Neither Biased Sex Ratio nor Spatial Segregation of the Sexes in the Subtropical Dioecious Tree Eurycorymbus cavaleriei (Sapindaceae)
Supported by the National Natural Science Foundation of China (30470185) and KIP Pilot Project of the Chinese Academy of Sciences (KSCX2-YW-N-061).
Abstract
Knowledge of sex ratio and spatial distribution of males and females of dioecious species is both of evolutionary interest and of crucial importance for biological conservation. Eurycorymbus cavaleriei, the only species in the genus Eurycorymbus (Sapindaceae), is a dioecious tree endemic to subtropical montane forest in South China. Sex ratios were investigated in 15 natural populations for the two defined ages (young and old). Spatial distribution of males and females was further studied in six large populations occurring in different habitats (fragmented and continuous). The study revealed a slight trend of male-biased sex ratio in both ages of E. cavaleriei, but sex ratio of most populations (13 out of 15) did not display statistically significant deviation from equality. All of the four significantly male-biased populations in the young class shifted to equality or even female-biased. The Ripley's K analysis of the distribution of males with respect to females suggested that individuals of the opposite sexes were more randomly distributed rather than spatially structured. These results suggest that the male-biased sex ratio in E. cavaleriei may result from the precocity of males and habitat heterogeneity. The sex ratio and the sex spatial distribution pattern are unlikely to constitute a serious threat to the survival of the species.
Dioecy is a breeding system characterized by the separation of sexual function into male and female individuals. Despite its relative rarity among flowering plants (∼6% of angiosperm species, Renner and Ricklefs 1995), dioecy is widespread in angiosperms, and has been an important subject for ecological and evolutionary studies (Bawa 1980; Renner and Ricklefs 1995). Dioecy is thought mostly to have evolved within flowering plants due to short-term benefits accruing to reduced inbreeding depression and/or more efficient allocation of resources (reviewed by Charlesworth 1999). However, a recent phylogenetic analysis of dioecious angiosperms demonstrated that dioecious clades have lower species richness than their cosexual sister clades, suggesting that dioecious plants may experience higher extinction rates and/or lower speciation rates than cosexual species (Heilbuth 2000). Due to spatial separation of the opposite sexes, dioecious plants may suffer from a severe disadvantage in reproductive success in comparison to cosexual ones, including reduced mate assurance (Pannell and Barrett 1998), a “seed-shadow handicap” (Heilbuth et al. 2001), a reliance on larger pollinator pools (Vamosi and Otto 2002) and a number of biological traits such as woody growth form (Vamosi and Vamosi 2005).
Sex ratio and spatial distribution patterns of individuals are of fundamental importance to reproductive strategies (Renner and Ricklefs 1995) and survival rates for dioecious plants (Freeman et al. 1976). For example, spatial segregation of the sexes (SSS), the non-random spatial distribution of the sexes, and availability of some limiting resources may cause sex ratio bias in some dioecious plants (reviewed by Bierzychudek and Eckhart 1988). Knowledge of sex ratio and sex spatial distribution in dioecious plants is therefore both of theoretical interest and of practical importance for biological conservation (Ewen et al. 2001; Tella 2001).
Eurycorymbus cavaleriei is a Tertiary relict and the sole member of its genus within the family Sapindaceae (Lo and Chen 1985; Fu and Jin 1992). It is a deciduous, broad-leaved dioecious tree endemic to China and widely scattered in subtropical montane mixed evergreen and deciduous broad-leaved forests, ranging from Taiwan in the southeastern China to Yunnan in the southwestern China. Although categorized as a rare species, E. cavaleriei is found in a range of diverse habitats within its natural distribution, with small (n < 200) and isolated populations in Fujian, Guangdong, Jiangxi and Hunan provinces, while a few large and continuous populations occur in Guangxi and Guizhou provinces in the southwestern China. The sex ratio and spatial distribution pattern of this species are unknown. The wide range and variable habitat distribution pattern of E. cavaleriei provide us with an opportunity to investigate the patterns of sex ratio and sex spatial distribution as a pioneer case study in China's mixed evergreen and deciduous broad-leaved forest.
In the present study, we investigated 18 natural populations of E. cavaleriei across its natural range in China, with the exception for Taiwan. The objectives were to examine the sex ratios in the populations and the spatial distribution of reproducing plants and to determine: (i) whether the sex ratios in different populations exhibited significant deviation from the expected 1:1 ratio of male to female; (ii) whether spatial segregation of the sexes occurs in this species; (iii) if so, whether the sex ratio bias was correlated with sex spatial distribution pattern and tree size or tree age; and (iv) whether the sex ratio and sex spatial structure of this species exhibited a significant difference between the two contrasting habitats.
Results
Sex ratios
Smaller diameter at breast height (DBH) values of males were found in 10 out of the 15 populations investigated (Table 1). On the whole, the mean DBH value of males was 12.18 ± 0.28 cm (mean ±SE) (n= 583), and that of females was 12.99 ± 0.31 cm (mean ±SE) (n= 497), suggesting that flowering males are usually smaller than females.
| Region | Population code | Elevation (m) | Sample size (n) | DBH (cm) | Location | Habitat | ||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Latitude (N) | Longitude (E) | |||||
| Nanping in Fujian | Mangdang (MD) | 420 | 24 | 10.7 (4.1) | 11.7 (5.4) | 26°39′ | 118°06′ | Fragmented |
| Changting in Fujian | Guilong (GL) | 480 | 30 | 10.1 (2.7) | 14.7 (8.1) | 25°45′ | 116°11′ | Fragmented |
| Jianning in Fujian | Gaofeng (GF) | 450 | 34 | 10.0 (6.2) | 10.1 (5.1) | 26°48′ | 116°56′ | Fragmented |
| Putian in Fujian | Dayang (DY) | 480 | 40 | 8.5 (2.1) | 8.3 (2.8) | 25°44′ | 119°01′ | Fragmented |
| Shixing in Guangdong | Zhandongshui (ZDS) | 390 | 27 | 13.2 (4.4) | 13.1 (6.1) | 24°43′ | 114°15′ | Fragmented |
| Longnan in Jiangxi | Jiulianshan (JLS) | 570 | 15 | 17.4 (6.8) | 8.9 (2.4) | 24°33′ | 114°27′ | Fragmented |
| Shimen in Hunan | Hupingshan (HPS) | 410 | 37 | 11.7 (4.0) | 12.3 (3.6) | 30°00′ | 110°38′ | Fragmented |
| Sangzhi in Hunan | Wangjiahe (WJH) | 550 | 94 | 11.1 (5.0) | 10.1 (3.9) | 29°44′ | 109°53′ | Fragmented |
| Xinning in Hunan | Houlongshan (HLS) | 320 | 218 | 7.4 (2.5) | 8.0 (3.0) | 26°42′ | 111°07′ | Fragmented |
| Xinning in Hunan | Shijiaotang (SJT) | 340 | 112 | 9.5 (2.5) | 9.2 (2.9) | 26°43′ | 111°07′ | Fragmented |
| Huanjiang in Guangxi | Hongdong (HD) | 570 | 129 | 14.9 (5.2) | 14.3 (6.0) | 25°08′ | 107°58′ | Continuous |
| Huanjiang in Guangxi | Dongxiang (DX) | 580 | 23 | 19.4 (5.7) | 16.2 (6.7) | 25°08′ | 107°59′ | Continuous |
| Libo in Guizhou | Laqiao (LQ) | 780 | 161 | 14.9 (5.8) | 13.8 (6.2) | 25°19′ | 107°57′ | Continuous |
| Libo in Guizhou | Banzhai (BZ) | 570 | 71 | 19.9 (7.6) | 18.8 (6.8) | 25°13′ | 107°59′ | Continuous |
| Libo in Guizhou | Xiaoka (XK) | 570 | 65 | 16.2 (7.9) | 13.2 (6.6) | 25°12′ | 108°01′ | Continuous |
| Mean DBH | 12.99 (0.31) | 12.18 (0.28) | ||||||
- DBH denotes mean value of diameter at breast height (cm), and standard deviations are in parentheses.
Male-biased sex ratios occurred in some populations throughout both classes (Table 2). In size class I (DBH ≤ 10 cm), four populations were significantly male-biased and one population (HLS) was female-biased (Table 2). However, only sex ratios of the populations ZDS and JLS differed significantly from 1:1 in size class II (DBH > 10 cm), with ZDS being male-biased and JLS female-biased. When the adult individuals of the two classes were pooled together, two populations exhibited significant male bias. Univariate anova showed that there were significant population (P= 0.000), sex (P= 0.051) and interaction (population × sex, P= 0.015) effects on DBH (Table 3).
| Population | Class I (DBH ≤ 10 cm) | Class II (DBH > 10 cm) | All adult individuals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Male | Female | G-test | n | Male | Female | G-test | n | Male | Female | G-test | |
| MD | 8 | 4 | 4 | NS | 16 | 8 | 8 | NS | 24 | 12 | 12 | NS |
| GL | 13 | 4 | 9 | NS | 17 | 11 | 6 | NS | 30 | 15 | 15 | NS |
| GF | 20 | 10 | 10 | NS | 14 | 6 | 8 | NS | 34 | 16 | 18 | NS |
| DY | 30 | 14 | 16 | NS | 10 | 7 | 3 | NS | 40 | 21 | 19 | NS |
| ZDS | 9 | 7 | 2 | NS | 18 | 14 | 4 | * | 27 | 21 | 6 | ** |
| JLS | 7 | 6 | 1 | * | 8 | 1 | 7 | * | 15 | 7 | 8 | NS |
| HPS | 13 | 6 | 7 | NS | 24 | 15 | 9 | NS | 37 | 21 | 16 | NS |
| WJH | 50 | 30 | 20 | NS | 44 | 26 | 18 | NS | 94 | 56 | 38 | NS |
| HLS | 178 | 76 | 102 | * | 40 | 20 | 20 | NS | 218 | 96 | 122 | NS |
| SJT | 69 | 42 | 27 | NS | 43 | 22 | 21 | NS | 112 | 64 | 48 | NS |
| HD | 32 | 23 | 9 | * | 97 | 53 | 44 | NS | 129 | 76 | 53 | * |
| DX | 3 | 2 | 1 | NS | 20 | 10 | 10 | NS | 23 | 12 | 11 | NS |
| LQ | 37 | 25 | 12 | * | 124 | 63 | 61 | NS | 161 | 88 | 73 | NS |
| BZ | 6 | 4 | 2 | NS | 65 | 36 | 29 | NS | 71 | 40 | 31 | NS |
| XK | 21 | 15 | 6 | * | 44 | 23 | 21 | NS | 65 | 38 | 27 | NS |
| Total | 496 | 268 | 228 | 584 | 315 | 269 | 1080 | 583 | 497 | |||
- Significant differences from a 1:1 ratio of males to females (based on goodness-of-fit tests (G-test)) are indicated by: *P < 0.05, **P < 0.01. DBH, diameter at breast height; NS, not significant.
| Source | Sum of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Population | 12 386.137 | 14 | 884.724 | 35.583 | 0.000 |
| Sex | 95.272 | 1 | 95.272 | 3.832 | 0.051 |
| Population × sex | 695.145 | 14 | 49.653 | 1.997 | 0.015 |
- Dependent cariable: DBH (diameter at breast height). df, degrees of freedom.
Spatial distribution patterns
The visual examination of the plant distribution in the analyzed six populations suggests that individuals of E. cavaleriei were significantly clumped in space within each population (Figure 1). The univariate Ripley's K analysis supports this conclusion, demonstrating that individuals are significantly more clustered in their habitats than would be expected at random (Figure 2). For each population, the L(d) statistic falls well above the upper confidence interval for most of the distances considered. The spatial pattern of individual distribution is highly similar in different populations. However, the bivariate analysis of the distribution of males with regard to females provides no evidence of spatial segregation of the sexes (Figure 2). The sexes were not significantly over-dispersed or regularly distributed at any scale. Instead, the significant clumping revealed by the univariate analysis is reflected to a lesser degree in each of the bivariate analyses. The sex spatial distribution patterns are highly consistent among natural E. cavaleriei populations, and there was no difference between the two types of habitats.
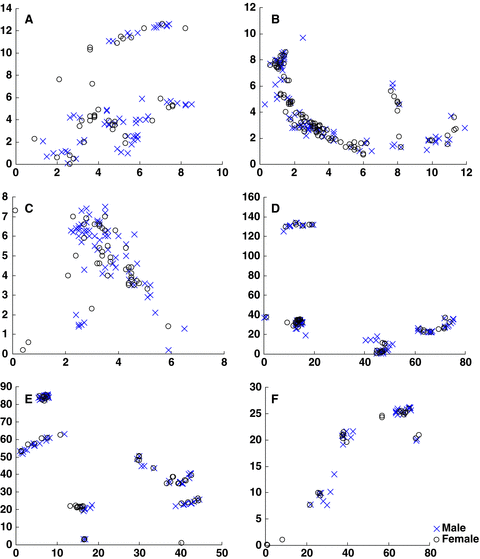
Maps of six large populations studied.Axes are in seconds of latitude (X) and longitude (Y). The origin was fixed on the point whose co-ordinates were the minimum values of latitude and longitude of plants measured by GPS in each population. The coordinates of each individual are represented by the differences between the actual values of latitude and longitude and the value at the origin, measured in seconds.(A) Wangjiahe (WJH). (B) Houlongshan (HLS). (C) Shijiaotang (SJT). (D) Hongdong (HD). (E) Laqiao (LQ). (F) Xiaoka (XK).
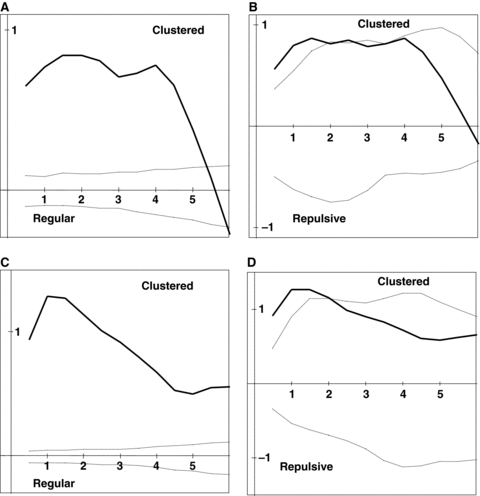
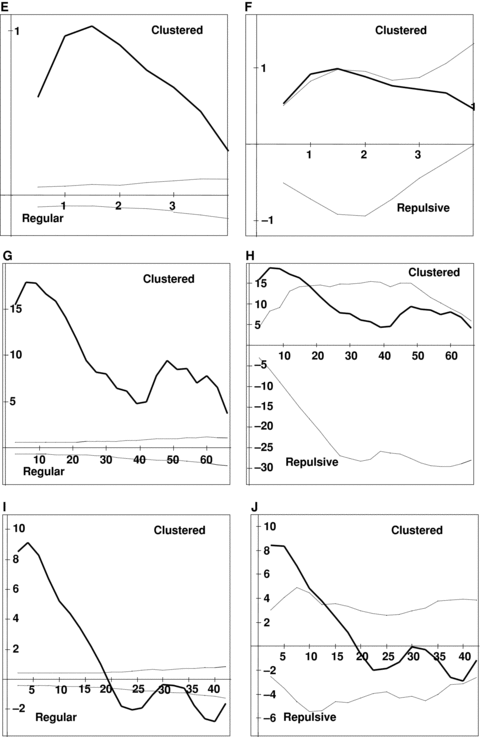
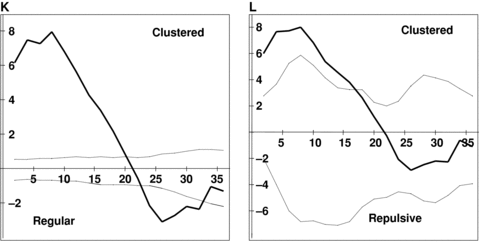
Results of Ripley's K analyses for six large populations studied.The dark line depicts the Ripley's K statistic (L(d)), and the dashed lines are the 95% confidence interval generated by Monte Carlo simulation of randomly distributed points. The univariate analysis addressed the distribution of all flowering individuals, regardless of sex, within populations. The bivariate analysis examined the distribution of males with regard to females. Note that any distance at which the L(d) statistic falls above the confidence envelope denotes clumping, and any distance at which it falls below the envelope denotes over-dispersal. (A) Wangjiahe (WJH) univariate. (B) WJH bivariate. (C) Houlongshan (HLS) univariate. (D) HLS bivariate. (E) Shijiaotang (SJT) univariate. (F) SJT bivariate. (G) Hongdong (HD) univariate. (H) HD bivariate. (I) Laqiao (LQ) univariate. (J) LQ bivariate. (K) Xiaoka (XK) univariate. (L) XK bivariate.
Discussion
Sex ratios can be linked to population dynamics (Gauquelin et al. 2002). Previous studies have found a variety of patterns of sex ratio in plant populations, being male-biased (Onyekwelu and Harper 1979; Barrett and Helenurm 1981; Nicotra 1998; Ortiz et al. 2002), female-biased (Crawford and Balfour 1983; Gauquelin et al. 2002; Stehlik and Barrett 2005; Stehlik et al. 2007) or not significantly different from the 1:1 proportion of male to female (Ahmed et al. 1990; Ortiz et al. 1998; Rottenberg 1998, 2000; Bram and Quinn 2000; Morellato 2004; Schmidt 2008; Togashi and Cox 2008). The biased sex ratios that have been observed in natural populations of diverse organisms may result from sex-based difference in a life history trait, that is, precocious reproduction of males (Allen and Antos 1993; Nicotra 1998). Meagher and Antonovics (1982) suggested that such a sex-specific difference in life history trait was likely to be a common cause of sex ratio bias.
Our results revealed a slight trend of male-biased sex ratio in E. cavaleriei, although sex ratios of most populations did not display statistically significant deviation from equality. All of the four significantly male-biased populations in the young class shifted to equality or were even female-biased. This suggests that male predominance in the young class in most populations may result from the precocious reproduction of males. Moreover, the mean DBH value of males was smaller than that of females, and the univariate anova showed that there was a significant difference of DBH between the sexes (Table 3). These results provided further evidence that males are likely to reach reproductive maturity at a smaller size than females (i.e., precocious over females) in this species. Early male maturation is a common characteristic in many dioecious plants, presumably due to lower reproductive costs in males than in females (Opler and Bawa 1978; Bullock and Bawa 1981; García and Antor 1995; Obeso 2002; Ueno et al. 2007). With increasing plant size, however, the number of flowering females increased, and consequently was nearly equal to the number of males. The results of E. cavaleriei demonstrate the fact that the sex ratio in natural populations could be fluctuating subject to population age.
Human activity may also affect the population sex ratio (Verdú and García-Fayos 1998). In E. cavaleriei, univariate anova revealed a significant population effect on DBH (P < 0.001) (Table 3), suggesting that plant size is different among populations. This pattern may result from human activities in many fragmented populations, which usually occur near villages. Individuals of E. cavaleriei are often logged by local residents as firewood, which might be an explanation for the unexpected female-biased sex ratio found in the young class of the HLS population.
Sex spatial distribution analysis exhibited that there was no evidence of spatial segregation of the sexes (SSS) in natural E. cavaleriei populations. Ripley's K analysis proved an excellent tool for analyzing the distribution of the sexes of a dioecious species. Because the analysis uses all possible pair distances to examine distributions as a function of scale, it is possible to determine the exact scale at which SSS is occurring (Nicotra 1998). The visual examination and the univariate Ripley's K analysis demonstrate that E. cavaleriei individuals are significantly clustered in their habitat regardless of the sexes (1, 2). These results can be explained by the seed dispersal pattern. In E. cavaleriei, seeds are mainly dispersed by gravity around female trees. Therefore, the small seed dispersal distance can lead to the clumped distribution of seedlings and young trees. However, the bivariate analysis of the distribution of males with regard to females suggests that individuals of the opposite sexes are usually distributed more at random than SSS and there was no difference of spatial pattern between fragmented and continuous habitats (Figure 2).
In many dioecious plant species, it has been documented that males and females are spatially segregated (reviewed by Bierzychudek and Eckhart 1988; Eppley et al. 1998; Bertiller et al. 2002). However, this may not reflect the true spatial distribution pattern of the sexes in the species studied (Eppley et al. 1998). In most previous studies, plants for which SSS has been documented can propagate asexually (Freeman et al. 1976; Hoffmann 1986; Iglesias and Bell 1989; Eppley et al. 1998). If male and female individuals randomly occupy differential microhabitats and can reproduce asexually, and/or male and female genets have differential rates of clonal growth in different habitats (Hoffmann 1986; Iglesias and Bell 1989), then such a patchy distribution would be expected, but the measured SSS may only show the spatial segregation of ramets rather than genets. However, this is not the case for E. cavaleriei, which can only reproduce sexually. It has not been observed that male and female individuals are occupying different microhabitats within individual populations, suggesting no difference in the spatial occupation between females and males. Therefore, SSS is unlikely to occur in natural E. cavaleriei populations.
In conclusion, most populations of E. cavaleriei exhibited a statistically equal sex ratio, suggesting little difference in growth or mortality between males and females. There was no evidence of spatial segregation of the sexes in the species. Apparently, the male-biased sex ratio is not a by-product of SSS in this species but a result from sex-based differentiation in life history trait (i.e. precocious reproduction of males). The sex ratio and the spatial distribution pattern of both sexes are unlikely to constitute a serious threat to the survival of E. cavaleriei. In order to explore the mechanism threatening the development of the natural populations, other mechanisms (e.g., ecological, genetic factors and their interaction) should be further studied. Genetic consequences resulting from isolated habitats and the effects of isolation on offspring fitness should be more emphasized in future work.
Materials and Methods
Study species
Eurycorymbus cavaleriei usually grows sparsely in lowland subtropical forests. Flowering occurs between April and June. It is a strictly dioecious species and flowers are small, sweet-scented, and arranged in umbellules, which occur in larger terminal corymbose panicles. E. cavaleriei is likely to be pollinated by unspecific insects as flowers were seen to be visited by a broad spectrum of pollinators including bees (Vespa manifica), butterflies (Pieris rapae) and flies (Eristalis ceralls) during a field survey we conducted. Capsules are globose, 3-partite. Fruits mature between July and October, and many of them remain on trees until the following year. Seeds are mainly dispersed by gravity, as shown by the fact that many seeds and seedlings are around maternal trees.
Field survey and sex ratio analysis
During the flowering season in 2005, field investigations were carried out in a whole range of distributions based on published data and herbarium records, and 18 populations of E. cavaleriei were identified and located. Three populations (data not shown) were excluded from analysis due to the small population size (n < 8). As a result, the sex ratio was analyzed in a total of 1 080 individuals sampled from 15 natural E. cavaleriei populations (Table 2). Gender can only be determined in reproductive plants. Within each population, for each individual plant that was flowering we recorded its gender, its DBH and its spatial coordinates (measured by GPS). Goodness-of-fit tests (G-tests) were used to examine significant deviation from a 1:1 sex ratio of male to female.
We grouped all adults into two DBH size classes: DBH ≤ 10 cm (Class I) and DBH > 10 cm (Class II) defined as young and old groups, respectively. The G-test was used to determine if the sex ratio in each size class within each population was significantly different from equality. In order to detect whether there was a significant difference in DBH of trees among different populations and between the sexes, we used univariate anova to assess the effects of population, sex (male or female) and their interactions on DBH by using SPSS version 11.0 (Miller and Campling 2002).
Spatial distribution analysis
Point pattern analysis was used to assess the spatial distribution of E. cavaleriei. Six populations (i.e. WJH, HLS, SJT, HD, LQ and XK, 1, 2), of which all flowering individuals were located and mapped, were used for these analyses.
First, a visual examination was used to test the spatial pattern of the populations. The X and Y axes are in seconds of latitude and longitude, respectively. The origin was fixed at the point whose coordinates were denoted by the minimum values of latitude and longitude of plants in each population. The coordinates of each individual were represented by the differences between the actual values of latitude and longitude and the value of the origin, expressed in seconds.
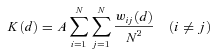
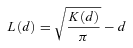
All calculations and simulations were carried out using multivariate analysis and graphical display software (ADS) in the ADE-4 computer package (Thioulouse et al. 1997). The software used for Ripley's K analysis outputs L(d) (the Ripley's K statistic) as well as a 95% confidence envelope for each distance analyzed (Mouer 1993). The confidence envelope is generated using 1 000 Monte Carlo simulations of random distributions based on plot-specific numbers of points and site dimensions.
Each population of E. cavaleriei was first analyzed using Ripley's univariate L(d) function, which included all reproductive individuals (regardless of sexes), and examined whether the population as a whole is randomly distributed in space. Each study plot was analyzed for distances up to half the length of the shortest side (Mouer 1993). When L(d) and the confidence envelope are plotted on the same axes, patterns of clumping and regularity become apparent. If L(d) exceeds the upper confidence interval for any distance class, those points are relatively closer together than expected, indicating clustering or clumping at those scales. If L(d) falls below the lower confidence interval for a distance class, the points are relatively further from one another than expected under a random distribution, indicating regularity, repulsion or over-dispersal of points. Values of L(d) within the confidence envelope indicate random distribution of points.
Ripley's bivariate L12(d) function was used to assess spatial association between the sexes, as described by Nicotra (1998). The intertype analyses were done for males relative to females and females relative to males. Only results from the analysis of male distribution relative to female are presented here, because the bivariate analysis of females relative to males produced an identical pattern. If the sexes of E. cavaleriei are spatially segregated, the Ripley's K analysis would indicate that males are further from females than expected at random, and the L12(d) statistic would fall below the lower confidence interval over some range of the distances considered, indicating repulsion between the sexes (Nicotra 1998).
(Handling editor: Song Ge)
Acknowledgements
The authors thank Professor Ying Wang for help with data analysis, Professor John Cram for checking the manuscript, and Hua Zeng and Dawei Li for help with data collection in the field. We also thank the following organizations for helping with field work: Mulun National Natural Reserve, Maolan National Natural Reserve, Jiulianshan National Natural Reserve, Guanshan National Natural Reserve, Hupinshan National Natural Reserve, Chebaling National Natural Reserve, Guilongshan Natural Reserve, Mingjiangyuan National Natural Reserve, and Mangdangshan Natural Reserve.




