Growth of Juvenile Green Sea Urchins, Strongylocentrotus droebachiensis, Fed Formulated Feeds with Varying Protein Levels Compared with a Macroalgal Diet and a Commercial Abalone Feed
Present address: 10 John Street, Apartment 212, Dundas, Ontario L9H 6J3, Canada.
Abstract
The effects of varying protein and carbohydrate levels in prepared diets on the somatic growth of juvenile green sea urchins, Strongylocentrotus droebachiensis, were examined. Ten diets were tested on 600 hatchery reared urchins (mean start weight = 0.11 g) for 6 mo with three replicate groups per diet. Nine of the diets were prepared specifically for urchins and varied in protein (16–40% protein) and carbohydrate (29–49% carbohydrate) levels. The other two diets consisted of a commercially available abalone diet and the kelp, Saccharina latissima. Weight measurements were carried out at 6-wk intervals, and at the end of the study urchins were individually weighed and a subsample from each treatment was analyzed for gonad weight and color. End weights after 6 mo ranged from 2.56 g for urchins fed the abalone diet to 6.11 g for urchins fed one of the prepared diets. Most of the prepared feeds outperformed kelp, and significant differences in growth were detected between some of the diets. In general, diets with lower protein levels (16–22% protein) and higher carbohydrate levels (>40% carbohydrate) produced the fastest growth. However, further diet refinement and/or use of finishing diets may be necessary to optimize gonad quality.
The green sea urchin, Strongylocentrotus droebachiensis, is highly valued in Japan and throughout Asia for the quality of its edible gonads, known as uni. This has led to significant fishing effort in many regions throughout the circumpolar range where S. droebachiensis is found. In the Gulf of Maine (USA and Canada), overfishing and resulting ecosystem changes are considered to be major causes of a significant decline in green sea urchin stocks (Harris et al. 2000; Steneck et al. 2004). The reduced urchin supply from capture fisheries and their high-market value have led to efforts to commercially farm S. droebachiensis and other urchin species (Robinson 2004). These efforts include sea-based stock enhancement and cage methods and land-based methods (Kirchhoff et al. 2008). Projects are currently underway in Norway, Canada, and the USA to develop methods for land-based echiniculture of green sea urchins and to evaluate the economic viability of these efforts (Kirchhoff et al. 2008; Hagen and Siikavuopio 2010; Pearce and Robinson 2010).
Successful land-based echiniculture will require the use of formulated diets. Although urchins can be grown in captivity using various species of macroalgae, this approach is unlikely to be environmentally sustainable or economically viable for commercial scale production (Lawrence et al. 2001). Macroalgae is relatively low in protein and energy and varies seasonally in nutrient profiles (Larson et al. 1980; Lobban and Harrison 1994; Kelly 2002; Schlosser et al. 2005). Formulated urchin feeds can be used to maximize somatic growth during the juvenile stages (McBride et al. 1998; Akiyama et al. 2001; Spirlet et al. 2001; Kennedy et al. 2005; Kennedy et al. 2007a, 2007b) or to improve gonad yield and quality during maturity (Walker and Lesser 1998; Robinson et al. 2002; Pearce et al. 2004). Ultimately, these diets will have to produce gonads with acceptable market quality. The use of different diets for different life stages will likely be required to culture urchins from hatchery to harvest (Kelly et al. 1998; Lawrence et al. 2001).
Many of the studies to date on the nutrition of urchins in culture conditions have examined the role of nutrients in promoting gonad yield and quality (Barker et al. 1998; Meidel and Scheibling 1999; Robinson et al. 2002; Shpigel et al. 2005; Siikavuopio et al. 2007). A number of other studies have compared somatic and gonad growth of urchins fed various formulated feeds with macroalgal diets, or compared the use of different algal species as feed (Cook et al. 1998; Russell 1998; Spirlet et al. 2001; Chang et al. 2005; Daggett et al. 2005; Lyons and Scheibling 2007). However, the specific nutrient requirements for optimal sea urchin somatic growth in aquaculture remain obscure. Kennedy et al. (2007a) have presented evidence that a lack of appropriate dietary minerals and pigments is a likely factor contributing to the shortcomings of prepared feeds in those cases where natural kelp diets have produced better somatic growth than prepared diets. Other recent studies have begun defining the gross levels of protein and carbohydrates required for somatic growth by urchins. McBride et al. (1998) observed no significant differences in growth of Strongylocentrotus franciscanus fed prepared diets with protein levels of 30, 40, and 50%, but they did see a decrease in feeding rate with increased protein levels. Fernandez and Boudouresque (2000) compared growth of Paracentrotus lividus given three feed types varying in quality (“vegetable,”“mixed,” and “animal”), and found that the higher protein feeds (“mixed” and “animal”) with relatively lower carbohydrate levels (28.9% protein/35.3% carbohydrate and 47.2% protein/15.9% carbohydrate, respectively) gave better results than the “vegetable” type feed (12.7% protein/58.2% carbohydrate). Akiyama et al. (2001) concluded that a dietary protein level of 20% was the optimum for Pseudocentrotus depressus when casein was the sole protein source. Hammer et al. (2006) observed similar results in a feeding study with the sea urchin Lytehcinus variegatus, where they determined that a 20% protein diet was more efficient than either a 9% protein or a 31% protein diet.
This study was conducted to determine the optimum protein level in the diet for young green sea urchins, S. droebachiensis. Given the importance of protein for growth and the expense of protein as a feed ingredient, this topic needs to be addressed to optimize the biological and economic efficiency of land-based green sea urchin culture. Eight formulated urchin diets with varying protein/carbohydrate levels were compared with one another and with the kelp, Saccharina latissima (previously Laminaria saccharina), a readily available species known to be consumed by green sea urchins (Vadas 1977; Daggett et al. 2005). A commercially available high protein abalone feed was included in the trial as a possible alternative diet for green urchins. Hatchery reared urchins with a starting test diameter (TD) of approximately 5.5 mm were used for the trial; hatchery reared urchins of this small size have rarely been used in previous formulated diet trials (Akiyama et al. 2001; Spirlet et al. 2001).
Materials and Methods
Urchins and Holding Conditions
The juvenile urchins used in the trial were selected on the basis of size from a population of approximately 10,000 9 mo post-settlement hatchery urchins reared at the Center for Cooperative Aquaculture Research (Franklin, ME, USA), where the feed trial also took place. The urchins had been reared almost exclusively on the kelp, S. latissima for the 9 mo prior to this study. The population as a whole varied between 3 and 15 mm TD, so to minimize variation and the effect of differential growth rates urchins with a TD of approximately 5.5 mm were selected from the population, weighed using an A&D Instruments digital scale to within 1 mg, and pooled to obtain 600 individuals with a mean start weight of 0.109 ± 0.011 g (CV ≤ 10.5%). The urchins were starved for 1 wk prior to the trial. Ten diets were tested, with three replicates per diet and 20 urchins per replicate. Replicates were segregated into slotted plastic hydroponic plant baskets (16.5 × 16.5 × 12.7 cm deep) randomly distributed into three shallow round fiberglass tanks supplied with a source of flow through seawater. Tank flows were equivalent to one tank turnover per 40 min to maintain water quality and avoid accumulation of metabolites. Oxygen levels were measured daily with an OxyGuard Handy Polaris probe (OxyGuard International A/S, Denmark) and ranged from 7.2 to 8.2 mg/L. Salinity was checked weekly with a refractometer and was stable throughout the trial at 30–32 ppt. Temperatures were maintained at 11.4 ± 1.2 C and the light regime was 8 L:16 D.
Study Diets
The nine formulated diets used in this trial were analyzed once for proximate composition and the kelp three times to account for seasonal variation. The proximate analyses were conducted by New Jersey Feed Labs, Inc. (Trenton, NJ, USA) according to AOAC methods 990.03 (protein by combustion), 920.39 (fat by ether extraction), 978.10 (fiber), 942.05 (ash), and 930.15 (moisture, using loss on drying at 135 C for 2 h). Carbohydrate levels were determined by subtraction from 100%. Eight of the diets were prepared urchin feeds formulated and produced at Texas A&M University (designated as 33, 34, 35, 36, 37, 38, 39, and 40). Protein sources for these diets were a proprietary mix of kelp, soybean, casein, fish, and squid; and the carbohydrate sources were wheat, kelp, and soybean. Lipid levels ranged from 3.6 to 6.2%, which are within a suitable range for meeting urchin growth requirements (Kelly 2002; Kennedy et al. 2007b). Each of the eight Texas A&M diets contained up to 28% marine ingredients, 28.7% plant ingredients, 1.1% carotenoids, 0.7% vitamin premix, 24 % mineral mix, 7.2% binder, and antifungal–antioxidant. The other two diets consisted of a commercial abalone feed (designated as AN for abalone noodles) (proprietary formulation by Adam & Amos Abalone Foods Pty. Ltd., Australia), and the kelp, S. latissima (designated as K for kelp). The kelp was collected fresh from a local pier about every 2 wk throughout the course of this study and maintained between collections in a chilled seawater tank.
The proximate diet analyses converted to a dry weight basis are summarized in Table 1 for all diets used in this study. The kelp was sampled at the beginning, middle, and near the end of the trial, corresponding to winter, spring, and summer, and the varying nutrient compositions likely reflect seasonal variations in growth, light, temperature, and nutrient regimes seen in the Gulf of Maine. For the purposes of analysis, a composite (average) nutrient profile was used for the kelp when comparing it to the formulated feeds. On a wet weight basis the kelp averaged 88% moisture, compared to a moisture content of 7–11.7% for the formulated diets. The kelp-fed urchins would thus have to consume significantly more kelp to match the nutrient intake of the urchins fed formulated diets in this study.
| Diet | Protein | Fat | Ash | Carbohydrate | 24-h stability |
|---|---|---|---|---|---|
| 39 | 16.0 | 4.6 | 37.7 | 41.7 | Disintegrated |
| 40 | 16.4 | 5.0 | 45.3 | 32.9 | Disintegrated |
| 33 | 17.1 | 4.7 | 45.3 | 32.9 | Disintegrated |
| 38 | 22.6 | 5.0 | 25.2 | 47.2 | Partially intact |
| 37 | 23.3 | 4.8 | 30.6 | 41.3 | Partially intact |
| 34 | 24.5 | 4.7 | 36.2 | 34.6 | Partially intact |
| 36 | 32.9 | 5.1 | 25.0 | 37.0 | Intact |
| 35 | 40.3 | 5.3 | 25.3 | 29.1 | Intact |
| AN (abalone feed) | 36.3 | 3.6 | 7.5 | 52.6 | Intact |
| Kelp (composite) | 23.6 | 3.2 | 35.0 | 38.2 | Intact |
| Kelp (February 19, 2008) | 24.4 | 7.3 | 40.2 | 28.1 | – |
| Kelp (May 6, 2008) | 32.9 | 0.6 | 35.2 | 31.3 | – |
| Kelp (June 25, 2008) | 13.4 | 1.8 | 29.6 | 55.2 | – |
- 1Texas A&M diets are ranked in order from low to high protein.
The diets were ranked based on percent protein levels (Table 1). Of the Texas A&M diets, diet 39 had the lowest protein content (16.0% dry weight) and diet 35 had the highest protein content (40.3%). The abalone feed had the second highest protein content (36.3%) of all the formulated feeds tested and also contained the highest levels of carbohydrates (52.6% dry weight). The abalone diet was also significantly lower in ash (7.5%) than all of the other diets used in this study (25–45.3% ash). In terms of average composition on a dry weight basis, kelp was most similar to the Texas A&M diet 34.
Feeding Levels
Initially, the urchins were fed the formulated feeds every 48 h, but this was increased to daily feeding after 2 wk into the trial. Feeding amounts were adjusted based on growth and 24-h consumption to maintain feeding at approximately 2% body weight and ad libitum (to satiation). The amount of feed added to each replicate was gradually increased through this study, but every replicate in each of the formulated feed treatments received the same quantity of feed at each feeding. Uneaten feed and feces was removed from all formulated feed replicates at each feeding and the tanks and replicate baskets were cleaned of biofilms once per week. The formulated diets varied in water stability so a scoring system was devised to evaluate stability. Diets that remained completely intact after 24 h were scored as a 3, diets that had disintegrated into a powder after 24 h were scored as a 1, and diets that were partially intact (broken into intact pieces and powder) were scored as a 2. Diets were scored daily at every feeding when the uneaten feed was removed.
The kelp-fed replicates were fed on the same schedule as the formulated feed replicates, but any kelp left over from the previous feeding was not removed. Instead, the amount of kelp added to the replicates was decreased or increased to maintain a constant supply of kelp in the baskets while preventing an excess from being left over. In this way, it was possible to approximate the total amount of kelp consumed by the urchins at the end of the experiment.
Urchin Measurements
Weight measurements were taken at d 0, 37, 83, 117, 142, and 216 when the trial was ended. This provided data that could be used to calculate the specific growth rates (SGRs) for five growth intervals. Survival/escapement was also recorded at these sampling intervals. Urchins were blotted dry before weighing. Mean individual wet weights were determined within replicates at d 37 and 83 by weighing all of the urchins in the replicate and dividing by the number of urchins. On all other sampling days individual urchin weights were recorded. Upon termination of this study, TDs were measured for each individual using digital calipers (model CD-6 PMX, Mitutoyo Corporation, Kawasaki, Japan), and five urchins per replicate were randomly selected and dissected to determine gonad wet weight.
Data Analysis
Whole wet weight, weight gain, SGR, and end TD were calculated using averages from each replicate basket (n = 3). The mean urchin wet weight per diet was calculated at each sampling interval using the pooled averages from diet replicates (n = 3). The average weight gain per urchin for each replicate between sampling intervals was calculated as: weight gain (g) = [whole wet weight (t2)–whole wet weight (t1)]. The mean gonad wet weight for each diet was calculated at the end of this study using pooled averages from the five urchins subsampled from each replicate. Urchin gonadal/somatic indexes (GSIs) were calculated as: GSI (%) = (wet gonad weight/whole wet weight) × 100. The mean GSI and mean end TD for each diet were calculated using the pooled averages from the diet replicates. Average SGR per urchin was determined for each replicate basket for each sampling interval according to the following equation: SGR(%) = ([ln (whole wetweight(t2))]–[ln(whole wet weight(t1))]/ [(t2)–(t1)]) × 100.
Multivariate repeated measures analyses were used to check for an interaction between diet treatment and time for whole wet weight, weight gain, and SGR. The interaction was significant (Wilks' λ: P < 0.05), so data from each measurement day were analyzed individually using a one-way ANOVA. For the endpoint data (TD, gonad wet weight, and GSI), a one-way ANOVA was performed for each response variable. Residuals from each ANOVA were then analyzed for normality and equal variance using the Shapiro–Wilk test for normality and Levene's equal variance test, respectively, and an acceptance level of P > 0.10 was adopted for both. There were occasional violations of Levene's test for equal variance, but this was disregarded when the normality assumption was satisfied, because differences between diets were often so great that a transformation applied to all measurement days would not likely have affected the results. In two cases where data were obtained over time in this study (whole wet weight at d 142 and weight gain from d 117 to 142), the assumptions of both equal variance and normality were not met (Levene's test: P < 0.01; Shapiro–Wilk: P < 0.01). Further statistical analyses in these instances were abandoned and only means are presented. For endpoint data, if either normality or equal variance assumptions were not met the residuals and outliers were examined and transformations were applied as needed. As such, gonad wet weight data were fourth root transformed and GSI data were square root transformed prior to any further statistical analyses, but original values are presented for ease of interpretation. When normality and variance assumptions were satisfied, the Ryan–Einot–Gabriel–Welsch Q (REGWQ) post-hoc test was used to make pair-wise comparisons among treatment means with a P < 0.05 level of significance.
Results
Survival and Growth
At the end of the trial, 12 animals (2%) were missing because of escapement or mortality, but losses were random across treatments and there were no significant differences in survival between treatments.
Significant differences between diets as reflected in urchin whole wet weight were evident by d 37 and continued throughout this study (Fig. 1). By d 83, the urchins fed with the Texas A&M diets 39 and 40 surpassed all others in weight, with the remaining treatments producing weights in the following descending order: 33, 38 > 37 > 34 > 36 > kelp > 35 > abalone noodles (ANOVA, P < 0.05). Day 117 weights were highest for diet treatments 33, 38, 39, and 40, with the remaining treatments producing weights in the following descending order: 37 > 34 > 36 > kelp > 35 > abalone noodles (ANOVA, P < 0.05). At d 216 the end weights ranged from 2.56 g (SE = 0.120) for the abalone diet to 6.11 g (SE = 0.243) for the Texas A&M diet 38 (Fig. 1). Diets 33, 37, 38, 39, and 40 were the top performing diets and showed statistically similar weight gains (Table 2). These diets were all ranked as low to intermediate in protein levels (Table 1). The remaining diets produced end weights in the following descending order: 34 > 36 > kelp > 35 > abalone noodles (ANOVA, P < 0.05). Throughout this study, the high protein diets (diets 35, 36, and AN) produced significantly less growth than the other diets, and the kelp treatment also produced significantly lower weight gains compared with the top performing diets. Growth of urchins fed diet 34 was statistically different from those on all other diets, and is best described as intermediate between the group containing the top five performing diets and the two lower performing groups. In terms of percent protein and carbohydrate (dry weight basis), this diet was the most similar to kelp (Table 1), although it outperformed the kelp in terms of growth (5.01 g vs. 4.47 g) (REGWQ test; P < 0.05).
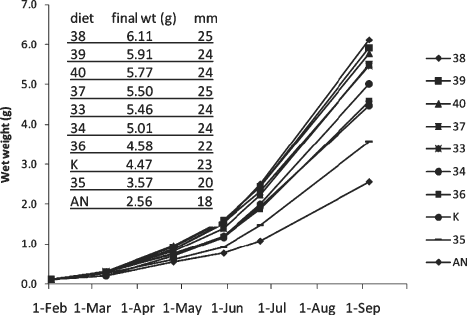
Growth of juvenile urchins fed formulated feeds or kelp. Urchins were weighed at d 0, 37, 83, 117, 142, and 216. Test diameters were measured at d 216.
| Diet | Protein level (%) | Mean weight gain (g) | Mean SGR | Mean percent gonad index |
|---|---|---|---|---|
| 39 | 16.0 | 5.81 ± 0.2a | 1.86 ± 0.00a | 17.1 ± 1.1c |
| 40 | 16.4 | 5.66 ± 0.25a | 1.84 ± 0.01a | 17.8 ± 1.1b |
| 33 | 17.1 | 5.35 ± 0.17a | 1.81 ± 0.01b | 22.5 ± 1.5d |
| 38 | 22.6 | 6.00 ± 0.24a | 1.87 ± 0.02a | 21.1 ± 0.5a |
| 37 | 23.3 | 5.39 ± 0.21a | 1.81 ± 0.01a | 21.8 ± 1.0a |
| 34 | 24.5 | 4.9 ± 0.07b | 1.78 ± 0.01c | 19.9 ± 0.3a |
| 36 | 32.9 | 4.47 ± 0.08c | 1.72 ± 0.01d | 20.3 ± 0.6a |
| 35 | 40.2 | 3.47 ± 0.08d | 1.62 ± 0.01e | 20.7 ± 1.3a |
| Abalone | 36.3 | 2.45 ± 0.12e | 1.47 ± 0.02f | 12.7 ± 1.4e |
| Kelp | 23.6 | 4.35 ± 0.13c | 1.71 ± 0.01d | 8.2 ± 0.5f |
- SGR = specific growth rate.
- 1Letters associated with each value indicate statistically significant differences among diets within each parameter (Ryan–Einot–Gabriel–Welsch Q test; P < 0.05).
Significant differences in end TD were also found among diet treatments in this study, with TDs ranging from 18 mm (SE = 0.51) for the abalone diet to 25 mm for prepared feeds 37 (SE = 0.43) and 38 (SE = 0.58) (Fig. 1). Prepared feeds 33, 34, 37, 38, 39, and 40 all produced similarly large end TDs, with the remaining treatments producing end TDs in the following descending order: kelp > 36 > 35 > abalone noodles (ANOVA, P < 0.05).
Specific Growth Rates
There were significant differences between the formulated diets and kelp in terms of SGRs during four of the five sampling intervals (Table 3). During the first interval (d 0–37), the urchins responded quickly to the introduction of formulated feeds and their growth rates surpassed those seen in the kelp replicates (ANOVA, P < 0.05). However, during the second interval (d 37–83), the growth rates seen in the kelp replicates (2.7%/d) surpassed those seen in any of the formulated feed replicates. The growth rates for the kelp-fed replicates remained relatively high until the fifth sampling interval (d 142–216), when they slowed to 1.13%. The average SGRs for the urchins fed formulated feeds during the first two growth intervals (from 0 to 83 d) exceeded 2% for every diet treatment, but during the third growth interval they declined to between 0.96 and 1.65%. The SGRs increased slightly during the fourth interval (117–142 d), and then decreased again during the final interval (142–216 d) to the lowest rates seen in the trial, to an average of 1.19%. The SGRs over the entire course of the trial (d 0–216) ranged from 1.47% (SE = 1.83 × 10−2) for the abalone diet to 1.87% (SE = 1.60 × 10−2) for Texas A&M diet 38, and were statistically similar for diets 37, 38, 39, and 40 (Table 2). The high protein diets and the kelp diet all showed significantly slower growth rates over the course of this study than those seen with the low and intermediate protein diets.
| 0–37 | 37–83 | 83–117 | 117–142 | 142–216 | |
|---|---|---|---|---|---|
| 33 | 2.70 ± 0.03a | 2.41 ± 0.02b | 1.65 ± 0.02a | 1.61 ± 0.01e | 1.16 ± 0.05 |
| 34 | 2.75 ± 0.02a | 2.00 ± 0.04e | 1.40 ± 0.10a | 2.02 ± 0.03a | 1.24 ± 0.03 |
| 35 | 2.45 ± 0.10b | 1.79 ± 0.03f | 1.21 ± 0.05b | 1.87 ± 0.07a | 1.20 ± 0.01 |
| 36 | 2.64 ± 0.05a | 2.06 ± 0.06d | 1.30 ± 0.06a | 1.87 ± 0.03a | 1.20 ± 0.01 |
| 37 | 2.80 ± 0.09a | 2.12 ± 0.01d | 1.56 ± 0.08a | 1.90 ± 0.07a | 1.22 ± 0.05 |
| 38 | 2.76 ± 0.10a | 2.31 ± 0.06b | 1.64 ± 0.17a | 1.97 ± 0.02a | 1.21 ± 0.05 |
| 39 | 2.73 ± 0.04a | 2.49 ± 0.04b | 1.61 ± 0.06a | 1.70 ± 0.04c | 1.20 ± 0.04 |
| 40 | 2.82 ± 0.05a | 2.47 ± 0.05b | 1.50 ± 0.09a | 1.67 ± 0.02d | 1.16 ± 0.06 |
| AN | 1.72 ± 0.06c | 2.20 ± 0.09c | 0.96 ± 0.06c | 1.37 ± 0.04f | 1.16 ± 0.04 |
| K | 1.62 ± 0.06c | 2.70 ± 0.03a | 1.50 ± 0.09c | 2.05 ± 0.07a | 1.13 ± 0.02 |
- 1Letters within columns indicate statistically significant differences among diets at the interval specified (Ryan–Einot–Gabriel–Welsch Q test; P < 0.05).
Gonadal–Somatic Index
Significant differences in the GSI were also found at the end of this study, with GSIs ranging from 8.23% (SE = 0.506) for kelp to 21.8% (SE = 0.458) for prepared feed 37 (Table 2). Prepared feeds 34, 35, 36, 37, and 38 all produced similarly large GSIs, with the remaining treatments producing GSIs in the following descending order: 40 > 39 > 33 > abalone noodles > kelp (ANOVA, P < 0.05). However, there was no statistically significant relationship between the GSI and the protein or carbohydrate level of the diet. Diets with intermediate or high protein levels produced similar GSIs, and all of the formulated feeds with the exception of the abalone noodles had GSIs that exceeded 15%. The kelp-fed urchins had a significantly lower GSI (8.23%) than that seen in any of the formulated feeds.
Feed Efficiency
The sum total of feed (grams wet weight) provided to each of the formulated feed replicates through the course of the trial was 179.5 g, whereas the kelp-fed replicates received 1040 g. The total amount of kelp actually consumed by the kelp-fed urchins could be closely approximated, but this could not be carried out for the formulated diets and therefore the true feed conversion ratios could not be calculated. However, it was possible to calculate and compare the ratio of total feed input per treatment to the total biomass gain per treatment as an approximate measure of feed efficiency. The mean total biomass gain per replicate for the top performing Texas A&M diet 38 was 120 g after 216 d (6 g/urchin), whereas the mean biomass gain per kelp-fed replicate was 88 g (4.4 g/urchin). Thus the ratio of feed input to biomass gain was 1.5/1 for the top performing formulated feed and 11.8/1 for the kelp. However, if the kelp is converted to a dry weight basis (average 88% moisture content), then this ratio improves to 1.4/1.
Feed Stability
The 24-h stability ratings in seawater varied between the diets but remained consistent for each diet over the course of this study. There was a clear relationship between protein levels and water stability: the low protein diets dissolved into a powder within 12–24 h, the intermediate protein diets broke up into small pieces and powder within 24 h, and the high protein diets remained entirely intact for 24 h or even longer (Table 1).
Discussion
TD has been traditionally favored as a proxy for measuring urchin growth (Swan 1961; Lang and Mann 1976; Raymond and Scheibling 1987; Devin et al. 2004; Pearce et al. 2005). However, as Ellers and Johnson (2009) point out, measuring diameter can be imprecise because urchins have spines, are not always exactly circular, and diameter measurements do not take into account potential height variation (some urchins may be more flattened than others). They recommended that weight be used in growth studies, and demonstrated that a formula incorporating the cube root of the weight could be used to estimate the nominal diameter of the urchin with six times the accuracy of a direct diameter measurement. Techniques utilizing image analysis software may increase the accuracy of TD measurements (Kennedy et al. 2007a, 2007b), but they require additional investment in equipment and time, and it remains to be tested whether this method provides a better measure of growth than weight. For these reasons, in this study weight was chosen as the primary measure of growth in addition to diameter. This had the further advantage of allowing for calculation of GSIs; an important consideration for urchins reared on formulated diets. Finally, the calculation of the SGR, which is widely used in aquaculture growth and feed studies, will yield very different results in urchins if TD is used instead of weight as the defining growth number. For example, in our study when weight was used to calculate the SGR we obtained a maximum SGR of 1.87%; for the same urchin the SGR based on TD growth was 0.7%.
As an urchins' growth is not linear over the course of its life span (Russell 1998; Lawrence 2000; Ellers and Johnson 2009), it is important that growth comparisons between studies be limited to urchins of similar size ranges. The top performing diet (38) in this study resulted in a net growth of 6 g (from 0.11 to 6.11 g) over the course of 216 d, with a corresponding SGR of 1.87%/d. In terms of TD, the urchins showed a net increase in TD of 19.5 mm (from 5.5 to 25 mm) over 216 d; a rate of increase equivalent to 2.7 mm/mo. The growth rates of the juvenile green urchins fed the formulated diets in our study compare favorably with growth rates of similar sized S. droebachiensis in the wild. Pearce et al. (2005) cite a number of studies estimating growth rates of S. droebachiensis in the wild, and reported a range of 0.2 mm to 1.2 mm/mo. Russell (2000) projected 2–3 yr from metamorphosis for green sea urchins to attain a TD of 20–25 mm in the field, whereas in the current study this was attained in 16.2 mo (9 mo to 5.5 mm + 216 d to 25 mm). The growth rates seen in our study also compare favorably with those seen in studies where similar sized green sea urchins were grown in controlled culture conditions. During a 490-d feeding trial with green sea urchins, Daggett et al. (2005) reported TDs of less than 20 mm and weights of less than 5 g at 200 d for green sea urchins grown on either formulated diets or macroalgae and with a starting size of about 9 mm. Kennedy et al. (2007a) reported a maximum SGR of 0.6% (based on TD) for wild collected juvenile green sea urchins fed a fortified formulated diet. The maximum TD-based SGR seen in our study was 0.7% (19.5 mm increase over 216 d). Hagen (2004) reported near exponential growth of hatchery reared S. droebachiensis, with an approximate “doubling time” in wet weight of 2.8 mo. Using his formula (doubling time = [time1–time0]/[log2 weight1–log2 weight0]), we saw a doubling time of 37.2 d for the fastest growing urchins in our study.
A strong correlation was seen between protein levels in the formulated diets and growth rates of the urchins, but there was no correlation between carbohydrate levels or carbohydrate/protein ratios and growth. The group of five top performing diets showed similar growth rates and they all had relatively low to intermediate protein levels (16–23.3% protein), as compared with the three high protein diets (32.9–40.3% protein) that only performed as well as, or even worse than, the kelp. This is in general agreement with other studies indicating that protein levels of 16–25% are optimal for urchin somatic growth (Akiyama et al. 2001; Hammer et al. 2004, 2006; Kennedy et al. 2005). It is also clear from the results seen here that formulated feeds can outperform kelp for urchins grown in culture. This is not always the case (McBride et al. 1998; Williams and Harris 1998), indicating that the nutritional composition of both the formulated feeds and the kelp is critical. In this study, the SGRs for the kelp-fed urchins varied between sampling intervals, and at the second sampling interval (d 37–83) they exceeded the SGRs seen for any of the formulated feeds (Table 3). This interval includes the period (May) when the proximate analysis of the kelp showed the highest protein levels (32.9%; dry weight basis) seen for kelp during the course of this study (Table 1). This shows that when seaweed is harvested at peak protein levels it can be effectively used for somatic growth. However, during the last sampling interval (d 142–216, July–September), the SGR for the kelp-fed urchins was 1.13%; the lowest SGR seen for the kelp-fed urchins during the study and ranking it at the bottom of all the diets for this interval. This interval coincides with reduced protein levels of 13.4% observed at the June proximate analysis for kelp. The correlation seen here between variable protein levels in macroalgae and urchin growth rates has been observed in other studies where macroalgae was used as an urchin feed (Vadas et al. 2000; Schlosser et al. 2005). Seasonal variation in seaweed nutritional quality (Larson et al. 1980; Lobban and Harrison 1994; Schlosser et al. 2005) underscores the need for developing formulated feeds suitable for commercial scale aquaculture.
As the trial progressed there was a general decline in the SGRs seen in all of the formulated feed treatments, beginning after d 83 of the trial during the third growth interval (Table 3). This decline was followed by an increased SGR for all treatments during the fourth growth interval (117–142 d), only to be followed by a further decline during the fifth growth interval (142–216 d). The increased SGR seen during the fourth interval may have been due to an increase in water temperature. For 30 d during the fourth interval the water temperature averaged 13.6 C and peaked at 15.6 C, as opposed to the average temperature of 10.8 C maintained during growth intervals 1–3 and 11.8 C during growth interval 5. The optimal temperature range for somatic growth and survival of early post-settled S. droebachiensis appears to be 9–13 C (Pearce et al. 2005). Devin et al. (2004) reported faster growth but decreased survival at 15 C for 3–5 mm TD green urchins. Kennedy et al. (2005) observed an acceleration in SGRs when the water temperatures increased in their feeding trial (14–16 C from 12 C). In the present trial, this relatively warm period of 30 d may have countered the overall trend of declining growth; a trend that was reasserted during the fifth interval once the water temperature was restored close to its former level.
The larger question is the cause of the overall trend of declining SGRs observed with the urchins fed formulated feeds after around 90 d into the trial. This phenomenon has been documented in other feed trials as well. Kennedy et al. (2007b) saw an initial increase in growth rates followed by a decline after mo 5 in juvenile green urchins fed prepared diets. Juvenile S. franciscanus fed formulated diets also showed declining growth rates after 5 mo (McBride et al. 1998), and the authors suggested that this may have been at least partially attributable to increased reproductive development.
Nutritive phagocytes in the gonads act as a site of nutrient storage and it is well documented in a number of species that mature urchins respond to increased food availability or quality with increased gonad production (Russell 1998; Walker and Lesser 1998; Lawrence 2000; Lawrence et al. 2001; Spirlet et al. 2001; Schlosser et al. 2005). In the case of mature urchins this is a desirable outcome, as the gonads are the marketable product, but for immature urchins the goal is to maximize somatic growth. Precocious gonad growth may result from a surplus of nutritional energy beyond what can be effectively utilized for somatic growth (Lawrence 2000). In this study, all of the formulated feeds resulted in significantly higher GSIs than those seen in the kelp-fed urchins (Table 2), and it is tempting to hypothesize that this gonad development came at the expense of somatic growth, resulting in declining growth rates as the feed trial progressed. The large gonads observed at the end of this study are indicative of precocious gonad development for this species.
At the time that the decline in SGR was observed, the urchins were approximately 1 yr post-metamorphoses and 12 mm TD. This is both younger and smaller than the 2–3 yr and 25 mm observed in the field where green sea urchins first reach reproductive maturity and their growth rates decline (Siversten and Hopkins 1995; Vadas and Beal 1999). The growth curve (TD–age relationship) generated by Russell (2000) for green urchins shows steady growth until around 35–40 mm before growth rates begin to decline. Hagen (2004) observed exponential growth rates in S. droebachiensis until the urchins were at least 6–7 g, and extrapolation of the curve indicated that they maintain this rate until they are about 2-yr old.
Precocious gonad growth has been observed with other species when they were fed formulated diets, including L. variegatus (Hammer et al. 2004), Psammechinus miliaris (Kelly et al. 1998), P. depressus (Akiyama et al. 2001), and Loxechinus albus (Olave et al. 2001). Hammer et al. (2004) suggested that a decrease in the rate of growth of L. variegatus fed high protein diets could have been due to the precocious gonad development they observed. Kennedy et al. (2005) saw large gonads but smaller TD in S. droebachiensis fed high energy prepared diets compared with urchins fed a lower energy kelp diet, and suggested that this was because of preferential allocation of energy into gonad production. However, Kennedy et al. (2005) note that several other nutritional factors could have also contributed to the poor somatic growth they observed. The evidence that there is a conflict between somatic growth and gonadal growth in prereproductive urchins remains inconclusive (Lawrence 2000). Both Minor and Scheibling (1997) and Meidel and Scheibling (1999) observed a parallel increase in gonadal and somatic growth in S. droebachiensis when there was an increase in diet quality or quantity. Cook et al. (1998) found that a high protein diet (salmon feed) promoted somatic and gonadal growth simultaneously in juvenile P. miliaris.
Although we observed some statistically significant differences between the diets in terms of gonad index (Table 2), these differences could not be attributed to protein or carbohydrate levels. This was the case even for the two high protein diets (35 and abalone feed) that performed worse than the kelp in terms of growth but produced higher GSIs than the kelp. Measurements of production efficiency and consumption rate were not utilized in this study, but have been effectively used in feed trials with other species, including S. franciscanus (McBride et al. 1998) and P. lividus (Spirlet et al. 2001). Further studies utilizing these and other tools are needed to examine the relationship between protein and energy levels in diets, precocious gonad development, and somatic growth in juvenile green sea urchins.
Limiting nutritional factors may provide an alternative explanation for the decline in SGRs we saw in this study. The juvenile urchins had been maintained on a diet of kelp for 9 mo prior to the start of this study. Kennedy et al. (2007b) proposed that urchins previously fed kelp and then used in formulated feed trials could have stored essential nutrients, such as minerals and pigments, which they can then draw upon during the first period of the feeding trial. Depletion of these stored nutrients would then cause a subsequent decline in SGRs if the diets were also lacking in those nutrients. Minerals, in particular magnesium and calcium, are required by urchins for test and spine growth (Okazaki 1956; Chen et al. 2000), and can become depleted over time. Kennedy et al. (2007a) hypothesized that inadequate mineral levels may have contributed to the poor performance sometimes seen with formulated feeds in previous studies. However, mineral levels in the eight Texas A&M diets used in this study were 24% dry weight, well in excess of the top level of 15% that gave good results for Kennedy et al. (2007a), so it is unlikely that mineral depletion was the cause of the declining SGRs.
Pigment has also been identified as an essential nutrient for sea urchins, particularly for normal gonad development. β-carotene is a major pigment in the gonads, test, and spines, and is a precursor for echinenone, which is responsible for the typical yellow to orange color of urchin gonads and important for reproductive success (Fox and Hopkins 1966; Griffiths and Perrott 1976; George et al. 2001). β-carotene appears to be also required for optimal somatic growth, at least for S. droebachiensis (Kennedy et al. 2007a). They saw improved somatic growth in juvenile green urchins when β-carotene was added to formulated diets at levels of 1.25% using Algro™ (a spray dried form of the microalgae Dunaliella salina). The addition of this pigment source increased the rate of somatic growth even in the absence of supplemental mineral premix, probably because the Algro™ also contributed 0.8% minerals to the diets (Kennedy et al. 2007a).
In this study, β-carotene was added to the Texas A&M formulated diets at levels of 1.1%, equivalent to the level used by Kennedy et al. (2007a). However, at the end of this study the gonad coloration was a pale off white, as opposed to the more typical orange color seen in the kelp-fed urchins. This suggests that β-carotene levels were either inadequate or that the β-carotene source (proprietary) was somehow lacking. Pigment depletion must therefore be considered as a possible cause for the decline in SGRs seen as this study progressed. The role of pigment sources and levels in urchin nutrition, although often addressed in gonad enhancement studies (Robinson et al. 2002), remains an area for further research in somatic growth studies (Lawrence et al. 2001).
A potentially negative consequence of using formulated diets is the effect they can have on gonad color and taste. The pale off-white gonad color we observed at the end of the trial in the formulated feed urchins is unacceptable for market quality, whereas the kelp-fed urchins had gonads that were a more suitable yellow/orange. This likely reflects an inadequacy in the pigment level or source in the formulated diets we used. Previous studies have documented the negative effects of formulated feeds on gonad color and flavor, as compared to the improvement in these sensory parameters when urchins are fed macroalgae. Senartna et al. (2005) observed that the taste and smell of gonads from wild collected purple sea urchins, Heliocidaris erythrogramma, were better than those fed formulated vegetable- or animal-based feeds. Siikavuopio et al. (2007) observed that increased protein levels in formulated diets resulted in an increased bitter taste in the gonads of S. droebachiensis. Shpigel et al. (2005) found that the urchin, P. lividus, fed a prepared diet for 8 wk followed by 4 wk of algal diet produced the optimal combination of gonad color and GSI. It remains to be seen whether this strategy can be used to efficiently grow hatchery derived green sea urchins in culture from juveniles to market acceptability, and this is currently the focus of our research efforts. The ideal diet for urchins in culture needs to provide for fast somatic growth without negatively affecting gonad yield or quality (Kelly 2002).
The need for a readily available commercial diet to use for our sea urchin aquaculture efforts was a primary factor for the inclusion of an abalone diet in the trial. Formulated diets have been developed for abalone and there are now several commercial sources (Hahn 1989; Fleming et al. 1996), whereas commercially available urchin feeds remain in short supply (Lawrence et al. 2001). Abalone is similar to sea urchins in that both feed primarily on macroalgae, and like urchins the energy metabolism of abalone is carbohydrate and protein-based rather than lipid-based (Fleming 1995; Bautista-Teruel and Millamena 1999). It thus seemed possible that formulated abalone diets could meet the nutritional requirements of sea urchins and might prove to be a convenient feed source until more urchin diets became available. In addition, abalone diets are typically high in protein and carbohydrate (Fleming et al. 1996), which allowed us to include a diet in the trial that had combined protein and carbohydrate levels higher than those found in any of the formulated Texas A&M diets (Table 1).
However, the abalone diet used here resulted in poor growth, underperforming the kelp diet and all of the Texas A&M diets, and it is therefore not a suitable feed for juvenile green sea urchins. As only a proximate analysis is available for the abalone feed we used, we have limited information on which to base an analysis of its poor performance. The low mineral levels seen in this diet, as reflected in an ash content of only 7.5%, indicates that there may have been inadequate levels of calcium and magnesium capable of supporting urchin test growth (Kennedy et al. 2007a). In addition, the abalone diet may have also had inadequate levels of β-carotene to support somatic growth of urchins. Although algae is often incorporated into abalone diets as a binder or feed attractant, thus contributing some level of carotenoids, dietary pigment levels do not appear to be an essential concern to the industry, and abalone diets are not typically supplemented with additional carotenoids (Fleming et al. 1996; Bautista-Teruel and Millamena 1999). In addition, other factors such as palatability, digestibility, and protein and lipid sources may have played a role. In particular, the abalone diet was notable for its extreme hardness and water stability, and it did not appear to be consumed by the urchins as readily as the other diets.
Despite the poor performance of the abalone diet seen in this trial, it might be premature to dismiss the use of abalone feed as an urchin feed. Formulations differ between manufacturers and for different life stages (Fleming et al. 1996; Bautista-Teruel and Millamena 1999), and a different abalone diet could possibly provide better results with urchins. There appears to be little if any previously published research carried out on this topic. Certainly, the history of the development of commercial abalone feeds after an initial industry reliance on seaweed provides a model for the further development of feeds for urchin aquaculture.
In this study, we observed a direct relationship between the protein level in the diet and the 24-h water stability, with stability increasing along with protein content. This complicated efforts to make a definitive recommendation regarding protein levels in green sea urchin feeds. The low and intermediate protein diets, which gave the best growth performance, also disintegrated more readily. The high protein diets (including the abalone feed) gave relatively poor growth performance but were highly stable, remaining intact after 24 h. There was also a noticeable (although unquantified) difference in texture between the low stability and high stability diets; the high stability diets were “harder” and more brittle than the low stability diets. This difference in texture and water stability may have affected the availability, palatability, or digestibility of the diet to the juvenile urchins.
Typically, a high degree of water stability is desirable for aquaculture feeds. As feeds disintegrate, they leach nutrients, become unavailable to the animal, and compromise water quality, particularly in closed recirculating systems. Pearce et al. (2002) looked at the effects of binder type and concentration in prepared diets on the gonad yield and quality in S. droebachiensis, and observed that more stable feeds increased gonad yields, possibly due to the longer period of time that the feed remained available to the urchins. They recommended gelatin as the optimum binder, at levels of 3–5%. However, Pearce et al. (2002) were working with mature adult urchins. Small juveniles, which at 5 mm have only recently switched from grazing on diatom films to feeding on macroalgae (Raymond and Scheibling 1987; Sakai et al. 2004; Pearce et al. 2005), may prefer or be better able to graze on disintegrated or softer diets versus intact and harder diets. Klinger (1982) did not find any difference in consumption rates in the urchin, L. variegatus, fed “soft” versus “hard” extruded feeds, but was working with larger individuals than those used in this study. The issues of appropriate texture, shape, palatability, and water stability in diets formulated for somatic growth of small juvenile urchins are all topics in need of further study.
Conclusion
The results from this study indicate that protein levels of 16–23% in formulated diets can support good somatic growth of small juvenile green sea urchins, and that formulated diets can outperform the kelp, S. latissima, as a primary diet. Kelp protein levels fluctuated seasonally, with the best growth of kelp-fed urchins seen when the kelp was at its highest protein level of 32.9%. Protein levels in the formulated diets in excess of 23% were of no benefit and indeed resulted in less growth. However, the variable water stability of the diets created some ambiguity in interpreting the results, and more work needs to be carried out to determine if urchins at this small size (5.5–25 mm) might benefit from softer or less water stable feeds. A commercially available abalone diet fed to urchins resulted in poor growth, but there are opportunities for further research regarding the use of abalone diets for urchins. All of the formulated diets resulted in precocious gonad growth, and the gonads had a pale off-white color that would make them unsuitable for market. Gonads of urchins fed kelp had a normal yellow/orange color. It may be the case that at least two diet formulations are required to grow green sea urchins in culture from settlement to harvest: a diet that promotes fast somatic growth during the juvenile stages, and a finishing diet to enhance gonad quality prior to harvest.




