The Characterization and Geographical Distribution of the Genes Responsible for Vernalization Requirement in Chinese Bread Wheat
Supported by the State Key Basic Research and Development Plan of China (2004CB117200) and Germplasm Conservation Project from Ministry of Agriculture.
Abstract
The frequency and distribution of the major vernalization requirement genes and their effects on growth habits were studied. Of the 551 bread wheat genotypes tested, seven allelic combinations of the three Vrn-1 genes were found to be responsible for the spring habit, three for the facultative habit and one for the winter habit. The three Vrn-1 genes behaved additively with the dominant allele of Vrn-A1 exerting the strongest effect. The allele combinations of the facultative genotypes and the discovery of spring genotypes with “winter” allele of Vrn-1 implied the presence of as yet unidentified alleles/genes for vernalization response. The dominant alleles of the three Vrn-1 genes were found in all ten ecological regions where wheat is cultivated in China, with Vrn-D1 as the most common allele in nine and Vrn-A1 in one. The combination of vrn-A1vrn-B1Vrn-D1 was the predominant genotype in seven of the regions. Compared with landraces, improved varieties contain a higher proportion of the spring type. This was attributed by a higher frequency of the dominant Vrn-A1 and Vrn-B1 alleles in the latter. Correlations between Vrn-1 allelic constitutions and heading date, spike length, plant type as well as cold tolerance were established.
Many temperate plant species, including Arabidopsis thaliana (At), wheat and barley require a period of cold treatment to effect the switch from vegetative to reproductive growth. This is formally termed as “vernalization requirement”. The adaptation of a number of temperate crop species to a wide range of latitudes has been achieved in large part by virtue of genetic variation. Allelic variation at the Vrn-A1, -B1 and -D1 homoeoloci are particularly important in determining the vernalization requirement of bread wheat (Nelson et al. 1995; Dubcovsky et al. 1998; Barrett et al. 2002; McIntosh et al. 2003). These genes are present on the homoeologous group 5 chromosomes, and the distribution of alleles has proven to be uneven across environments (Gotoh 1979; Kato et al. 1988; Kosnar and Bromova 1993; Iwaki et al. 2000, 2001). The Vrn-1 constitution of a genotype can be determined by genetic segregation of flowering time among the progeny (no vernalization requirement) × winter (vernalization-requiring) type crosses by the use of established tester lines, or by comparisons based on near-isogenic lines. However, a simple segregation analysis only discriminates between the two parental alleles (vernalization-requiring versus non-requiring). Since the Vrn-1 genes have recently been isolated, further allelic variants can now be characterized at the sequence level (Yan et al. 2003, 2004a; Fu et al. 2005). The distribution and combination of alleles among 117 hexaploid spring wheats from Argentina and California have recently been analyzed using just this approach (Yan et al. 2004a; Fu et al. 2005). Wheat has conventionally been categorized as either spring or winter types, and the Vrn-1 allele combinations which determine these categories are well understood. However, a third type, the facultative wheat, which is widely distributed across the temperate zones of Asia and Europe, is less well described at the genetic level.
Wheat is grown widely throughout China, despite the substantial variation in both latitude and climate. Cultivation occurs at altitudes varying from −150 m to >4 000 m, with mean annual rainfall ranging from 20 mm to 900 mm and mean temperature fluctuating between 8 °C and 17 °C during the growing season (Cui et al. 1991). The production area in China has been divided into ten ecological zones, which encompass the full environmental range experienced by wheat worldwide. For this reason, the eco-geographical distribution of alleles and allelic combinations of Vrn-1 in China is an important area of study. Zhang et al. (2008) reported the distribution of the alleles in 266 Chinese improved spring and winter varieties. Here, we report a survey of the spring, facultative and winter types of wheat in a collection of landraces and varieties, with respect to allelic constitution at both Vrn-1 and Vrn-2 genotype. Our aims were to investigate whether specific Vrn-1 alleles and/or allelic combinations appear to be characteristic of a particular ecological region(s), and to explore the relationship between environmental adaptation and some key phenotypic traits.
Results
Growth habit
According to Gardner and Barnett's (1990) classification criteria, the entries were grouped as 222 spring, 118 winter, and 211 facultative types (Table 1). Of the Chinese varieties, 36.6% were spring, 18.6% winter and 44.7% facultative types. Spring types predominated among both the Australian (100%) and the Chinese entries from the Northeastern Spring Wheat Region (NeS) (100%), Northern Spring Wheat Region (NS) (90%) and South China Winter Wheat Region (SCW) (83%); facultative types were common in major regions of production in China, the Middle and Low Yangtze Valley Winter Wheat Region (MLYW) (59%), Northern Winter Wheat Region (NW) (54%) and Yellow and Huai River Winter Wheat Region (YHW) (53%). See Figure 1A for a map of the ten regions.
| Locality | Growth habit | Total | ||||
|---|---|---|---|---|---|---|
| Spring | Facultative | Winter | ||||
| North America | 30 | 2 | 9 | 41 | ||
| Europe | 17 | 5 | 24 | 46 | ||
| Oceania | 8 | 0 | 0 | 8 | ||
| China | NeS | Landraces | 9 | 0 | 0 | 9 |
| Improved varieties | 11 | 0 | 0 | 11 | ||
| NW | Landraces | 0 | 10 | 11 | 21 | |
| Improved varieties | 0 | 26 | 20 | 46 | ||
| NS | Landraces | 10 | 2 | 0 | 12 | |
| Improved varieties | 8 | 0 | 0 | 8 | ||
| YHW | Landraces | 3 | 33 | 6 | 42 | |
| Improved varieties | 32 | 55 | 38 | 125 | ||
| MLYW | Landraces | 9 | 20 | 0 | 29 | |
| Improved varieties | 10 | 9 | 1 | 20 | ||
| NwS | Landraces | 8 | 4 | 1 | 13 | |
| Improved varieties | 11 | 3 | 1 | 15 | ||
| SwW | Landraces | 6 | 25 | 1 | 32 | |
| Improved varieties | 26 | 2 | 0 | 28 | ||
| SCW | Landraces | 6 | 2 | 0 | 8 | |
| Improved varieties | 4 | 0 | 0 | 4 | ||
| SkWS | Landraces | 6 | 1 | 3 | 10 | |
| Improved varieties | 1 | 2 | 2 | 5 | ||
| QTSW | Landraces | 7 | 7 | 0 | 14 | |
| Improved varieties | 0 | 3 | 1 | 4 | ||
| Total number | Landraces | 64 | 104 | 22 | 190 | |
| Improved varieties | 103 | 100 | 63 | 266 | ||
| Total number | 167 | 204 | 85 | 456 | ||
| Total number | 222 | 211 | 118 | 551 | ||
- Wheat accessions from China were grouped into ten ecological regions, based on locality and planting environment. MLYW, Middle and Low Yangtze Valley Winter Wheat Region; NeS, Northeastern Spring Wheat Region; NS, Northern Spring Wheat Region; NW, Northern Winter Wheat Region; NwS, Northwestern Spring Wheat Region; QTSW, Qinghai-Tibet Spring-Winter Wheat Region; SCW, South China Winter Wheat Region; SkWS, Sinkiang Winter-Spring Wheat Region; SwW, Southwestern Winter Wheat Region; YHW, Yellow and Huai River Winter Wheat Region.
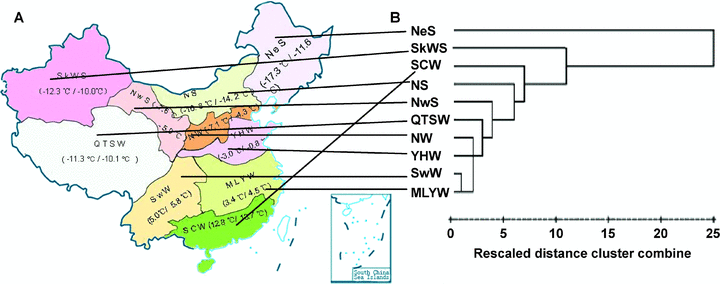
The geographical distribution and cluster analysis of the ten Chinese ecological regions.(A) The geographical distribution of the ten Chinese ecological regions. Mean January temperature in the 1950s and the 2000s are shown in parentheses.(B) Cluster analysis of ecological regions based on Vrn-1 genotype.MLYW, Middle and Low Yangtze Valley Winter Wheat Region; NeS, Northeastern Spring Wheat Region; NS, Northern Spring Wheat Region; NW, Northern Winter Wheat Region; NwS, Northwestern Spring Wheat Region; QTSW, Qinghai-Tibet Spring-Winter Wheat Region; SCW, South China Winter Wheat Region; SkWS, Sinkiang Winter-Spring Wheat Region; SwW, Southwestern Winter Wheat Region; YHW, Yellow and Huai River Winter Wheat Region.
Allelic status at Vrn-A1, -B1 and -D1 and its relationship with growth habit
The allelic status at the Vrn-1 loci was determined for all 551 entries (Table 2). The dominant alleles were found in 69 entries for Vrn-A1, 85 for Vrn-B1 and 258 for Vrn-D1. All of the entries carrying the dominant Vrn-A1 locus exhibited a spring growth habit; 76.5% of the entries carrying Vrn-B1 were of spring type and the remainders were all facultative. Of the entries carrying Vrn-D1, 41.6% were of spring type and the remainder was facultative (Table 2). Thus, for assuring spring habit, Vrn-A1 was the most effective allele, followed by Vrn-B1 and Vrn-D1. All accessions having any two of these alleles were spring types, indicating that the alleles can act additively. All 118 winter type entries had the allelic constitution vrn-A1 vrn-B1 vrn-D1. Of the 222 spring type entries, 41.4% were vrn-A1 vrn-B1 Vrn-D1, 12.1%Vrn-A1 vrn-B1 vrn-D1, 12.1%Vrn-A1 Vrn-B1 vrn-D1, 11.7%vrn-A1 Vrn-B1 vrn-D1, 9.4%vrn-A1 Vrn-B1 Vrn-D1 and 6.3%vrn-A1 vrn-B1 vrn-D1. The combinations Vrn-A1 vrn-B1 Vrn-D1 and Vrn-A1 Vrn-B1 Vrn-D1 were both rare. The 221 facultative types were composed of vrn-A1 vrn-B1 Vrn-D1 (61.1%), vrn-A1 vrn-B1 vrn-D1 (35.1%) and vrn-A1 Vrn-B vrn-D1 (3.8%) (Table 2).
| Allelic combination | Total | Growth habit | |||||
|---|---|---|---|---|---|---|---|
| Spring | Facultative | Winter | |||||
| No. | Ratio (%) | No. | Ratio (%) | No. | Ratio (%) | ||
| vrn-A1 vrn-B1 vrn-D1 | 206 | 14 | 6.8 | 74 | 35.9 | 118 | 57.3 |
| vrn-A1 vrn-B1 Vrn-D1 | 221 | 92 | 41.6 | 129 | 58.4 | 0 | 0.0 |
| vrn-A1 Vrn-B1 vrn-D1 | 34 | 26 | 76.5 | 8 | 23.5 | 0 | 0.0 |
| vrn-A1 Vrn-B1 Vrn-D1 | 21 | 21 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Vrn-A1 vrn-B1 vrn-D1 | 26 | 26 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Vrn-A1 vrn-B1 Vrn-D1 | 13 | 13 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Vrn-A1 Vrn-B1 vrn-D1 | 27 | 27 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Vrn-A1 Vrn-B1 Vrn-D1 | 3 | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 551 | 222 | 40.3 | 211 | 38.3 | 118 | 21.4 |
The growth habit of 225 entries was not satisfactorily explained by current models of the action of present knowledge of the Vrn-1 genes. The 88 vrn-A1 vrn-B1 vrn-D1 entries would have been expected to be of winter type, but 14 were spring and 74 facultative types had the same Vrn-1 genotype. Similarly, 137 vrn-A1 Vrn-B1 vrn-D1 and vrn-A1 vrn-B1 Vrn-D1 entries were not the spring type, as predicted, but rather facultative. This outcome suggests the presence of an as yet undescribed allelic variation at Vrn-1 and/or the existence of other genes interacting with the Vrn-1 genes in determining the vernalization response.
The distribution of Vrn-1 alleles and allelic combination
Among the Chinese ecological regions, the identity and frequency of the dominant alleles of Vrn-1 differed significantly (χ2, degrees of freedom d.f. = 18, P < 0.000 1). In all but one of the ecological regions, Vrn-D1 was the most frequent (53.3%, ranging from 26.9% to 91.7%), followed by Vrn-B1 (12.3%) and Vrn-A1 (11.2%) (Table 3). The exception was NeS (Vrn-A1– 80%, Vrn-B1– 55%, Vrn-D1– 30%). In contrast to this region, Vrn-A1 was absent in NW, MLYW, and the Qinghai-Tibet Spring-Winter Wheat Region (QTSW). However, in SCW, Vrn-A1 was twice as frequent as Vrn-B1, while in the Northwestern Spring Wheat Region (NwS), the frequencies of Vrn-B1 and Vrn-A1 were comparable. Vrn-A1 and Vrn-B1 were more common in North America, Argentina and Australia (χ2, d.f. = 4, P= 0.139 5). Most of the European accessions derived from Italy, and the distribution of alleles was similar to that found in the Chinese materials (χ2, d.f. = 2, P= 0.337 8). Vrn-A1a was significantly more frequent than Vrn-A1b, while Vrn-A1c was only present in a single spring type accession (from North America). The distribution of the three alleles was uneven among the Chinese ecological regions (Table 3). Thus, Vrn-A1b was only sporadically present in NwS (10.7%), SCW (8.3%), the Southwestern Winter Wheat Region (SwW) (1.7%) and YHW (0.6%). Vrn-A1a was more common than Vrn-A1b, and its geographic distribution was wider.
| Locality | Total | Vrn-D1 | Vrn-B1 | Vrn-A1 | Vrn-A1a | Vrn-A1b | Vrn-A1c | |
|---|---|---|---|---|---|---|---|---|
| North America | 41 | 9.8 | 46.3 | 48.8 | 41.5 | 4.9 | 2.4 | |
| Argentina | 62 | 41.9 | 66.1 | 56.5 | n/a | n/a | n/a | |
| Oceania | 8 | 0.0 | 37.5 | 50.0 | 37.5 | 12.5 | 0.0 | |
| Europe | 46 | 23.9 | 15.2 | 4.3 | 4.3 | 0.0 | 0.0 | |
| China | NeS | 20 | 30.0 | 55.0 | 80.0 | 80.0 | 0.0 | 0.0 |
| NW | 67 | 26.9 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | |
| NS | 20 | 60.0 | 25.0 | 15.0 | 15.0 | 0.0 | 0.0 | |
| YHW | 167 | 49.1 | 6.6 | 3.0 | 2.4 | 0.6 | 0.0 | |
| MLYW | 49 | 77.6 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| NwS | 28 | 53.6 | 35.7 | 32.1 | 21.4 | 10.7 | 0.0 | |
| SwW | 60 | 68.3 | 16.7 | 10.0 | 8.3 | 1.7 | 0.0 | |
| SCW | 12 | 91.7 | 16.7 | 33.3 | 25.0 | 8.3 | 0.0 | |
| SkWS | 15 | 46.7 | 20.0 | 6.7 | 6.7 | 0.0 | 0.0 | |
| QTSW | 18 | 72.2 | 11.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Total | 456 | 53.3 | 12.3 | 11.2 | 9.9 | 1.3 | 0.0 |
- Data regarding Vrn-1 promoter regions extracted from Yan et al. (2004a), and regarding intronic sequence from Fu et al. (2005). Vrn-A1, Vrn-B1 and Vrn-D1 represent the dominant alleles of A, B, D loci of Vrn-1 gene. Vrn-A1 is composed of Vrn-A1a, Vrn-A1b and Vrn-A1c. Vrn-A1a and Vrn-A1b represent alleles having, respectively, an insertion and a deletion in the Vrn-A1 promoter; Vrn-A1c is an allele having a deletion in the first intron of Vrn-A1. Vrn-B1, Vrn-D1 has a deletion in their respective first intron. The data regarding Argentinean wheat extracted from Fu et al. (2005). MLYW, Middle and Low Yangtze Valley Winter Wheat Region; NeS, Northeastern Spring Wheat Region; NS, Northern Spring Wheat Region; NW, Northern Winter Wheat Region; NwS, Northwestern Spring Wheat Region; QTSW, Qinghai-Tibet Spring-Winter Wheat Region; SCW, South China Winter Wheat Region; SkWS, Sinkiang Winter-Spring Wheat Region; SwW, Southwestern Winter Wheat Region; YHW, Yellow and Huai River Winter Wheat Region.
The frequency and distribution of allelic combinations differed significantly both between and within the ecological regions (χ2 test, P= 0.0000). The gene diversity present in each ecological region was reflected by the richness (Ri) and genetic diversity (DI) of the Vrn-1 genotypes. Ri varied from 3 (NW and MLYW) to 8 (NwS) (Table 4). DI is determined by the number and frequency of allelic combinations. On the basis of Ri and DI, the ranking of diversity in the various regions was NwS (8, 0.79) > NeS (6, 0.73) > SwW (7, 0.64) > Sinkiang Winter-Spring Wheat Region (SkWS) (4, 0.67) > YHW (6, 0.59) > NS (5, 0.59) > SCW (4, 0.58) > QTSW (4, 0.5) > NW (3, 0.41) > MLYW (3, 0.36). The highest Ri and DI occurred in NwS, the largest spring wheat production region, where the climate is very heterogeneous, which is consistent with an earlier study, showing that among the five spring wheat production regions of China, the highest level of phenotypic diversity was found in NwS (Dong et al. (2003). The lowest diversity was present in MLYW, where the environment and climate are both relatively homogeneous. The diversity of allelic combinations across the ecological regions presumably reflects differences in the level of heterogeneity for soil and climatic factors within each region. The combination vrn-A1 vrn-B1 Vrn-D1 dominated in seven (NS, YHW, MLYW, NwS, SwW, SCW, QTSW) of the ten regions. However, in NeS, the most common combination was Vrn-A1 Vrn-B1 vrn-D1, while in NW and SkWS, vrn-A1 vrn-B1 vrn-D1 was preferred.
| Allele combinations | NeS | NW | NS | YHW | MLYW | NwS | SwW | SCW | SkWS | QTSW |
|---|---|---|---|---|---|---|---|---|---|---|
| vrn-A1vrn-B1 vrn-D1 | 0.0 | 71.7 | 5.0 | 44.9 | 20.4 | 17.9 | 18.3 | 0.0 | 46.7 | 22.2 |
| vrn-A1vrn-B1Vrn-D1 | 10.0 | 26.9 | 60.0 | 45.5 | 77.6 | 35.7 | 56.7 | 58.3 | 26.7 | 66.7 |
| vrn-A1Vrn-B1vrn-D1 | 0.0 | 1.5 | 20.0 | 4.8 | 2.0 | 3.6 | 10.0 | 8.3 | 0.0 | 5.6 |
| Vrn-A1vrn-B1vrn-D1 | 30.0 | 0.0 | 10.0 | 1.2 | 0.0 | 7.1 | 1.7 | 0.0 | 6.7 | 0.0 |
| Vrn-A1vrn-B1Vrn-D1 | 5.0 | 0.0 | 0.0 | 1.8 | 0.0 | 3.6 | 6.7 | 25.0 | 0.0 | 0.0 |
| Vrn-A1Vrn-B1Vrn-D1 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.6 | 0.0 | 8.3 | 0.0 | 0.0 |
| vrn-A1 Vrn-B1 Vrn-D1 | 10.0 | 0.0 | 0.0 | 1.8 | 0.0 | 10.7 | 5.0 | 0.0 | 20.0 | 5.6 |
| Vrn-A1 Vrn-B1 vrn-D1 | 40.0 | 0.0 | 5.0 | 0.0 | 0.0 | 17.9 | 1.7 | 0.0 | 0.0 | 0.0 |
| No.of the tested varieties | 20 | 67 | 20 | 167 | 49 | 28 | 60 | 12 | 15 | 18 |
| Ri (the richness) | 6 | 3 | 5 | 6 | 3 | 8 | 7 | 4 | 4 | 4 |
| DI (diversity index) | 0.73 | 0.41 | 0.59 | 0.59 | 0.36 | 0.79 | 0.64 | 0.58 | 0.67 | 0.5 |
- Data extracted from Yan et al. (2004a) and Fu et al. (2005). Vrn-A1, Vrn-B1 and Vrn-D1 are the dominant alleles, while vrn-A1, vrn-B1 and vrn-D1 are the recessive alleles lacking an indel in either the promoter (-A1) or the first intron (-A1, -B1, -D1). MLYW, Middle and Low Yangtze Valley Winter Wheat Region; NeS, Northeastern Spring Wheat Region; NS, Northern Spring Wheat Region; NW, Northern Winter Wheat Region; NwS, Northwestern Spring Wheat Region; QTSW, Qinghai-Tibet Spring-Winter Wheat Region; SCW, South China Winter Wheat Region; SkWS, Sinkiang Winter-Spring Wheat Region; SwW, Southwestern Winter Wheat Region; YHW, Yellow and Huai River Winter Wheat Region.
The similarity between the ecological regions, as reflected by the Vrn-1 allele combination frequencies, was consistent with their geographic location. For example, MLYW and SwW clustered closely with one another, and were very dissimilar to NeS (Figure 1A). A cluster analysis based on the Vrn-1 genotype (Figure 1B) also showed that most of the winter wheat regions (MLYW, SwW, YHW, NW, QTSW) were similar to one another, and were quite distinct from the spring wheat production area SCW. These later five similar regions also border one another (Figure 1A). This kind of information provides a basis for the choice of germplasm to be transferred between breeding programs in different ecological regions.
Allelic variation within the Vrn-2 CCT domain
No Vrn-2 deletions (Vrn-A2c) were observed in the present set of accessions. The mutation present in einkorn (Vrn-A2b) was also present in some of the bread wheat, but only in the A genome copy Vrn-A2. The frequency of Vrn-A2b Vrn-B2a Vrn-D2a was very low (10.0% in spring accessions, 6.0% in facultative accessions and 9.8% in winter accessions) and its occurrence was mostly independent of the Vrn-1 genotype. The frequency of Vrn-A2b Vrn-B2a Vrn-D2a among the spring types was the highest in NWS (29.2%), followed by NeS (17.6%) and SwW (15.2%). It was absent in SCW, SkWS and QTSW. Among the facultative types, it was only present in SkWS, NW, QTSW and MLYW, and occurred among winter types from NW and YHW, Europe and North America.
Comparison between landraces and improved varieties
The proportion of spring types among the improved varieties was higher than that among the landraces in YHW, MLYW, NwS, SwW, NS and SCW (Table 1). Across these six regions, altogether 30.4% of the landraces and 45.8% of the improved varieties were of the spring type. Of the landraces in MLYW, YHW and SwW, respectively 31.0%, 7.1% and 16.1% were spring types, while among the improved varieties, the equivalent frequencies were 50.0%, 25.6% and 93.1%. In these three regions, facultative types included 63.7% of the landraces and 34.3% of the improved varieties. In YHW, MLYW and SwW, spring types survived poorly when planted in the autumn during the 1950s, so that at that time, the facultative types predominated. A larger proportion of spring types were planted where January mean temperatures were higher (e.g., in YHW, MLYW and SwW (Figure 1A).
The frequencies of the three dominant Vrn-1 alleles among both the landraces and improved varieties in YHW, MLYW, NwS, SwW, NS and SCW are illustrated in Figure 2. For the spring varieties (Figure 2A1), the frequency of the dominant allele was Vrn-D1 (90.2%), Vrn-A1 (14.6%) and Vrn-B1 (4.9%) among the landraces, and 64.1%, 34.8% and 22.8%, respectively, among the improved varieties. Thus in the shift from landraces to modern cultivars, the frequency of Vrn-A1 and Vrn-B1 increased but that of Vrn-D1 decreased. As a result, the greater representation of spring types among the improved varieties was achieved by an increasing frequency of the alleles Vrn-A1 and Vrn-B1. The distribution of each dominant allele in the landraces and improved varieties among the facultative types was very similar to that of the spring types (Figure 2A2). The decline of facultative types among the improved varieties related to a reduced frequency of the Vrn-D1 allele. The frequencies of the various allelic Vrn-1 combinations are illustrated in Figure 2B. The appreciable decline in the frequency of the genotype vrn-A1 vrn-B1 Vrn-D1 among the improved varieties ensured that vrn-A1 Vrn-B1 vrn-D1 occurred much more often among the modern improved varieties than among the landraces.
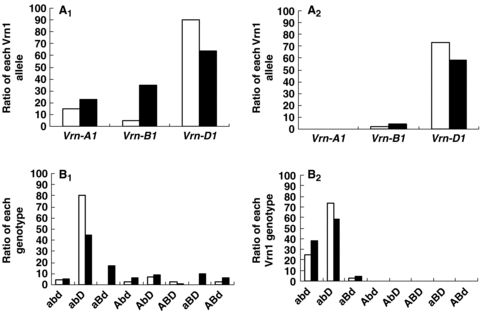
The frequency of Vrn-1 dominant alleles and allelic combinations in landraces and improved varieties in six ecological regions.(A1, B1)Spring types;(A2, B2) Facultative types.(□) Landrace; (▪) Breeding line. abd, vrn-A1 vrn-B1 vrn-D1; abD, vrn-A1 vrn-B1 Vrn-D1; aBd, vrn-A1 Vrn-B1 vrn-D1; aBD, vrn-A1 Vrn-B1 Vrn-D1; Abd, Vrn-A1 vrn-B1 vrn-D1; AbD, Vrn-A1 vrn-B1 Vrn-D1; ABd, Vrn-A1 Vrn-B1 vrn-D1; ABD, Vrn-A1 Vrn-B1 Vrn-D1.
The association between Vrn-1 and phenotypic traits
The vernalization response was associated with differences in heading date (HD), spike length (SL), plant type at the seedling stage (PTS), and freezing tolerance (FT) (Table 5). These traits were differentially expressed according to which allele (or allele combination) was present at the Vrn-1 loci. We found that in the presence of Vrn-A1, the vernalization treatment had a greater effect on SL, FT and PTS than in the presence of Vrn-D1. But there was no significant correlation between the effect of vernalization requirement on HD and the Vrn-A1 or Vrn-B1 loci, and neither was there significant correlation between the effect of vernalization requirement on SL and Vrn-B1 loci. In comparison of the role of three dominant alleles, Vrn-D1 had a significantly lesser (P < 0.1%) effect on PTS and FT than did either Vrn-A1 or Vrn-B1. The effect of Vrn-B1 was also less than that of Vrn-A1, although this difference was not statistically significant (P > 5%). Thus for PTS and FT, the role of Vrn-A1 was the largest, while Vrn-D1 was the smallest. Allelic status at Vrn-1 also appeared to affect the level of resistance to powdery mildew, yellow rust and stem rust (data not shown); this was likely to be a pleiotropic effect, operating through an association between plant development and disease resistance.
| Loci | HD (d) | SL (cm) | PTS | FT | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ±SD | F-value | Mean ±SD | F-value | Mean ±SD | F-value | Mean ±SD | F-value | |
| vrnA | 180.12 ± 2.72 | 10.76 ± 1.82 | 4.21 ± 1.45 | 2.88 ± 0.92 | ||||
| VrnA | 180.83 ± 2.78 | 0.71NS | 12.22 ± 1.33 | 1.46* | 6.45 ± 1.11 | 2.24*** | 3.79 ± 0.70 | 0.91*** |
| vrnB | 180.28 ± 2.71 | 10.83 ± 1.82 | 4.17 ± 1.45 | 2.86 ± 0.92 | ||||
| VrnB | 179.74 ± 2.88 | −0.54NS | 11.55 ± 1.54 | 0.72NS | 6.35 ± 1.06 | 2.18*** | 3.76 ± 0.71 | 0.9*** |
| vrnD | 181.54 ± 2.85 | 10.37 ± 1.50 | 3.96 ± 1.45 | 2.77 ± 0.91 | ||||
| VrnD | 179.16 ± 2.60 | −2.38* | 11.39 ± 1.94 | 1.02* | 4.87 ± 1.45 | 0.91*** | 3.15 ± 0.92 | 0.38*** |
- FT, freezing tolerance; HD, heading date; PTS, plant type at seedling stage; SL, spike length. NS, not significant. *P < 0.05; ***P < 0.001.
Discussion
Novel gene/alleles affecting the vernalization response of wheat
In the present study, the growth habit of 225 accessions (around 41%) was not completely predicted by their allelic status at the Vrn-1 genes, suggesting that there were novel allele and /or genes related to vernalization in wheat. In Arabidopsis, 27 genes have been related to the vernalization response (Levy and Dean 1998). Therefore, it is unreasonable to expect that the only genes affecting the vernalization response of wheat are Vrn-1, Vrn-2 and Vrn-3 (Yan et al. 2006). Kane et al. (2005) reported that TaVRT2, a member of the MADS-box transcription factor family, is known to interact with Vrn-1 and Vrn-2, and is a putative repressor of the floral transition in wheat. It has also been established that VIN3-like plant homeo domain (PHD) finger genes are upregulated by vernalization (Fu et al. 2007). Some as yet uncharacterized genes affecting the vernalization response of wheat have also been assigned to locations on the chromosomes of homoeologous groups 1, 3 and 6 (Miura and Worland 1994; Islam-Faridi et al. 1996; Law et al. 1998). In addition, genetic background interacts with Vrn-1 genotype to affect growth habit (Danyluk et al. 2003). Work on these not yet predicted accessions will discover more genes and alleles responsible for vernalization and will facilitate our understanding on the mechanisms of the vernalization responses in wheat.
The relationship between allelic status at Vrn-1 and growth habit
One-hundred percent of accessions surveyed that carried the dominant Vrn-A1 locus were spring types, whereas only 76.5% of accessions with the dominant Vrn-B1 loci and only 41.6% with the dominant Vrn-D1 loci were spring types. This result suggested that the “spring” allele at Vrn-A1 clearly had a stronger effect than the ones at either Vrn-B1 or Vrn-D1. The reason that Vrn-A1 was favored by the spring wheat may be due to its higher expression level, which has been shown in a set of spring types near isogenic lines (Trevaskis et al. 2003), and is consistent with the role of Vrn-1 as a positive regulator for flowering time (Yan et al. 2003). We discovered the major polymorphism on Vrn-A1 was at their promoter regions, whereas for Vrn-B1 and Vrn-D1 the polymorphism mainly lies in the first intron. The mutation at the Vrn-A1 promoter, especially at the CArG-box, may affect the binding of TaVRT2, a MADS-domain transcription factor that acts as a repressor of Vrn-1 genes (Kane et al. 2007). We therefore conclude that the dominant “spring” allele of Vrn-A1 is a major determinant for the spring growth habit, improved from the previous opinion in which all three alleles were considered to exert similar effect on wheat flowering time control (Pugsley 1971, 1972; Law et al. 1976). Experiments are underway to confirm the effect of sequence mutations on the binding capacity of the major flower suppressor TaVRT2 at the promoter region of the Vrn-1 genes.
The genotype, frequency and distribution of the facultative habit
Of the Chinese accessions tested here 44.8% belonged to facultative type, which were even more than spring type (36.6%) and winter type (18.6%), suggesting that the facultative type was most important among Chinese varieties. We first reported here that there are three Vrn-1 genotypes for facultative wheat, vrn-A1 vrn-B1 Vrn-D1, vrn-A1 Vrn-B1 vrn-D1 and vrn-A1 vrn-B1 vrn-D1. The first two of these are expected to be of spring type, and the last one to be winter type, thus their presence in a facultative type implies that the expression of Vrn-1 is modulated in some way. Of the three genotypes, 61.1% of the facultative wheats were of the genotype vrn-A1 vrn-B1 Vrn-D1, which was mainly distributed in YHW, MLYW, SwW and the south part of NW; 35.1% of them had the genotype vrn-A1 vrn-B1 vrn-D1, which was mainly distributed in the south part of NW and YHW; only 3.8% of the facultative accessions had the genotype vrn-A1 vrn-B1 vrn-D1, and these were distributed in YHW and SwW. Wheat is sown in autumn in all of these regions and harvested in the summer. The temperature in the winter in these regions is low but not so severe that the crop cannot survive.
The association between Vrn-1 and phenotype
The nature of the association between vernalization requirement and frost tolerance is still unclear. Genetic studies have shown that FT in wheat is quantitatively inherited and Fr-1, a major set of frost resistance homoeoloci, has been mapped to chromosomes 5A, 5B and 5D, and is closely linked to Vrn-1 (Galiba et al. 1995; Snape et al. 1997; Iwaki et al. 2002; Toth et al. 2003). In diploid wheat, a second locus Fr-A2 has also been defined in the distal region of the long arm of chromosome 5A (Vagujfalvi et al. 2003). Kobayashi et al. (2004) have suggested that a functional Fr-A1 allele (or another as yet unknown gene) linked to recessive Vrn-A1, rather than the vernalization gene itself, may play a major role in regulating low temperature inducible gene expression in wheat. In the present study, the allelic constitution at the Vrn-1 genes was significantly correlated with seedling FT, since damage was consistently higher for carriers of the spring type alleles. Similarly, Danyluk et al. (2003) showed that the expression of WAP1 was negatively associated with the degree of FT. Others have also suggested that pleiotropic action of the Vrn loci may explain the association between low-temperature tolerance and winter habit (Allen and Fowler 2006). Other members of the flowering pathway may also be involved in the determination of FT. The individual Vrn-1 loci had a differential effect on FT. Thus the negative effect of Vrn-D1 on FT was significantly lower than that of Vrn-A1 or Vrn-B1, while that of Vrn-A1 was higher (although not significantly so) than that of Vrn-B1. Likewise the Vrn-A1/Fr-A1 interval exerted a larger negative effect on cold acclimation and freezing tolerance than did the Vrn-B1/Fr-B1 interval (Kobayashi et al. 2004).
Premature or overly delayed initiation of reproduction has a negative effect on reproductive success. Varieties carrying Vrn-D1 grew slowly and exhibited a stronger tolerance to low temperatures than did those carrying either Vrn-A1 or Vrn-B1. On the other hand, Vrn-D1 carriers grew faster at the seedling stage than those carrying the winter type Vrn-1 alleles. As a result, Vrn-D1 genotypes over-wintered successfully and matured normally in the following year. Wheat varieties grown in subtropical regions commonly carry Vrn-D1, and the presence of this allele has been associated with higher yield in such environments (Stelmakh, 1993). The present data also establishes an association between SL and allelic status at Vrn-1, such that carriers of Vrn-A1 or Vrn-D1 tend to produce longer spikes than do those with Vrn-B1. As a result, the Vrn-D1 genotype (and specifically the combination vrn-A1 vrn-B1 Vrn-D1) is very prevalent in China.
The proportion of spring types among the improved varieties has increased in the past decades
The proportion of spring types among the improved varieties is higher than among the landraces, and at the same time, the proportion of Vrn-A1 and Vrn-B1 carriers has increased. This trend may be driven by global warming. Between the 1950s and the 2000s, the mean temperature during January, the coldest month throughout, increased by >2.1 °C (http://cdc.cma.gov.cn) in China. Higher winter and autumn temperatures favor spring types over winter ones. However, if the spring habit is too strong, the plant will enter the reproductive phase too early, resulting in cold damage during winter or early spring. Such a situation has occurred frequently in China over recent years, for example in 2005, when over 3 M ha of mostly spring wheat suffered cold damage. Thus choice of growth habit has become a particularly critical breeding aim. The salient outcome of the present study is the association between Vrn-1 and phenotype, which has some potential practical breeding applications. For example, appropriate molecular markers linked to Vrn-1 can now be used to accelerate the development of wheat showing wide adaptation and good freezing tolerance.
Materials and Methods
Plant materials
A collection of 551 wheat accessions (summarized in Table 1) was provided by Chinese Academy of Agricultural Sciences (CAAS). Of these, 456 represented materials originating from one of the ten wheat ecological regions in China, and the remaining 95 were introductions from Europe, America and Australia. The Chinese accessions were classified into one group of 190 landraces and another of 266 improved varieties.
Phenotype
Following the criteria of Gardner and Barnett (1990), a two stage trial was carried out: first, all entries were grown at 20 °C under a 16 h photoperiod, an environment where spring types flowered at least one month before winter types, and where entries having a strong vernalization requirement failed to reach flowering by the time the experiment was terminated. Second, all of the vernalization-requiring entries were subjected to a period of 2, 4 and 6 weeks at about 4 °C. Entries exposed to 2–4 weeks vernalization, which flowered significantly earlier than they did in the non-vernalization treatment were classified as facultative types; those which required a 6-week vernalization treatment were classified as “winter” types. Heading date (HD) was defined as the number of days between germination and the emergence of the spike from the boot. Spike length (SL) was measured at maturity. Plant type at the seedling stage (PTS), prior to the vernalization treatment, was scaled from prostrate (1) to erect (5). Freezing tolerance (FT) was assessed in early spring on a scale of 1 (no damage) to 5 (death).
Genotype
Genomic DNA was isolated from young leaves using the cetyl trimethyl ammonium bromide (CTAB) method (Saghai-Maroof et al. 1984). Primers and polymerase chain reaction (PCR) conditions for Vrn-1 were as described elsewhere (Yan et al. 2004a,b; Fu et al. 2005). For Vrn-2, based on the Vrn-A2 sequence of Triticum monococcum, we designed the primer, got Vrn-S2 and Vrn-D2 from Triticum spelta L. and Aegilops tauschii; and then, using the primer amplified the hexaploid wheat and comparing with Vrn-A2, Vrn-S2 and Vrn-D2 from diploid wheat, obtained respectively Vrn-A2, Vrn-B2 and Vrn-D2 sequences of hexaploid wheat. A cleaved amplified polymorphic sequences (CAPS) marker for Vrn-2 was applied, as described by Yan et al. (2004b). PCR products were electrophoresed through 1% or 1.2% agarose gels, and visualized by ethidium bromide staining.
Data analysis
Simpson's Index of Diversity (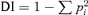 ) was used to calculate gene diversity in each ecological region, where pi is the frequency of the ith allele across all accessions from a given region. Cluster analysis was carried out by using SPSS software, in the following sequence: Classify – Hierarchical cluster – Measure – Interval (Squared Euclidean Distance).
) was used to calculate gene diversity in each ecological region, where pi is the frequency of the ith allele across all accessions from a given region. Cluster analysis was carried out by using SPSS software, in the following sequence: Classify – Hierarchical cluster – Measure – Interval (Squared Euclidean Distance).
(Handling editor: Chun-Ming Liu)
Acknowledgements
We thank Drs Jorge Dubcovsky and Liu-Ling Yan for providing valuable information and advice; Professor John Snape and Mike Gale for revising the manuscript; Drs Zhao Liu and Jian-Fang Chai for helpful discussion; Drs Chen-Yang Hao, Lan-Fen Wang and Lei Zhang for help with data analysis; and Dr Wing Cheung (DNA LandMarkers, Canada) for linguistic revision.




