Photosynthetic and Biochemical Effects of Cold Storage on Marine Benthic Diatoms of the Mexican Pacific Coast
Abstract
We determined the effects of 16 wk of storage at low temperature (4 C) in darkness on the viability, growth, photosynthetic parameters, and biochemical composition of four diatom cultures. Significant differences in cell density and proximal composition were observed for all diatoms throughout storage. Cell density increased with time of storage for all diatoms. Protein content increased for Navicula incerta, Nitzschia laevis, and Navicula sp., whereas lipid content increased during storage in only N. incerta. When the stored diatoms were used as inocula in fresh medium, they increased their viability, generating a lag phase for Nitzschia thermalis var. minor, N. incerta, and Navicula sp. cultures. There were noted species-specific modifications in proximal composition, ash-free dry weight, and photosynthetic parameters in response to storage. We conclude that N. thermalis, N. incerta, N. laevis, and Navicula sp. can be stored at 4 C for 16 wk and are viable in new cultures.
The culture of microalgae constitutes a fundamental step in the aquaculture production of mollusks, crustaceans, and fish larvae (Lavens and Sorgeloos 1996). The production costs of algae biomass can account for 15–85% of total hatchery management costs, depending on the culture method and scale of production (Borowitzka 1997).
Another important task in culturing microalgae is continuous maintenance, in which cells are transferred to new medium prior to attaining the stationary growth phase (usually weekly); this step depends on the specific growth rate and culture conditions. The refrigeration of live microalgae (4 C in darkness) is an alternative method to reduce the time that is needed for continuous maintenance of live microalgae cultures (Cañavate and Lubián 1997; Chen 2001; Ponis et al. 2008; Núnez-Zarco and Sánchez-Saavedra 2011).
Environmental culture conditions can alter the growth and physiology of microalgal cultures (Muller-Feuga et al. 2007). Light is one of the most important variables that determine the quantity and quality of algal culture biomass (Mercado et al. 2004; Muller-Feuga et al. 2007). Light irradiance plays an important role in photosynthesis, but the requirements of light vary greatly according to each species and conditions, such as depth and density of the microalgae (Dubinsky and Stambler 2009; Verma et al. 2010). These parameters have an influence in the quality of the microalgae culture (Jiménez et al. 2003; Carvalho et al. 2006).
However, in total darkness, certain diatoms can survive from several weeks to months (Smayda and Mitchell-Innes 1974; Peters and Thomas 1996) without experiencing resting spore formation. Although many benthic diatoms are capable of facultative heterotrophy (Lewin and Lewin 1960), other diatoms maintain their metabolic integrity for extended periods in the dark by relying on alternative carbon sources, such as cellular carbohydrates, proteins, and lipids (Manoharan et al. 1999), and glucan (Smith et al. 1998).
The aim of this study was to measure the viability and photosynthetic rate of four marine benthic diatoms that were stored by refrigeration for 16 wk to circumvent the maintenance of live cultures.
Materials and Methods
We experiment with four marine benthic diatoms, obtained from a collection of the Biology and Culture of Microalgae Group, Department of Aquaculture, Center for Scientific Research and Higher Education at Ensenada (CICESE): Nitzschia thermalis var. minor and Nitzschia laevis, isolated from Ejido Eréndira, Baja California (BC), by Correa-Reyes (2001); Navicula incerta, isolated from Todos Santos Bay, BC (Carbajal-Miranda 2002); and Navicula sp., isolated as the epiphyte of Macrocystis pyrifera from Todos Santos Bay, BC, by Sánchez-Saavedra (2006). All diatoms were collected from the Mexican Pacific Coast.
The diatoms were maintained in triplicate as monospecific, nonaxenic batch cultures, without any exchange in medium, in progressive volumes of 150 and 800 mL of f/2 medium (Guillard and Ryhter 1962) and supplied with the recommended silicate concentration for f medium (0.107 mol/L Si). The culture conditions were 20 ± 1 C, salinity 33 ± 1 g/L, and 24 h of 110 µE/m2/s continuous light irradiance, provided by cool white fluorescent lamps. The pH was not controlled and ranged from 8.6 to 8.7. Diatom cultures were harvested at the start of the stationary growth phase.
For storage, cells from each diatom species were collected from 800 mL of f/2 medium and divided into 12 100 mL aliquots in glass flasks. The glass flasks were maintained at 4 C and stored in black plastic bags to generate darkness as described by Sánchez-Saavedra (2006).
The viability of each diatom after storage was evaluated at 16 wk in 5 mL triplicate samples from cultures in 250 mL Erlenmeyer flasks with 200 mL of fresh f/2 medium. For each flask, 2 mm glass pearls (7.3 g) were used to dislodge the cells from the walls and bottom. Viability was measured as the capacity of the stored cells to increase their density in fresh medium. As a control, we measured the growth rate of fresh diatom cultures (without storage).
Clumps of benthic diatoms were dislodged ultrasonically (3 min at 100 KHz) with an L & R Solid State/Ultrasonic model T-9B per Voltolina (1985). To determine cell density, the samples were counted using a Neubauer hemocytometer (Hausser Scientific, Horsham, PA, USA) and compound microscope (Olympus CX31, Tokyo, Japan). The specific growth rates (µ: divisions/d) of the fresh culture controls and stored cells was measured per Fogg and Thake (1987).
The proximal composition (percentage of organic dry content) of each diatom was measured before storage (Week 0) and after 16 wk of storage. Protein was extracted with 1.0 N NaOH at 100 C for 15 min as reported (Correa-Reyes 2001; Aguilar-May 2002) and measured per Lowry et al. (1951). Carbohydrates were extracted per Whyte (1987) and measured per Dubois et al. (1956). Lipid content was determined by colorimetric method per Pande et al. (1963) after extraction, per Bligh and Dyer (1959).
The standards (SIGMA, Glendale, CA, USA) were bovine serum albumin (98%) for proteins, glucose (99%) for carbohydrates, and tripalmitin (99%) for lipids. Total and ash-free dry weights (AFW) were measured per Sorokin (1973).
We determined photosynthesis rates in the stored algae cultures in which viability was measured by oxygen evolution method using a Clark type oxygen electrode (Yellow Spring Instruments, Yellow Springs, OH, USA) in a 7 mL Plexiglas chamber at 20 C and under 30–2500 µE/m2/s and maintained under darkness to measured respiration. A sample of each triplicate diatom culture was placed in the chamber after 10 min of preincubation in darkness. Maximum oxygenic photosynthesis (Pmax), the initial slope of photosynthesis (α), and the threshold of irradiance-saturated photosynthesis (Ik) were calculated using a nonlinear direct fitting algorithm of the data to the exponential equation. Chlorophyll was measured per Parsons et al. (1984). All photosynthetic parameters for each diatom species were normalized to chlorophyll content on each day.
The data on proximal composition, cell viability, AFW, and photosynthetic parameters were analyzed by one-way ANOVA. The assumptions of normality and homogeneity of variances were considered before each statistical data analysis. Statistical significance was determined by Tukey a posteriori test at P < 0.05. All statistical analyses were performed using Statistica 7.0, and all graphs were generated with SigmaPlot 10.
Results
On storage, the four benthic diatoms experienced significant differences (P < 0.001) in cell density (Table 1). All diatoms maintained viability throughout the 16 wk of storage. N. laevis (9307 ± 372 × 104cells/mL) and N. thermalis var. minor (5015 ± 420 × 104 cells/mL) had the highest density (P < 0.001) at week 16 in storage, whereas N. incerta had the lowest density (4091 ± 882 × 104 cells/mL) (Table 1). For storage cells, the growth rate was highest for N. laevis (0.12 ± 0.01) and lowest for N. thermalis var. minor (0.04 ± 0.01) after 16 wk of storage (P < 0.001) (Table 1). However, when the storage cells are used to produce new cultures, the growth rate was highest for N. laevis (0.46 ± 0.02) and lowest for N. incerta (0.13 ± 0.02) (P < 0.001) (Table 1).
| Diatoms | Time | Cell density (cells/mL) | Growth rate (µ) |
|---|---|---|---|
| Nitzschia thermalis var. minor | |||
| Storage (wk) | 0 | 22 ± 57b | |
| 16 | 5015 ± 424a | 0.04 ± 0.01 | |
| Viability in fresh medium (d) | 20 | 162 ± 2 | 0.16 ± 0.04 |
| Navicula incerta | |||
| Storage (wk) | 0 | 54 ± 11b | |
| 16 | 4091 ± 882a | 0.05 ± 0.01 | |
| Viability in fresh medium (d) | 16 | 682 ± 117 | 0.13 ± 0.02 |
| Nitzschia laevis | |||
| Storage (wk) | 0 | 64 ± 4b | |
| 16 | 9307 ± 372a | 0.12 ± 0.01 | |
| Viability in fresh medium (d) | 4 | 139 ± 2 | 0.46 ± 0.02 |
| Navicula sp. | |||
| Storage (wk) | 0 | 68 ± 15b | |
| 16 | 4869 ± 149a | 0.05 ± 0.01 | |
| Viability in fresh medium (d) | 4 | 57 ± 3 | 0.31 ± 0.04 |
- 1Values with different letters indicate significant differences between weeks 0 and 16 (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b, n = 3).
The proximal composition of the benthic diatoms throughout storage is shown in Figure 1. The four diatoms showed significant changes in proximal composition (P < 0.001). Navicula sp. (53.2 ± 1.4%) and N. laevis (44.3 ± 3.3%) had the highest protein percentages and the highest carbohydrate percentages (N. laevis, 10.3 ± 2.9%, and Navicula sp., 10.1 ± 1.1%) at week 16. N. thermalis var. minor (14.1 ± 1.47%) and N. incerta (17.3 ± 0.5%) contained the highest percentage of lipids at week 16 (Fig. 1). N. incerta had the lowest percentage of organic content (Week 0) (Fig. 1).
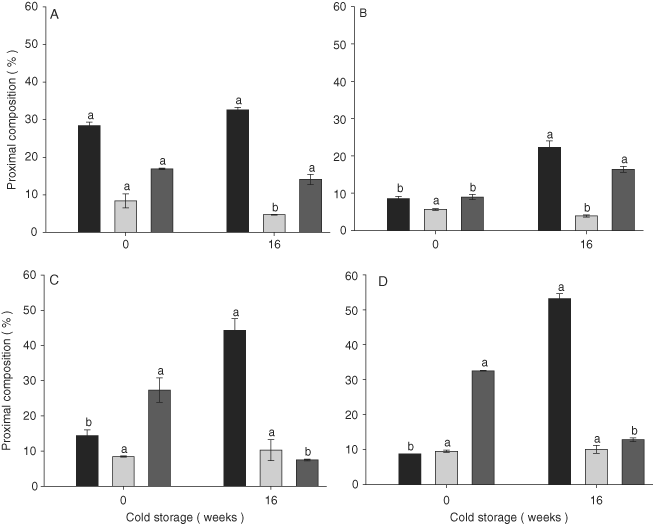
Proximal composition of (
 ) proteins, (
) proteins, (
 ) carbohydrates, and (
) carbohydrates, and (
 ) lipids in Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) after 16 wk of storage. The bars are standard deviations, and values with different letters indicate significant differences between weeks 0 and 16 (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b, n = 3).
) lipids in Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) after 16 wk of storage. The bars are standard deviations, and values with different letters indicate significant differences between weeks 0 and 16 (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b, n = 3).
Mean AFW was affected by storage in N. thermalis var. minor, N. laevis, and Navicula sp. (P < 0.001); AFW in N. incerta did not change. N. laevis and Navicula sp. had decreased AFW, and that of N. thermalis rose after storage (Table 2). N. incerta (348.40 ± 52.80 µg × 106 cells) had the highest AFW at Week 0 and N. thermalis var. minor (36.32 ± 3.11 µg × 106 cells) had the lowest value (Table 2).
| Storage time (wk) | Diatom strains | |||
|---|---|---|---|---|
| A | B | C | D | |
| 0 | 36.32 ± 3.11b | 348.40 ± 52.80a | 160.96 ± 11.16a | 171.78 ± 22.30a |
| 16 | 115.48 ± 3.41a | 326.69 ± 13.96a | 44.66 ± 1.52b | 84.38 ± 5.07b |
- 1Values with letters indicate significant differences between weeks 0 and 16 (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b, n = 3).
The viability of fresh cultures that were obtained with the cells after 16 wk of storage differed among the four diatoms (Fig. 2). N. laevis and Navicula sp. had a lag growth phase of 4 d, and N. thermalis var. minor had an 8-d lag phase. However, N. incerta began exponential growth when the culture was initiated. The number of days of exponential growth increased from 8 to 20 for N. thermalis var. minor, 8 to 16 for N. incerta, 4 to 8 for Navicula sp., and 0 to 4 for N. laevis (Fig. 2). N. incerta had the highest cell concentration in fresh medium (6.82 × 106 cells/mL) on day 16; the lowest value was observed in N. laevis (0.18 × 106cells/mL) on day 20 of the culture (Fig. 2).
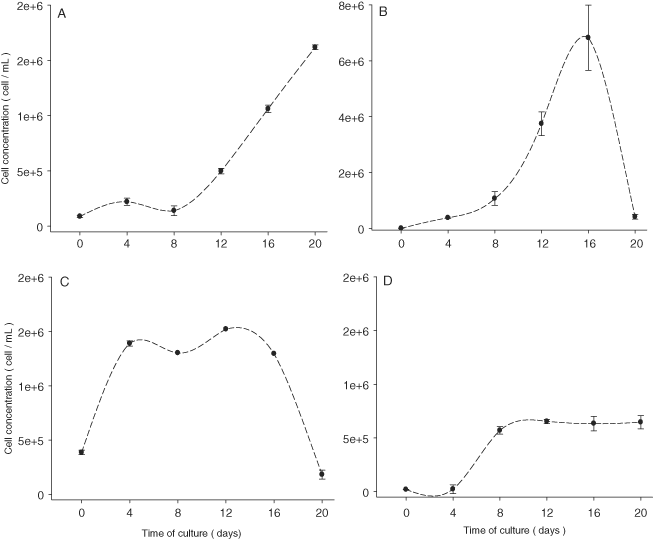
Mean cell concentration values of Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) in fresh f/2 media culture for 20 d after 16 wk of storage.
With regard to photosynthetic parameters affected by storage (P < 0.002), the N. thermalis and Navicula sp. cultures maintained similar mean Pmax values during the lag phase. The Pmax of N. laevis and N. incerta increased in the first 8 d of culture (Fig. 3).
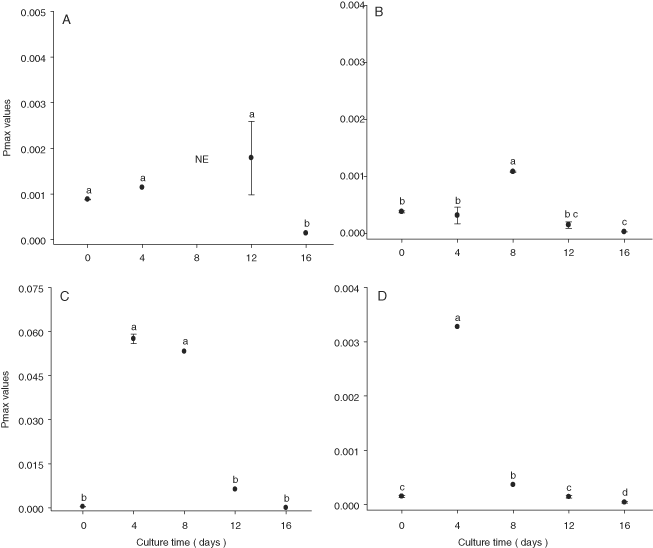
Mean Pmaxvalues (moles O2/pg Chl a/min) of Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) after 16 wk of storage. The bars are standard deviations, and values with different letters indicate significant differences (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b > c > d, n = 3), NE, not evaluated.
The mean Ik values of N. thermalis var. minor, N. laevis, and Navicula sp. increase during the first days of culture (P < 0.001) (Fig. 4). N. incerta experienced a decrease in Ik value over time (Fig. 4). Mean α values tended to increase over the first days of culture for N. thermalis var. minor and N. incerta, whereas Navicula sp. not show a clear trend during the time (Fig. 5).
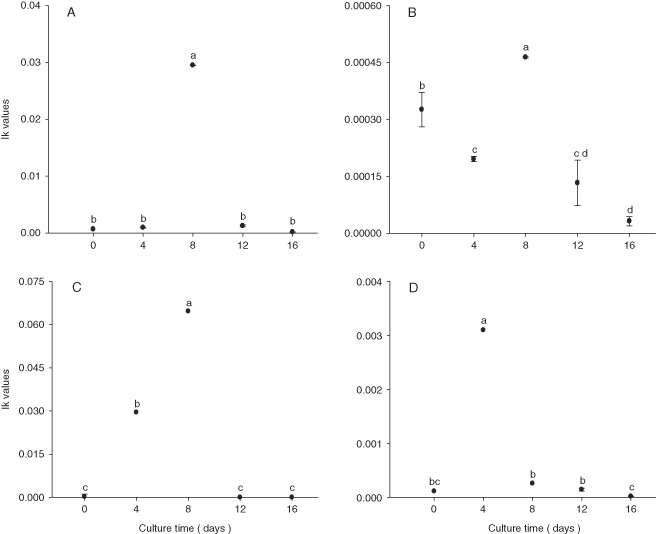
Mean Ikvalues (µE/m2/s) of Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) after 16 wk of storage. The bars are standard deviations, and values with different letters indicate significant differences (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b > c > d, n = 3).
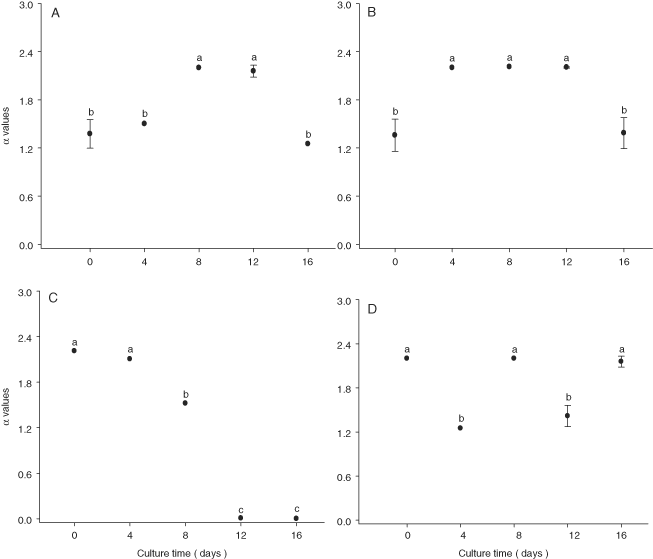
Meanαvalues (moles O2/pg Chl a/min) of Nitzschia thermalis var. minor (A), Navicula incerta (B), Nitzschia laevis (C), and Navicula sp. (D) after 16 wk of storage. The bars are standard deviations, and values with different letters indicate significant differences (by one-way ANOVA and Tukey a posteriori test, P < 0.001: a > b > c, n = 3).
Discussion
The densities of the four diatoms studied increased after 16 wk of storage at low temperature (4 C) refrigeration and darkness. The AFW of N. incerta did not change but decreased in the other diatoms throughout storage. The changes in cell density, growth rate, and AFW of the four diatoms were similar compared with those of N. incerta after 4 wk of cold storage (Núnez-Zarco and Sánchez-Saavedra 2011). However, the growth rate of the diatoms was lower versus those of fresh cultures in other studies (without refrigerated storage) (Correa-Reyes 2001; Aguilar-May 2002; Carbajal-Miranda 2002). Furthermore, the changes in temperature were attributed to the availability of twice the nutrients in the medium that was used by these groups, whereas we used f/2 medium per Guillard and Ryhter (1962).
Microalgal cultures are most commonly conserved by perpetual maintenance under controlled environmental conditions. The maintenance of metabolically active microalgae cultures is one of three objectives of the culture: conservation of the stock culture, attainment of a specific morphological and physiological status, and mass culture (>200 mL liquid media) (Lorenz et al. 2005). The main limitations of perpetual transfer are the possibility of contamination of axenic cultures and the finding that serial subculturing is a consumables-intensive process (Day et al. 1997; Muller-Feuga et al. 2007).
Several methods have been used to preserve cells, the most common of which is lyophilization (Kirsop and Doyle 1991) and storage at low and ultra low temperatures with cryoprotective agents, such as methanol, glycerol, and dimethyl sulfoxide (DMSO), to prevent cell disruption due to crystallization of ice (Day et al. 1997; Day and Brand 2005).
We did not use cryoprotective agents, and the changes in proximal composition of the stored cultures that we noted were species specific. The principal changes were the increase in protein percentage at week 16 in N. incerta, N. laevis, and Navicula sp. and the rise in lipid values in N. incerta. Carbohydrate content was similar or decreased after storage. The changes in the proximal composition of the diatoms might have been caused by their growth by a heterotrophic strategy, which could have induced a rise in proteins. The increase in lipids in N. incerta was possibly affected by a strategy to compensate for the effect of low temperature on membrane permeability, as has been described in other species (Chen 2001; Martz et al. 2006).
The Antarctic zone is characterized by low temperatures and prolonged darkness (up to 10 mo), and several diatom species can survive there by inducing resting spore formation, and others in their vegetative stage (Peters and Thomas 1996), or by sealing membranes and reducing respiration to negligible rates, condensed cytoplasm in physiological dormancy (Hargraves and French 1983).
Storage of diatom cells for 16 wk increased their viability in fresh medium; only N. laevis failed to show a lag growth phase. These differences might be due to induced metabolic responses in acclimatizing to the new medium. Apparently, N. laevis adapted more easily to modifications in temperature and light darkness.
Low temperature causes rigidification of the membrane lipid bilayers and loss of ion permeability (Los and Murata 2004). In cold environments, psychrophilic (optimal growth at or below 15 C) and psychrotrophic organisms (can grow at temperatures below 15 C) alter their fatty acid composition to regulate the membrane fluidity at low temperatures, such as by incorporating polyunsaturated, short-chain, branched, or cyclic fatty acids (White et al. 2000).
Wheat leaves have been proposed to suppress the carboxylation of ribulose 1,5-bisphosphate at low temperatures, leading to increased formation of glycine and serine through the glycolate pathway. Alternatively, ribulose-1,5-bisphosphate carboxylase-oxygenase can undergo reversible cold inactivation (Sawada and Miyachi 1974). Diatoms from the ice edge in the Bearing Sea, including Thalassiosira sp., Navicula sp., Nitzschia sp, and Chaetoceros spp., grow slowly at 0 C, which correlates with one or more of these changes in ribulose-1,5-bisphosphate carboxylase-oxygenase (Baalen and O Donnel 1983). The changes in proximal composition in the stored diatoms could have been caused by a similar physiological mechanism.
Photosynthetic cells activity at low temperatures and in darkness is affected by the maintenance and regulation of various metabolic pathways (Descolas-Gros and de Billy 1987). A major goal of survival in periods of prolonged darkness is the maintenance of the photosynthetic antenna units, which is vital for efficient photosynthesis on return to favorable light conditions. Certain diatoms that have been maintained in the dark for 3 mo remain photosynthetically active and recover their metabolic activity (Peters and Thomas 1996).
Exposure of the four benthic diatoms to cold conditions for 16 weeks affected their growth curves and photosynthetic activity in fresh medium. The similar Pmax values among the four diatoms on the first day of culture in fresh medium are related to the effect of storage at low temperature on the lag phase of growth. The changes in photosynthetic activity at low temperature were due to increases in ATP and adenylate reserves, compensating for the low biochemical reaction rates and the imbalance between the energy that was absorbed and utilized.
The low Pmax values could have resulted from low substrate production that cannot be detected in quantities of net photosynthesis (Andersen 2005; Morgan-Kiss et al. 2006). Photosynthetic organisms have many mechanisms of acclimating to extreme light environments (from total darkness and extreme shade to photoinhibitory levels). Adsorption of light energy (a temperature-independent process) must be tightly coordinated with the temperature-dependent synthesis of photochemically formed energy products (NADP and ATP) and downstream catabolic consumption of these energy sources during growth and metabolism (Morgan-Kiss et al. 2006).
The diatoms that we stored in the dark at 4 C used a similar strategy to survive as phytoplankton cells from the Antarctic zones, proximal composition, and photosynthetic parameters.
We have demonstrated that the diatoms N. thermalis var. minor, N. laevis, N. incerta, and Navicula sp. can survive in the dark for 16 wk at 4 C and be used as inocula to produce new cultures, and their proximal composition and photosynthetic parameters vary in a species-specific responses.
Acknowledgments
This work was supported by Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE, Project 6554) and Consejo Nacional de Ciencia y Tecnología de México (CONACyT, Project SEP-CONACyT 130074). Thanks to the editorial bureau Blue Pencil Science for English editing of this article.




