important role in its tolerance to hypoxia (Childress 1975).
| Species | S crit (%) | Temperature (C) | References |
|---|---|---|---|
| Nektonic crustaceans | 1.9–13.1a | 5.5–10.0 | Childress (1975) |
| Austropotamobius pallipes | 25.6a | 15 | Taylor (1981) |
| Carcinus maenas | 25.5a | 10 | Taylor (1981) |
| C. maenas | 38.5a | 18 | Taylor (1981) |
| Cherax tneuimanus | 41.1b | 22 | Villarreal (1990) |
| C. tneuimanus | 53.0b | 26 | Villarreal (1990) |
| Penaeus californiensis | 31.6b | 35 | Villarreal and Ocampo (1993) |
| Nephrops norvegicus | 12.5c | 12 | Schmitt and Uglow (1998) |
| Maja brachydactyla | 35.4 | 18 | Current study |
| M. brachydactyla | 48.6 | 26 | Current study |
- aPO2 (mmHg) values transformed into percentage of oxygen saturation from the equation: % Sat. = (PO2/PO2 Sat.) × 100, where PO2 is the partial oxygen pressure measured and PO2 Sat. is the partial oxygen pressure at saturation. PO2 Sat. = (PA–PV) × %O2, where PA and PV are atmospheric pressure and water vapor pressure, respectively, at the experimental temperature in mmHg, and %O2 is the percentage of atmospheric oxygen (20.9%).
- bOxygen concentration values (mgO2/L) transformed into percentage of oxygen saturation calculating saturation point according to Weiss (1970).
- cPO2 values expressed as KPa transformed into mmHg (1 mmHg = 0.133 KPa) and then into percentage of oxygen saturation.
In general, as observed in this study, temperature has been seen to have a negative effect on the regulatory capacity. In M. brachydactyla, for example, Scrit was estimated at 35.4% Sat. at 18 C and 48.6% Sat. at 26 C; in C. maenas figures of 25.5% Sat. at 10 C and 38.5% Sat. at 18 C have been obtained (Taylor 1981); while in Cherax tneuimanus it was 41.1% Sat. at 22 C and 53.1% Sat. at 26 C (Villarreal and Ocampo 1993). According to Taylor (1981), temperature would provoke adverse effects in the affinity of hemocyanin for oxygen, both directly through the lower pH of hemolymph and directly through an alosteric effect on the hemocyanin molecule. A decrease in the pH of body fluids decreases the affinity of hemocyanin for oxygen, thereby affecting enzymatic systems and metabolic processes (Reiber and Birchard 1993). However, other studies have found that l-lactate could increase hemocyanin oxygen affinity in a number of crustaceans (Truchot 1980; Bouchet and Truchot 1985), or even the presence of hemocyanin insensitive to pH (Mauro and Moore 1987). Furthermore, hemocyanin oxygen affinity is modulated by the presence of many compounds such as ammonia, trimethylamine, urate, and CO2 (Lallier et al. 1987; Sanders et al. 1992). As a consequence, the oxygen levels of water need to be higher to maintain the hemolymph saturated with oxygen at high temperatures.
The lethal concentrations obtained as a result of hypoxia in M. brachydactyla varied greatly (6.8–19.3%) and were significantly correlated with temperature. As in the case of Scrit, the higher the temperature, the more pronounced the consequences of hypoxia and therefore higher LSs were registered. These results are similar to those obtained in several species of fish (Schurmann and Steffensen 1992; Secor and Gunderson 1998) and cephalopods (Cerezo Valverde and García García 2005). According to Frederich and Pörtner (2000), high temperatures favor the establishment of anaerobic metabolism in M. brachydactyla because of the negative effect on hemocyanin oxygenation.
As regards the influence of body weight, a negative correlation was observed with LS, the lowest LS values obtained in the largest specimens (N° 2 and 13) and suggesting a lower degree of sensitivity compared with the small specimens. These results could be explained by the higher oxygen requirements of smaller crustaceans per unit of body weight (Carvalho and Phan 1997; Cerezo Valverde et al. 2009).
As in previous studies in crustaceans, in the recovery period following hypoxia, M. brachydactyla showed increased oxygen consumption. Hypoxia implies lower adenosine triphosphate and phospagen, glycogen and glutamate, and higher lactate and alanine levels in tissues (Bridges and Brand 1980b; Hill et al. 1991; Hervant et al. 1997). The metabolic processes required to re-establish prehypoxia conditions would be responsible for this oxygen debt. According to Bridges and Brand (1980b), this debt would depend on the duration and severity of the hypoxia.
In conclusion, survival oxygen levels can be suggested for this species. On the basis of the results, M. brachydactyla possesses mechanisms to combat hypoxia, although they would be incapable of regulating oxygen consumption below 36% at 18 C and 49% at 26 C. Below these levels, anaerobic metabolism would be evident and the animals would only survive a short length of time. We recommend suitable values of oxygen of above 65% Sat. (applying safety margin of 10%) and cool temperatures (<20 C) for M. brachydactyla maintenance in recirculation water system or fisheries.
Acknowledgments
This project was financed by JACUMAR Spanish National Plans for Aquaculture. Thanks are due to the Institut de Recerca i Tecnología Agroalimentaries (IRTA, Tarragona, España) and Centro de Investigación y Formación Acuícola Pesquera (CIFAPA) “Aguas del Pino” (Huelva, España) for their collaboration in obtaining the animals used.




