Uncovering Genetic Relationships using Small Molecules that Selectively Target Yeast Cell Cycle Mutants
Abstract
Genetic analysis in budding yeast has shown that multiple G1 cyclins and cyclin-dependent kinases control cell cycle entry, polarized growth, and spindle pole duplication. The G1 cyclins Cln1 and Cln2 associate with the cyclin-dependent kinase Cdc28 to facilitate cell cycle progression and development of the cleavage apparatus. We have developed a chemical genetic approach toward the discovery of compounds that target G1 control pathways by screening for compounds that selectively kill a yeast strain lacking the G1 cyclins Cln1 and Cln2. A class of small molecules was identified that is highly toxic toward the cln1Δcln2Δ double mutant and has relatively little effect on wild-type yeast. We call these compounds ‘clinostatins’ for their selectivity toward the cln1/2 deletion strain. Clinostatins were used in a genome-wide chemical synthetic lethality screen to identify other genes required for growth in the presence of the drug. Other deletions that were sensitive to the drug include members of the protein kinase C(PKC)-dependent MAP kinase pathway. These results suggest an approach for combining chemical synthetic lethality and chemical genomic screens to uncover novel genetic interactions that can be applied to other eukaryotic pathways of interest.
A classic genetic method for elucidating biologic pathways is to screen for mutants that enhance the effects of a pre-existing mutation. Mutations in two different genes are said to be synthetically lethal if either mutation is viable in a wild-type background, but the combination of both mutations results in lethality. Genes related by synthetic lethality tend to operate in similar or redundant pathways (1), and systematic genome-wide synthetic lethality screens have revealed new relationships between genes and pathways (2).
Late G1 signaling in yeast is dependent on two main cyclin-cyclin-dependent kinase (CDK) pathways. One is defined by the interaction between Cln1 and Cln2 with Cdc28. Another pathway involves the cyclins Pcl1 and Pcl2, which interact with the CDK Pho85 (3). A cln1/2Δpcl1/2Δ null mutant shows a deficiency in bud emergence and is unviable, while pcl1/2Δ and cln1/2Δ cells are viable (4), suggesting that Cln–Cdc28 and Pcl–Pho85 complexes represent at least partially redundant G1 signaling pathways (Figure 1). Late G1 CDK activity stimulates the degradation of the S-phase inhibitor Sic1 (5) and is required for the establishment of cell polarity and bud emergence (4).
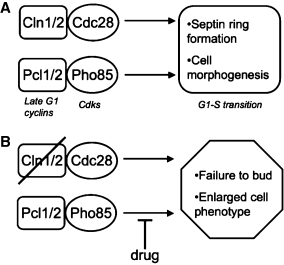
(A) Parallel G1-S cyclin-CDK pathways in yeast. (B) Concept of chemical synthetic lethality screening, in which compounds that selectively kill a cln1,2Δ deletion mutant might act by targeting the parallel pathway via Pcl1/2-Pho85.
Extending the concept of synthetic lethality to small molecules, a compound that exhibits selective toxicity to a particular deletion mutant is likely to target the same pathway, or a pathway that is parallel to or linked by epistasis to the deleted gene. The concept of ‘chemical epistasis’ has been used to study the effects of drugs on specific yeast deletion mutants in the context of DNA repair and checkpoint processes (2,6–10), and chemical modifier screens have been performed in yeast leading to small molecule enhancers and suppressors of the drugs FK506 (11) and rapamycin (12). Here, we demonstrate the application of chemical screening toward the study the cln branch of G1 cell cycle signaling in yeast. Our results point to the potential generality of targeting a pathway of interest using chemical epistasis and chemical genomics screening to uncover new genetic relationships.
Results and Discussion
We employed a high-throughput screening approach to identify compounds that were selectively toxic to either a cln1Δcln2Δ deletion strain (cln1/2Δ) or a pcl1Δpcl2Δ deletion strain (pcl1/2Δ). About 3099 compounds from the National Cancer Institute (NCI) diversity, mechanistic, and natural product libraries were screened for compounds that inhibited growth in liquid culture of each mutant strain relative to wild type. Compounds were screened in 384-well plates, and the OD544 for each well was measured as a function of time using a standard plate reader. Although several compounds showed moderate selective toxicity, only one compound (1, Figure 2) exhibited a >10-fold inhibition of mutant growth relative to wild type. Compound 1 inhibited growth of the cln1/2Δ yeast mutant with an IC50 of 0.7 μm (Figure 2) and exhibited only slight toxicity to both the pcl1/2Δ and wild-type strains (IC50 >50 μm). The sensitivity of cln1/2Δ appeared to be specific to compound 1 and not due to a general sensitivity of cln1/2Δ to toxic compounds, as other known cytotoxic compounds (wortmanin, benomyl, and rapamycin) were found to be equally growth inhibitory to cln1/2Δ, pcl1/2Δ, and wild type (data not shown).

(A) Structure of compound 1. (B–D) Yeast growth curves in the presence of 1. Yeast strains wild type (B); pcl1/2Δ (C); cln1/2Δ (D), were treated with different concentrations of 1 and growth was measured as a function of absorbance at 544 nm. (E) Spot assays of each yeast strain onto agar containing 1 at the indicated concentrations.
To monitor the lethal phenotype in the presence of compound, cln1/2Δ cells were grown in the presence of 1 at 20 μm on agar plates. After 24 h, the cells arrested as microcolonies of about 4–20 cells. These cells were dramatically enlarged over untreated cln1/2Δ cells, and all cells arrested in an unbudded state (Figure 3). Many cells underwent lysis after becoming enlarged. This phenotype was distinct from observations in untreated cln1/2Δ cells, where a small population of cells delayed in G1 and became slightly enlarged, but eventually progressed in the cell cycle. No obvious phenotype was observed at the same concentration of 1 for either wild-type or pcl1/2Δ cells.

Images (DIC) of microcolonies of cln1,2Δ cells grown in the presence of 1 at 20 μm on agar.
Compound 1 is a derivative of naphthazarin (5,8-dihydroxy-1,4-naphthoquinone) with a 2-pyridylpiperazine group linked to the naphthoquinone ring. To probe the structural basis for its biologic selectivity, a series of derivatives was synthesized in which the pyridine ring was substituted with other aryl substituents (Figure 4). Derivatives in which the pyridyl group was replaced with either a pyrimidine (2) or a trifluorylmethylpyridine ring (3) were nearly equal in potency and selectivity as the original compound 1. Placement of a 4-cyano group on the pyridine ring provided 4, a compound with significantly increased potency against the cln1/2Δ cells (IC50 = 150 nm). Like 1–3, compound 4 was highly selective for the cln1/2Δ deletion mutant and had no discernable effect on either wild type or pcl1/2Δ cells up to 50 μm. Remarkably, compound 5, in which the pyridine ring was replaced with a benzene ring, was approximately 50-fold less potent than 1 against the cln1/2Δ mutant. As the inactive phenyl derivative 5 differed from the original compound 1 by only one nitrogen atom, compound 5 was used as a negative control in subsequent experiments. We call this new class of compounds ‘clinostatins’ for their ability to selectively kill cln yeast deletion mutants.
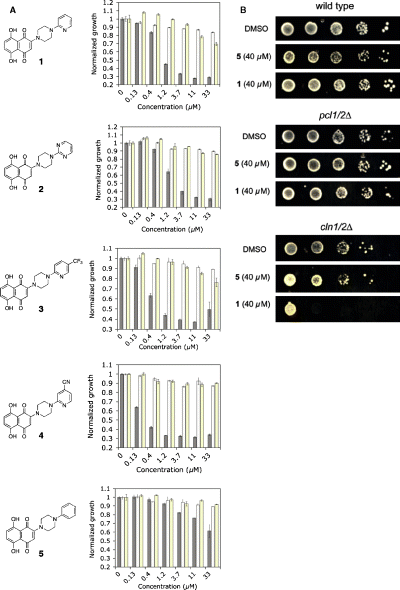
(A) Concentration dependence data for compounds 1–5 against cln1,2Δ (solid bars), wild type (hashed bars), and pcl1,2Δ (yellow bars) yeast strains. Yeast growth was measured after 24 h as a function of absorbance at 544 nm. (B) Spot assays comparing the effects of compounds 1 and 5 against the three yeast strains.
In order to further characterize 1 with respect to its targeted pathway(s) in yeast, a chemical genomics screen was performed to measure the effect of 1 on growth rates of 4819 viable haploid deletion mutants (Figure 5). At 20 μm, 1 completely inhibited the growth of cln1/2Δ cells but had negligible effect on wild type or pcl1/2Δ cells (Figure 4). Compound 5 had little effect on the growth of cln1/2Δ cells at 20 μm and was therefore used as the negative control. Thus, cell growth was measured as a function of time for each deletion mutant in the presence of 1 or 5, and the resulting ratios were normalized to the sensitivity of the cln1/2Δ deletion mutant (Figure 5A). Remarkably, only two strains, slt2Δ and ume6Δ, were as sensitive to 1 as cln1/2Δ. Slt2 is a protein kinase C (Pkc1)-regulated MAP kinase that is implicated in stress response, maintenance of cell integrity, nutrient signaling, and cell cycle control (13). Ume6 is a key transcription factor that regulates early meiotic genes and is a negative regulator of unscheduled meiosis during vegetative growth (14). We confirmed the sensitivities of slt2Δ and ume6Δ to compound 1 individually using spot assays (Figure 5B).
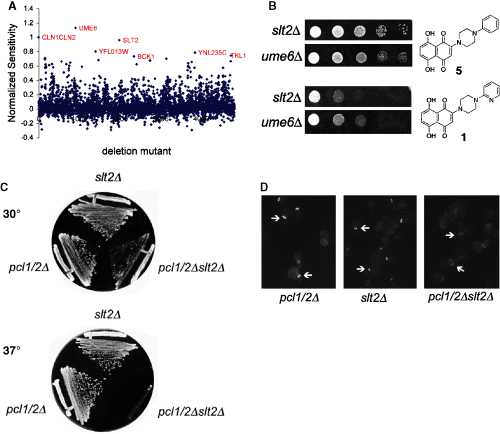
(A) Results of chemical genomics experiment. Yeast deletion mutants that showed significant sensitivity to 1 relative to control compound 5 are shown in red. (B) Spot assays for two strains, ume6Δ and slt2Δ identified in the chemical genomics experiment as being sensitive to 1 relative to 5. Compounds were added to the plates at 20 μm. (C) Temperature sensitivity of pcl1/2Δslt2Δ triple mutant. (D) pcl1/2Δslt2Δ triple mutant shows widened and diffuse septin distribution at bud necks. Septins were visualized using anti-cdc11 antibody (Santa Cruz Biotech) conjugated to Alexa-fluor.
In addition to cln1/2Δ, slt2Δ, and ume6Δ, among the top five most sensitive strains to 1 was bck1Δ. Bck1 is the MAP kinase kinase kinase (MAPKKK) that is upstream of Slt2 in the arm of the yeast MAP kinase cascade that is dependent on Pkc1. As, like pcl1/2Δ, 1 is synthetically lethal with the cln1/2Δ double mutant, we hypothesized that 1 might act on components of the Pcl1/2-Pho85 pathway that communicate with the MAP kinase cascade. To assess this hypothesis genetically, we created the triple null mutant pcl1Δpcl2Δslt2Δ. The pcl1Δpcl2Δslt2Δ triple mutant exhibited a synthetic growth defect and was unviable at 37 °C (Figure 5C), with gross defects in cytokinesis including widened bud necks and a failure to separate at cytokinesis (Figure 5D), supporting a role for the MAP kinase cascade during bud neck formation that is at least partially functionally redundant with Pcl1/2 signaling. Neither ume6 nor bck1 showed a synthetic interaction with pcl1/2 (data not shown).
A PubChem search of compound 1 shows reported activity in the NCI Yeast Anticancer Drug Screen (http://dtp.nci.nih.gov/yacds/index.html) and is cytotoxic to human breast and melanoma cancer cell lines in the low micromolar range. The yeast strains that were sensitive to 1 in the NCI screen are DNA damage-related mutants, including the double mutants mlh1Δrad18Δ and sgs1Δmgt1Δ, and single mutants mec2-1Δ and rad50Δ. The absence of rad50 as a hit in our genome-wide sensitivity screen may be due to our use of 5 as a control, in which only those deletions related to the observed cln1/2 sensitivity would be expected to score as sensitive. Nonetheless, the suggested connection between 1 and DNA repair pathways may be real. Cell integrity genes slt2 and bck1 are synthetic lethal with rad50, as well as the PAK family kinase cla4, which is involved in septin ring assembly. The identification of the molecular target(s) of 1 in yeast may be useful in the subsequent identification of its relevant target(s) in human cancer cells.
The G1 arrest induced by compound 1 supports the hypothesis that the compound is affecting pathways prior to bud emergence. The genomic evidence points to a compound target that is functionally redundant with components of the Pkc1-dependent MAP kinase cascade. In an slt2 (MAPKKK) or bck1 (MAPK) deletion background, the compound showed a significant toxic effect. To extrapolate from the hypothesis that 1 targets a PCL1/2-dependent pathway, we reasoned that we should see a genetic interaction between PCL1/2 and the most sensitive strains from the genomic analysis. The pcl1Δpcl2Δslt2Δ deletion displayed a significant synthetic growth defect, having gross abnormalities in cytokinesis and disorganized budding at 37°, while neither of the individual mutants, pcl1/2Δ or slt2Δ, exhibited a growth defect at 37°. These results support the previously established link between the PKC-dependent MAP kinase pathway and the G1 cyclins. For example, slt2 mutants exhibit defects in polarized cell growth (15) and augment the cell division defect of a partially inactivated cdc28 allele, and both slt2 and bck1 were shown to be synthetically lethal with pho85 (16). In addition, overexpression of PCL1 and PCL2 (but not CLN1 or CLN2) suppresses some of the osmolytic sensitivity of an slt2 mutant strain (17). However, despite the observation that bck1 and ume6 were highly sensitive to 1, neither gene showed a synthetic genetic interaction with pcl1/2. This suggests that the target of 1 is not functionally redundant with Pcl1/2 and may operate downstream of the Pcl cyclins or in a parallel pathway.
Given that pho85, the Pcl1/2-dependent CDK, is synthetically lethal with slt2 and bck1, as well as the meiotic transcription factor ume6, we hypothesized that the clinostatins may target Pho85 directly. Inhibition of Pho85 activity by small molecules causes nuclear accumulation of a Pho4-GFP fusion protein (18), and we used this assay to ask whether addition of clinostatins affect Pho85 activity in vivo. Clinostatins had no effect on Pho4-GFP localization (data not shown), suggesting that clinostatins do not target Pho85 in yeast. We are currently working to elucidate the target(s) of clinostatins using a combination of biochemical and genetic approaches.
Conclusions and Future Directions
The combined use of chemical synthetic lethal screening followed by chemical genomic profiling of hit compounds allows for the identification of new genetic relationships even without prior knowledge of the small molecule's biologic target(s). Although the genetic connection between the Pkc1-MAP kinase pathway and G1 cyclins was known previously, this study provides a proof-of-concept that such connections can be made independently of the knowledge of a compound's molecular target.
The ability to monitor lethal phenotypes as a function of time and dosage presents distinct advantages over classical genetic synthetic lethal combinations, which often provide only binary results. The temporal and dosage control of small molecules allow phenotypic data to be added to the classical synthetic genetic interaction map, providing an extra layer of information in the analysis of pathway relationships. As the map of known synthetic lethal interactions continues to expand, linking chemical sensitivities to genetic interaction data offers the possibility of ‘triangulating’ onto small molecule targets (7). In addition, clustering drugs according to their genomic sensitivity profiles in yeast can provide valuable insights into their mechanisms of action (19). The screening of larger chemical libraries against other yeast deletion mutants is likely to uncover new chemical-genetic relationships relevant to many cellular processes of interest.
Materials and Methods
Yeast strains and materials
The following yeast strains were used in this study:
-
DK186: MATa his3-11 leu2-3, 112 trp1-1 ura3-52 ade2-1 can1-100 GAL+ bar1
-
KA61: MATa his3-11 leu2-3, 112 trp1-1 ura3-52 ade2-1 can1-100 GAL+ bar1 cln1::TRP1 cln2::LEU2
-
DK573: MATαhis3-11 leu2-3, 112 trp1-1 ura3-52 ade2-1 can1-100 GAL+ bar1 pcl1::NAT pcl2::KAN
Additional deletion strains were obtained from the Open Biosystems MATa haploid deletion collection, and the Pho4-GFP strain was obtained from Invitrogen. The strain SL010 (pcl1Δpcl2Δslt2Δ) was generated by using standard genetic procedures to cross strains DK573 and the MATa slt2 haploid deletion strain obtained from Open Biosystems. After selection for diploids, sporulation was induced and tetrads were dissected. The triple mutant was identified by scoring tetrads for the nutritional markers associated with each deletion.
Chemical screening
The NCI Diversity, Mechanistic, and Natural Products libraries were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI. Compounds were reformatted into 384-well plates at 1 or 10 mm in DMSO prior to screening. The initial screen used yeast diluted into YPD + adenine liquid media to OD544nm <0.01. Compound was added using the V&P Scientific Pin Tool Robot Model VP 903B (pin tool VP384FP3S100) to a final concentration of approximately 5 or 50 μm in 384-well polystyrene plates. Growth levels were analyzed after 24 h for wild type and 36 h for cln1/2Δ, pcl1/2Δ using a plate reader (Wallac Victor2) measuring absorbance at 544 nm. Hits were considered as those with a differential reading between wild type and the mutant of interest of >10-fold.
Spot assays
Compound at 10 mm in DMSO was infused into agar media to give a final concentration of 20 μm. A series of dilutions were performed on the yeast strains and plated at 2 μL per spot. Plates were incubated at 30 °C for 36 h.
Phenotypic analysis
Yeast strains were grown on agar containing 20 μm compound or DMSO as a negative control at 30 °C. Thin agar sections were removed and placed on glass slides with coverslips. Cell phenotypes were observed and photographed at various intervals over 24 h.
Chemical genomic screen
Compound in DMSO was added to YPD to a final concentration of 20 μm. This solution was then added at 80 μL per well into 96-well plates. Yeast strains were transferred into compound/YPD plates using a 96-well metal pin array. Deletion plates were transferred initially into 50 μL YPD in order to provide the appropriate yeast dilution, and then immediately from this plate the yeast were pin-transferred into the assay plate containing compound. The plates were agitated in the plate reader and absorbance was measured at 544 nm after 24–48 h of growth. Multiple time-points were used in order to account for slow-growing strains. In each set of experiments, control plates containing wild type and cln1/2Δ yeast were run in parallel. Growth inhibition of different strains was normalized to these control plates, i.e. the ratio of the absorbance of the cln1/2Δ strain in the presence of 1 versus 5 was set equal to 100% inhibition, and growth ratios of all deletion strains were plotted as a fraction of this. The top 50 most sensitive strains were verified by repeating the drug sensitivity screen on these strains.
Acknowledgments
This work was supported by the National Institutes of Health (1 R01 CA104569-03) and California Institute for Quantitative Biomedical Research (QB3). We thank William Sullivan for insightful discussions and Patrick Cleveland (V&P Scientific) for technical support. We also thank the Developmental Therapeutics Program at the National Cancer Institute for supplying libraries and reagents.




