Polyamine Accumulation in Transgenic Tomato Enhances the Tolerance to High Temperature Stress
Supported by the State Key Basic Research and Development Plan of China (2009CB119000), the National Natural Science Foundation of China (30571268), and the Hi-Tech Research and Development Plan of China (G2006AA100108).
Abstract
Polyamines play an important role in plant response to abiotic stress. S-adenosyl-l-methionine decarboxylase (SAMDC) is one of the key regulatory enzymes in the biosynthesis of polyamines. In order to better understand the effect of regulation of polyamine biosynthesis on the tolerance of high-temperature stress in tomato, SAMDC cDNA isolated from Saccharomyces cerevisiae was introduced into tomato genome by means of Agrobacterium tumefaciens through leaf disc transformation. Transgene and expression was confirmed by Southern and Northern blot analyses, respectively. Transgenic plants expressing yeast SAMDC produced 1.7- to 2.4-fold higher levels of spermidine and spermine than wild-type plants under high temperature stress, and enhanced antioxidant enzyme activity and the protection of membrane lipid peroxidation was also observed. This subsequently improved the efficiency of CO2 assimilation and protected the plants from high temperature stress, which indicated that the transgenic tomato presented an enhanced tolerance to high temperature stress (38 °C) compared with wild-type plants. Our results demonstrated clearly that increasing polyamine biosynthesis in plants may be a means of creating high temperature-tolerant germplasm.
Tomato (Lycopersicon esculentum Mill.) is one of the most popular and widely consumed vegetables grown worldwide. High temperature is a major factor limiting productivity and it adversely affects the vegetative and reproductive phases of tomato, ultimately reducing yield and fruit quality (Dinar and Rudich 1985; Sato et al. 2001; Pressman et al. 2002).
Polyamines (PAs) are small ubiquitous compounds that have been implicated in the regulation of many physiological processes and a variety of stress responses in plants (Bouchereau et al. 1999; Yang et al. 2007). PAs, spermidine (Spd), spermine (Spm) and putrescine (Put) accumulate under abiotic stress conditions. The enhanced level of polyamines plays an important role in the protective response of plants to various abiotic stresses (Kumar et al. 2006). However, the mechanism of action of PAs in abiotic stress response is not clearly understood. It has been suggested that PA involvement in abiotic stress adaptation could be due to their roles in osmotic adjustment, membrane stability, free-radical scavenging and regulation of stomatal movements (Liu et al. 2007).
The genetic manipulation of polyamine metabolism is one of the approaches to elucidate the functional role that polyamines play under stress. Unfortunately, there are only few reports on responses of plants expressing PA metabolic genes to environmental stress condition (Rajam et al. 1998; Kumar et al. 2006), although it has been shown that an increased tolerance to environmental stress was observed overexpressing PA biosynthetic genes, such as arginine decarboxylase (Masgrau et al. 1997; Roy and Wu 2001; Capell et al. 2004), ornithine decarboxylase (Kumria and Rajam 2002), S-adenosylmethionine decarboxylase (Torrigiani et al. 2005) and spermidine synthase (Franceschetti et al. 2004; Kasukabe et al. 2004, 2006) in rice, tobacco, Arabidopsis and sweet potato plants.
S-Adenosyl-l-methionine decarboxylase (SAMDC, EC 4.1.1.50) is one of the key regulatory enzymes in the biosynthesis of polyamines. SAMDC catalyzes S-adenosylmethionine (SAM) to form decarboxylated SAM, which provides the aminopropyl groups for subsequent Spd and Spm. It is believed that the synthesis of Spd and Spm is mainly regulated by the level of SAMDC. Overexpression of heterologous SAMDC in plants generally results in improving the tolerance to abiotic stress, including salt (Roy and Wu 2002), drought (Waie and Rajam 2003), acidic and oxidant stress (Wi et al. 2006). However, there are few reports on its role in heat-stress protection in higher plants. The SAMDC primary sequences in higher plants are similar. Therefore, the SAMDC gene isolated from Saccharomyces cerevisiae was selected and introduced to tomato plants to avoid the homologous depression in the present work. The response of transgenic plants to high temperature stress was investigated in order to obtain some fundamental information on the role of PAs during heat stress. Indeed, overexpression of yeast SAMDC gene (ySAMDC) in transgenic tomato plants led to an increase in PAs content and enhanced the tolerance to high temperature stress. Our results provide helpful information in order to better understand the physiological function of polyamines under abiotic stress in tomato plant.
Results
Transformation and regeneration
The visible shoots emerged in the co-cultivated explants after 7–10 d of culture on selection medium. The growth of shoots derived from leaf explants was slow and the shoots rooted in rooting medium after more than 80 d of culture. However, the plantlets grew well when transferred to pots for 20–30 d, and all of the transgenic plants showed normal flower and fruit formation (Figure 1).

The yeast S-adenosyl-l-methionine decarboxylase (ySAMDC) transgenic plantlets of tomato were obtained by Agrobacterium tumefaciens mediated method.(A) HygR buds were induced.(B) Screen HygR shoots.(C) HygR shoots were formed from buds.(D) Rooting of HygR shoots.(E) Regenerated plants transferred to the pot.(F) Fruit of HygR plant.
Transgene integration and expression
The presence of ySAMDC in putative transgenic plants was confirmed by polymerase chain reaction (PCR) analysis. The expected amplified product of 1.2 kb, specific to the ySAMDC gene was obtained (Figure 2A). The PCR positive plants were analyzed by Southern hybridization to identify the integration and copy number of the transgene. The transgenics showed one or two copy insertions of the transgene in tested plants (Figure 2B).
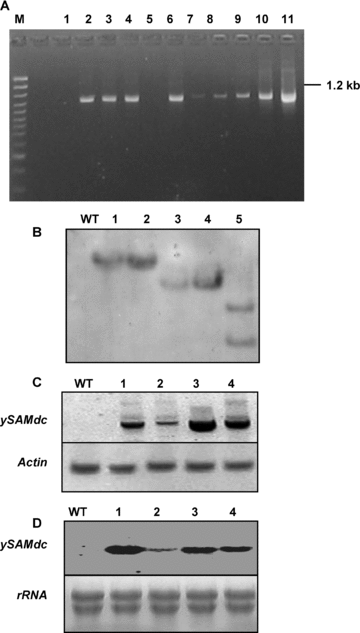
Molecular characterization of transgenic tomato plants.(A) Polymerase chain reaction (PCR) analysis of putative transgenic plants using primers specific to the yeast S-adenosyl-l-methionine decarboxylase (ySAMDC) gene. 100 bp DNA ladder (M), DNA from wild-type (WT) plant, transformed plantlet with the empty plasmic pCAMBIA1301, different putative transgenic plants and positive p35S-SAMDC vector.(B) Southern analysis of EcoRI-digested genomic DNA for checking transgene copy number using the hpt gene as a probe. DNA from wild-type plants and different transgenic lines (A7, B2, B6, Q5, T9).(C) Reverse transcription (RT)-PCR analysis for transgene expression at transcript level using primers specific to yeast SAMDC gene (upper) and actin primer. The RNA from wild-type plants and different transgenic plants (A7, B2, Q5 and T9).(D) Northern blot with the probe specific to ySAMDC gene. RNA from wild-type plants and different transgenic plants (A7, B2, Q5 and T9).
The transgenic lines showed high transgene expression at the transcript level by reverse transcription (RT)-PCR using ySAMDC gene-specific primers, while not in wild type plants (Figure 2C). The inserted ySAMDC was further proved by Northern blot analysis, transgenic plants showed high transgene expression at the transcript level (Figure 2D). The expression levels were, however, variable among the transgenic lines. The highest transcription level was observed in A7 and Q5, while relatively low in B2.
Electrolyte leakage
Leaf electrolyte leakage (EL) was measured to evaluate cell membrane stability, so EL is suggested to be closely related to heat tolerance in higher plant. The amount of leakage increased with time of exposure to heat stress condition in all lines; however, EL in transgenic plants was significantly (P < 0.05) less than in wild-type plants after 4 h treatment stress. After 12 h treatment, the EL in wild-type plants was 87.7%, whereas that in transgenic tomato lines B2 and Q5 was 64.3% and 43.1%, respectively (Figure 3), which suggested that overexpression of SAMDC in tomato plants resulted in enhanced protection of cell membrane permeability.
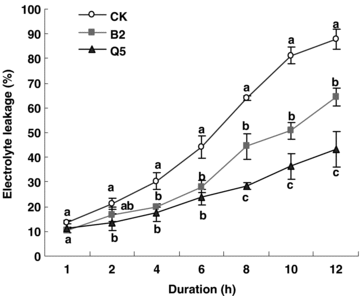
Electrolyte leakage of leaf discs of wild-type (WT), and transgenic yeast S-adenosyl-l-methionine decarboxylase (ySAMDC) (Lines B2 and Q5) tomato plants following heat stress (42 °C) of varying durations (1, 2, 4, 6, 8, 10 and 12 h).Values are means of four repeats ± standard error (SE). Different letters (in column) indicate significant differences (P < 0.05) between means within each sampling time with Duncan's multiple range test.
Polyamine metabolism
The transgenic lines showed significant increase in Spd and Spm levels when compared with wild-type plants and pCAMBIA1301 plants (Table 1). In wild-type plants, the Spd and Spm contents were 35.2% and 40%, respectively, higher under high temperature stress than under non-stressed conditions. However, in the transgenic lines, Spd contents were increased by 104% and 145% for B2 and Q5, respectively, while Spm contents increased by 93.9% and 51.7%, when compared with non-stressed conditions. The levels of accumulated polyamines were much higher in all transgenic tomato plants than in wild-type ones. On average, Spd and Spm in transgenic lines were increased by 134% and 66.1%, respectively, under high temperature conditions compared with wild-type plants. Different transgenic lines showed variations in the increased levels of PAs when exposed to high temperature. Q5 showed higher increase of PAs than B2, which may correspond to the high level of SAMDC RNA shown in Figure 2.
| Plants | Spermidine | Spermine | Putrescine | |||
|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | Control | Stress | |
| Non-transgenic | 128 ± 32a,b | 173 ± 25a | 40 ± 9.4a | 56 ± 7.2a | 170 ± 26a,b | 203 ± 39a,b |
| Transformed plant with pCAMBIA1301 | 113 ± 21a | 165 ± 37a | 32 ± 5.9a | 47 ± 12a | 201 ± 34a,b | 196 ± 17a |
| Transgenic B2 | 184 ± 43b | 376 ± 55b | 49 ± 11a,b | 95 ± 16b | 137 ± 43a | 236 ± 33a,b |
| Transgenic Q5 | 177 ± 35b | 434 ± 64b | 60 ± 14b | 91 ± 11b | 213 ± 45b | 262 ± 42a |
- Values (nmol/g fresh weight) represent average of data from three independent experiments and are shown as means ± standard error (SE) Different letters in columns indicate significant differences (P < 0.05) between means of different transgenic lines and wild-type with Duncan's multiple range test.
Transgenic lines showed significant difference in Put level grown under control conditions. Put content increased 25.3% in Q5 but decreased 19.4% in B2 compared with the wild-type plants (Table 1). Under high temperature treatment, Put increased 72.3% and 23%, respectively, in B2 and Q5 compared with the control conditions, and there was no significant difference between two transgenic lines. On the contrary, Put level in the non-transformed plants and pCAMBIA1301 plants remained unchanged.
CO2 assimilation and chlorophyll fluorescence parameters
Photosynthetic gas exchange parameters were examined in transgenic plants, which were kept at 28 °C for 3 d after having been exposed to high temperature of 38 °C for 4 d (Figure 4). Net photosynthetic rate (Pn) was not significantly different in transgenic lines compared with wild-type plants grown under control conditions. Although Pn was 20.9% lower in B2 than Q5, the difference was not significant (P= 0.064). Pn significantly decreased in all plant types when exposed to 38 °C for 4 d. However, the decrease in Pn was much greater in wild-type plants than in transgenic plants. Pn in transgenic plants was significantly higher than wild-type plants after high temperature treatment. Moreover, Pn in transgenic lines recovered near to normal levels when the plants were brought to optimal growth conditions for 3 d, while not in wild-type plants. Similar results were also observed in the stomatal conductance (Gs) and internal CO2 concentration (Ci). Gs decreased 31.3% and 20% in B2 and Q5, respectively, compared with 56.3% in wild-type plants under high temperature. Ci decreased by 23.6% and 13% in line B2 and Q5, respectively (Figure 4), while it reached 37.9% in wild-type plants. Gs and Ci in transgenic plants were significantly higher than in wild-type plants after high temperature treatment. These results indicated that the tolerance of CO2 assimilation rate to high temperature was greatly increased in transgenic plants.
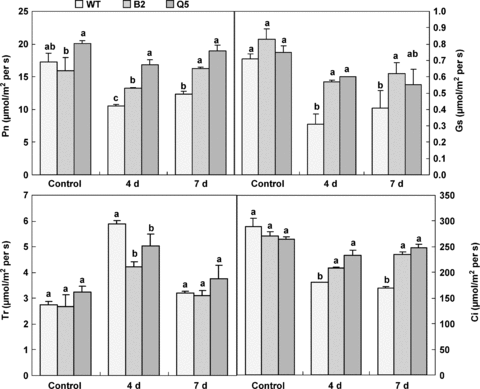
Net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr) and internal CO2 concentration (Ci) in tomato leaves from the non-transgenic plants (WT), transgenic line B2, and Q5 treated with high temperature conditions (38/30 °C) for 4 d (4 d), and then transferred to normal conditions (28/22 °C) for recovery for 3 d (7 d).The control plants were grown in normal conditions (28/22 °C) at all times. Data are the means of three independent repeats ± standard error (SE) shown by vertical error bars. Different letters indicate significant differences (P < 0.05) between means within each sample day with Duncan's multiple range test.
The transpiration rate (Tr) was not significantly affected in transgenic plants under control growth conditions. Increased Tr was observed in wild-type and transgenic tomato plants when they grew under high temperature of 38 °C for 4 d. However, Tr increase in transgenic lines was significantly lower than that in wild-type plants. After 3-d recovery, Tr decreased in all examined plants to near the normal level and showed no significant difference between transgenic and non-transgenic plants.
The ratio of variable fluorescence (Fv) to maximum fluorescence (Fm) was measured to estimate leaf photochemical efficiency. The maximum quantum efficiency of photosystem II (PSII), as given by Fv/Fm, was not influenced by transgene of ySAMDC under normal growth conditions. Fv/Fm decreased slightly after high-temperature treatment for 4 d. Such decline was not observed in transgenic line Q5 (Figure 5). However, the difference between transgenic plants and non-transgenic plants was not significant under high temperature conditions and during the period of recovery.
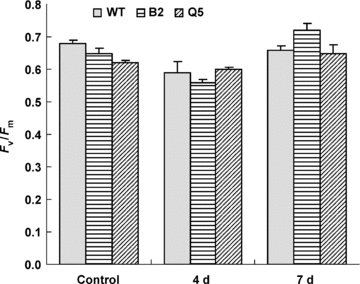
Effects of high temperature treatment and recovery on Fv/Fm in tomato leaves from the non-transgenic plants (WT), transgenic lines B2 and Q5.Data are the mean of three independent repeats ± standard error (SE) shown by vertical error bars.
Activities of antioxidant enzymes and malondialdehyde content
Antioxidant enzymes including superoxide dismutase (SOD), catalases (CATs), peroxidase (POD) and ascorbate peroxidase (APX) scavenge active oxygen species to protect plant cells through a complex antioxidant system (Scandalios 1993). The activities of SOD, APX, guaiacol peroxidase (G-POD) and CAT significantly increased in all lines after exposure to high temperature (Figure 6). GPOD and CAT were even increased by more than twofold. In general, the activities of SOD, APX, GPOD and CAT increased in transgenic lines; however, the extent of increase of SOD and CAT in two transgenic lines was significantly different. SOD activity was significantly higher in Q5 than that in B2 and wild-type plants, while CAT activity was higher in B2 than in Q5. Activities of SOD, APX, GPOD and CAT decreased after recovery in normal temperature for 3 d. There was no significant difference between transgenic and wild-type plants after recovery.
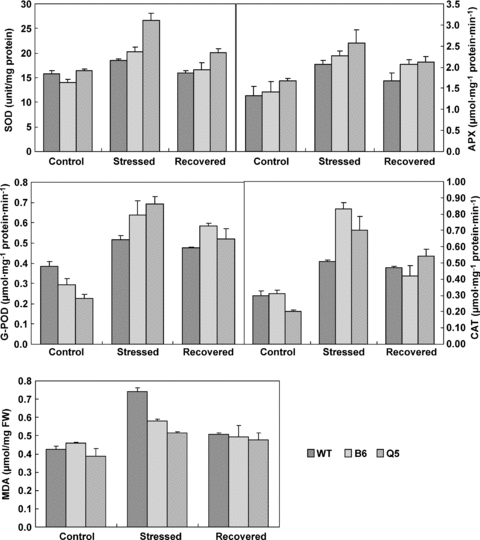
Effects of high temperature treatment on the activity of superoxide dismutase (SOD), guaiacol peroxidase (G-POD), ascorbate peroxidase (APX), catalase (CAT) and malondialdehyde (MDA) content in tomato leaves from the non-transgenic plants (WT), transgenic lines B2 and Q5.Data are the mean of three independent repeats ± standard error (SE) shown by vertical error bars.
Malondialdehyde (MDA) is the product of membrane lipid peroxidation. Increasing MDA content in plant cells indicates damage of cell membranes (Scandalios 1993), which can lead to inhibition of photosynthesis and respiration processes, and thus reduced plant growth. MDA content was significantly increased and almost doubled in wild-type plants after high temperature treatment for 4 d (Figure 6). MDA also increased in transgenic lines after heat-stress, but the increase was significantly lower than wild-type plants. After recovering in normal temperature for 3 d, MDA content in all tested plants decreased approximately to the normal level, and there was no significant difference between transgenic and wild-type plants.
Discussion
It is known that the stress tolerance is associated with maintenance of high concentrations of polyamines. Increased levels of Spd and Spm under stress conditions have been implicated in radical scavenging mechanisms (Lester 2000). Transgenic plants overexpressing PAs gene with increased PA levels showed tolerance to salinity, drought, cold or acidic stress (Roy and Wu 2002; Waie and Rajam 2003; Wi et al. 2006). Recently, Prabhavathi and Rajam (2007) reported that transgenic eggplants with oat arginine decarboxylase (ADC) gene exhibited increased polyamine content and enhanced tolerance levels to multiple abiotic stresses.
In the present study, transformation with ySAMDC in tomato plant significantly increased PAs accumulation, especially Spm and Spd under high temperature conditions. Spd and Spm increased 2.4-fold and 1.7-fold, respectively, on average in transgenic lines compared with wild-type plants (Table 1). Increased Spd and Spm levels are usually associated with enhanced plant tolerance to unfavorable conditions (Jiménez-Bremont et al. 2007). Spm accumulation is associated with the stabilization of the membranes and cell constituents through binding with negatively charged groups (Ha et al. 1998). On the other hand, we found that increase in Put was not significantly different between transgenic plants and wild-type plants, although Put also increased when exposed to high temperature. These results suggested that plants overexpressing SAMDC genes with high endogenous PA levels would be very important for abiotic stress tolerance, which may be implemented by PA involvement of signal transduction pathways associated with this process (Sairam and Tyagi 2004; Kasukabe et al. 2004; Vinocur and Altman 2005; Alcázar et al. 2006).
Although the genetic engineering of the synthesis of polyamine to tolerate abiotic stress appears promising (Alcázar et al. 2006; Vinocur and Altman 2006), there are still no reports on the enhanced tolerance of photosynthesis to high temperature stress. Photosynthesis is, among the plant functions, the most sensitive to high temperature damage (Havaux 1993; Camejo et al. 2005). Heat-stress caused a significant reduction in Pn. However, this was only partly a result of stomatal closure, since carboxylation was also limited (Figure 4). However, the decline in assimilation rates in transgenic plants was smaller compared with wild-types, which suggested that plants in these treatments were able to better use internal carbon dioxide. Such a protective influence of these compounds may explain the higher stomatal conductance, carboxylation efficiency (Figure 4). On the other hand, the antitranspirant action observed in this study confirmed previous reports for polyamine. A lower Tr rate helped to conserve water in heat-stressed plants (Rajasekaran and Blake 1999). The results showed that Pn responses to high temperature were partially reversed by enhanced polyamine level in SAMDC tomato plants; however, the promotive effects of polyamine were not the result of a more favorable PSII. High temperatures induced no significant damage to PSII, as given by Fv/Fm. SAMDC transgene had no significant effect on the chlorophyll fluorescence parameter. Our results are in agreement with previous studies that PSII is not affected at moderately high temperature (35 °C–45 °C) although PSII has long been considered the most temperature-sensitive component of photosynthesis (Havaux 1993; Haldimann and Feller 2005). Hence, the promoting action of polyamine on Pn may have been triggered by other processes. There are many data that strongly support the hypothesis that polyamines play a more complex role in the regulation of structure and function of the photosynthetic apparatus. Thus, there was strong indication that polyamines hold a pivotal role in photosynthesis, since they have been reported to be capable of simulating a photosynthetic apparatus adapted to high temperature conditions through the enhanced thermostability of thylakoid membranes (Kusano et al. 2007). Another possible explanation is that the apparent increased protection of photosynthesis from SAMDC could result from an effect on carotene accumulation, which directly affected the xanthophyll cycles. Enhanced carotene content in SAMDC transgenic plants provides the clues that polyamine could apparently affect carotene biosynthesis (Mehta et al. 2002).
As antioxidants, polyamines may protect against oxidative degradation and membrane damage, resulting in maintenance of homeostasis in plant cells (Rodríguez-Kessler et al. 2006). Enhancing the PA accumulation was found to be associated with increased antioxidant enzyme activities under stress conditions. Wi et al. (2006) proved that overexpression of SAMDC in tobacco could induce high mRNA levels of several antioxidant enzymes, such as ascorbate peroxidase, superoxide dismutase and glutathione S-transferase in transgenic plants. PAs were shown to function mainly as a scavenger of free superoxide radicals under conditions of weak stress, whereas under conditions of strong stress they mainly acted as positive modulators of antioxidant genes (Tkachenko and Nesterova 2004; Liu et al. 2007). In the present study, an increase in Spd, Spm and the total free polyamine level were found under high temperature treatment, which was accompanied by the markedly increased antioxidant enzyme activity and decreased MDA content in transgenic plants. The stress increased lipid peroxidation and this was significantly alleviated by overexpression of the ySAMDC gene. There was less lipid peroxidation relative to wild-type plants. These results suggested that a possible mechanism of heat tolerance was due to an increase in polyamines with marked increases of antioxidant enzyme activities and alleviation of the membrane damage caused by reactive oxygen species (ROS) during heat stress. Liu et al. (2007) have demonstrated that Spd and Spm levels in tomato leaves could have a protective role against heat stress-induced ROS.
In summary, in the present paper, an ySAMDC cDNA from yeast was expressed in tomato, the transgenic progeny (T2), belonging to two different lines, were compared with wild-type and the empty vector-transformed (pCAMBIA1301) controls in terms of PA metabolism, photosynthetic parameters and response to heat stress. The introduction of the ySAMDC gene into tomato increased the PAs accumulation and antioxidant enzyme activity and also enhanced CO2 assimilation, and therefore enhanced the tolerance to heat stress.
Materials and Methods
Plant material and plasmid
The seeds of tomato (Lycopersicon esculentum Mill.) variety “zhongshu No.6” were obtained from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China. The Agrobacterium tumefaciens strain LBA4404 containing binary plasmid pCAMBIA1301 with the SAMDC gene from Saccharomyces cerevisiae under the control of cauliflower mosaic virus CaMV35S promoter and nopaline synthase terminator, and hygromycin phosphotransferase (hpt) as plant selection marker was used for tomato transformation (Ding et al. 2006). An empty pCAMBIA1301 vector was also transferred into the A. tumefaciens strain LBA4404 as a positive control.
Tomato transformation and regeneration
Cotyledon explants, collected from about 6–7 d seedlings, were used for transformation. The Agrobacterium culture grown to an optical density (O.D.) (A600) of 0.1–0.2 was used for infection (10 min) on 2-d-old precultured explants, grown on shoot regeneration medium (SRM), Murashige and Skoog (MS) medium supplemented with 1 mg/L benzylaminopurine (BAP) and 0.15 mg/L indole-3-acetic acid (IAA) for 2 d. After infection, the explants were co-cultured on SRM for 2 d, and then transferred to selection medium (i.e. SRM containing 7.0 mg/L hygromycin[Hyg]+ 300 mg/L Cefotaime [Cef]) and cultured for 35 d with one sub-culture. The small shoots obtained on selection medium were subjected to proliferation on MS medium fortified with 0.5 mg/L BAP and 0.1 mg/L IAA. The well-grown shoots were excised and transferred to the rooting medium (half-strength MS with 0.05 mg/L 1-naphthaleneacetic acid (NAA) + 7.0 mg/L Hyg + 300 mg/L Cef). The rooted plants were transferred to pots containing peat : perlite in a 2:1 ratio and covered with polythene bags for 1 week to maintain high humidity for hardening in the growth chamber and then transferred to a greenhouse (Ding et al. 2006).
Polymerase chain reaction
The putative transgenic plants were analyzed by PCR for the integration of the transgene. DNA was isolated from the leaf explants by the Cetyl Trimethyl Ammonium Bromide (CTAB) method. About 100 ng of DNA from untransformed plants, as well as putative transgenic lines, was taken and mixed with 100 mM of primer pair, 7.5 μL PCR buffer, 100 mmol/L dNTP mix and 0.5 U of Taq polymerase (MBI Fermentas, Burlington, Ontario, Canada).The PCR program included denaturation at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 45 s and synthesis at 72 °C for 45 s and finally one cycle of 7 min at 72 °C. The primer pairs specific for the amplification of a 1.2-kb fragment of the ySAMDC gene were 5′-CGGAGCTCACATGGCTGTCACCATAAAAGAATTGA-3′ and 5′-GCCGCGGATCCTTTTCATATTTTCTTCTGCAATTTC-3′.
Southern blot hybridization
Tomato genomic DNA (10 μg) was restricted with EcoRI to detect the copy number of the transgene. Southern blots were prepared by the standard procedure (Sambrook et al. 1989) using Hybond-N Nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The hpt gene probes were prepared using 32P-labeled dCTP by nick translation as the manufactures guidelines (Gibco-BRL, Gaithersburg, MD, USA). Hybridization was carried out for 18–22 h at 65 °C. Signals were detected by exposure of storage phosphor screens, which were scanned in a Typhoon 9400 (GE Healthcare, Piscataway, NJ, USA).
RNA extraction, RT-PCR and Northern blot
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) as a template and the cDNA was made using SMART PCR cDNA Synthesis Kit according to the manufacturer's instructions (Clontech, Mountain View, CA, USA). The 20 μL of reaction mixture included 1× PCR reaction buffer, 400 mmol/L of dNTPs, 1.2 mmol/L of each primer, 2 U Taq Polymerase and 1.2 μL cDNA. The reaction mixture was heated to 95 °C for 10 min, followed by 30 cycles of 30 s denaturation at 94 °C, annealing at 53 °C for 30 s, extension at 72 °C for 1 min and final extension for 10 min. The PCR products were analyzed on 1% agarose gel.
Twenty micrograms of total RNA were electrophoresed through a 1.2% (w/v) denaturing formaldehyde/agarose gel, blotted to a Nitran filter by the capillary blot method. Prehybridization and hybridization were carried out in 50% formamide buffer using an [32P]-labeled SAMDC cDNA probe at 42 °C. Yeast specific SAMDC cDNA fragment was labeled using Random Priming Labeling Kit (Promega, Shanghai, China) with α-[32P] dCTP. Washing of the filter was carried out first with 2× standard saline citrate (SSC), 0.5% sodium dodecyl sulfate (SDS) for 7 min and then 1× SSC, 0.5% SDS at 55 °C for 4 min.
Electrolyte leakage
The T1 seeds obtained from primary transgenic plants were screened with 7 mg/L hygromycin in seedlings for PCR analysis. Two independent lines (B2 and Q5) of the hygromycin-resistant progenies (T2) from self-fertilized T1 transgenic tomato plants were selected for the following study. The leaf discs (0.80 cm in diameter) were punched out with a cork borer from the youngest fully expanded leaves of two T2 transgenic lines and the wild-type plants of 40-d-old plantlets grown under normal conditions (28 °C/22 °C). The leaf discs were immersed in a test tube containing 10 mL of deionized, distilled water. The base of the tube was submerged in a water bath at 42 °C and removed after 1, 2, 4, 6, 8, 10 and 12 h for testing. Following heat treatment, electrolyte leakage was measured using a conductivity meter (Greisinger Electronic, Regenstauf, Germany). Determination of percent electrolyte leakage was calculated according to the method of Ahn et al. (1999). Means for all values are an average of three subsamples in each plant with four replications.
High temperature treatment
The seeds of T2 transgenic lines, empty vector-transformed line and wild-type plants were surface-sterilized and sowed in vermiculite-filled plastic egg trays in the greenhouse of Zhejiang University, China. After 3 weeks, the seedlings were transferred to 10 cm individual plastic pots with the media of peat, perlite and vermiculite mixture (7:2:1 in volume). The pots were incubated in a greenhouse maintained at 28 °C/22 °C (day/night) with photosynthetic photon flux density (PPFD) of 1 000 μmol/m2 per s, a relative humidity of 75% to 80% and a light : dark cycle of 14:10 h. Nutrient solution were supplied once every 3–4 d, and the solution included Ca(NO3)2 5.0 mmol/L, KNO3 4.0 mmol/L, KH2PO4 1.0 mmol/L, MgSO4 2.0 mmol/L, Fe-ethylenediaminetetraacetic acid (EDTA)70.0 μmol/L, MnSO4 10.0 μmol/L, H3BO3 50.0 μmol/L, ZnSO4 0.7 μmol/L, CuSO4 0.2 μmol/L, (NH4)6Mo7O24 0.01 μmol/L.
To avoid complications resulting from differences in plant size and reproduction stage, all stress assays were carried out with 35-d-old seedlings. At this stage no apparent differences in plant size and growth were observed between the wild-type and transgenic plants (data not shown).
The treatments were conducted in a climate chamber with 38 °C/30 °C (day/night), 80% relative humidity, 16 h light/day (photonflux of 350 μmol/m2 per s) After 4 d of high-temperature treatment, the seedlings were recovered for 3 d at the temperature 28 °C/22 °C. Samples on the 4th (stressed) and 7th days (recovered) after high-temperature treatment were used for the evaluation of photosynthesis characteristics and heat tolerance. The control plants were maintained under a constant temperature of 28 °C/22 °C. All of the measurements on physiological and biochemical parameters were carried out on the youngest fully expanded leaves.
Analysis of polyamines
Polyamines were estimated in seedlings of the wild-type and transgenic lines with and without high temperature treatment according to the protocol of Minocha et al. (1990). Tomato leaves (0.4 g) from five seedlings were pooled and powered with nitrogen, then extracted in 10 volumes of 4% perchloric acid (PCA) and centrifuged at 20 000 g for 30 min at 4 °C. Aliquots (0.2 mL) of the supernatant containing free polyamines were dansylated, extracted in tobuene and analyzed by high performance liquid chromatography (HPLC) (Shimadzu CS-9301, LC-7A, Japan) on a reverse phase C18 column (Spherisorb ODS2, 5-μm particle size, 4.6 × 250 mm, Waters, Wexford, Ireland) using a programmed acetonitrile : water step gradient, respectively, with a 1 mL/min flow rate. Eluted peaks were detected with a fluorescence spectrophotometer at excitation and emission wavelengths of 360 and 506 nm and their areas were recorded and integrated relative to those of standard PAs (Sigma, St. Louis, MO, USA). Three extractions of polyamines were made from each sample and each extraction was quantified by HPLC in duplicate.
Photosynthetic and chlorophyll fluorescence parameter
Gas exchange parameters were determined on a fully expanded attached leaf of tomato seedlings by using an infra red gas analyzer (CIRAS-1-PP systems, Hitchin, Herts, UK). For the measurement of net photosynthetic rate (Pn), intracellular CO2 content (Ci) and stomatal conductance (Gs), air temperature, relative humidity, CO2 concentration and PPFD were maintained at 25 °C, 80–90%, 400 μL/L and 1 000 μmol/m2 per s, respectively. The measurements on these photosynthetic parameters lasted approximately 10 min, during which no significant recovery was observed on these parameters.
Chlorophyll fluorescence was measured using a portable pulse modulated fluorimeter (Model FMS-2, Hansatech, Norfolk, UK). After a dark adaptation period of 20 min, minimum fluorescence (Fo) was determined under a weak pulse of modulating light over 0.8 s and maximal fluorescence (Fm) was induced by a saturating pulse of light (8 000 μmol/m2 per s) applied over 0.8 s. The measurements were carried out on the attached leaves of tomato seedlings. The maximal efficiency of PSII photochemistry was determined as the ratio of variable to maximal chlorophyll fluorescence (Fv/Fm) (Krause and Weis 1991).
Activities of antioxidant enzymes and MDA content
For the enzymatic activity analysis, 0.5 g fresh leaf samples were homogenized in an ice bath in 5 mL 25 mmol/L HEPES buffer (contained 0.2 mmol/L EDTA, pH 7.8) and 2% polyvinylpyrrolidone. The homogenate was centrifuged at 13 000 g for 20 min at 4 °C. Supernatant obtained was used for enzyme analysis. All assays were carried out in a UV/visible light spectrophotometer (Shimadzu UV-2410 PC). Protein content was determined according to the method of Bradford (1976), which uses bovine serum albumin as the standard. Four separate extractions were carried out for each treatment for assay of the activities of all enzymes. The APX, CAT and SOD were determined according to our previous report (Lu et al. 2008). The method according to Cakmak and Marschner (1992) was used to determine the activity of G-POD with some modifications. The contents of the reaction mixture were: 25 mmol/L phosphate buffer (pH 7.0), 0.05% guaiacol, 10 mmol/L H2O2 and enzyme extract. Increase in absorbance at 470 nm caused by guaiacol oxidation was used to measure activity.
Thiobarbituric acid (TBA) test was used to measure MDA. Leaves were homogenized, centrifuged in a potassium phosphate buffer (pH 7.8) for 20 min at 12 000 g, and 1 mL of the supernatant was incubated in boiling water for 30 min. The tubes were placed in an ice bath to stop the reaction after which the samples were centrifuged at 1 500g for 10 min and the absorption read at 532 nm. The value for non specific absorption at 600 nm was subtracted.
Data analysis
The data reported in tables and figures are means of the values with standard error (SE) and examined statistically by anova using SAS software (SAS, 1996, SAS Institute, Cary, NC, USA). Means were compared for significant differences between treatments according to Duncan's multiple range test at P < 0.05.
(Handling editor: Zhizhong Gong)
Acknowledgements
The authors are thankful to Dr Riaz Ur Reman (Quaid-i-Azam University) for his kind proofreading of this article.




