Additive and Over-dominant Effects Resulting from Epistatic Loci Are the Primary Genetic Basis of Heterosis in Rice
Supported by the State Key Basic Research and Development Plan (973) of China (2007CB109002).
Abstract
A set of 148 F9 recombinant inbred lines (RILs) was developed from the cross of an indica cultivar 93-11 and japonica cultivar DT713, showing strong F1 heterosis. Subsequently, two backcross F1 (BCF1) populations were constructed by backcrossing these 148 RILs to two parents, 93-11 and DT713. These three related populations (281BCF1 lines, 148 RILs) were phenotyped for six yield-related traits in two locations. Significant inbreeding depression was detected in the population of RILS and a high level of heterosis was observed in the two BCF1 populations. A total of 42 main-effect quantitative trait loci (M-QTLs) and 109 epistatic effect QTL pairs (E-QTLs) were detected in the three related populations using the mixed model approach. By comparing the genetic effects of these QTLs detected in the RILs, BCF1 performance and mid-parental heterosis (HMP), we found that, in both BCF1 populations, the QTLs detected could be classified into two predominant types: additive and over-dominant loci, which indicated that the additive and over-dominant effect were more important than complete or partially dominance for M-QTLs and E-QTLs. Further, we found that the E-QTLs detected collectively explained a larger portion of the total phenotypic variation than the M-QTLs in both RILs and BCF1 populations. All of these results suggest that additive and over-dominance resulting from epistatic loci might be the primary genetic basis of heterosis in rice.
Rice is the staple food for more than half of the world's population. Rice production has been further enhanced after the “Green Revolution” due to the successful exploitation of heterosis since the late 1970s (Yuan 1992; Khush 2001). However, study of the genetic basis of heterosis dropped behind exploitation of heterosis long ago. Though many theories, such as dominance (Bruce 1910; Keeble and Pellew 1910; Jones 1917), over-dominance (Shull 1908, East 1936) and epistasis (Stuber 1994; Goodnight 1999), have been proposed to explain the genetic basis of heterosis, its mechanism has not been elucidated entirely yet.
Recent advances in genome research involving a number of molecular-marker techniques and the availability of high-density molecular linkage maps, together with developments in analytical methods (Lander and Botstein 1989; Zeng 1994), facilitated the analysis of the genetic basis of quantitative traits. Many quantitative trait loci (QTLs) mapping studies were conducted recently to gain insight into the genetic basis of heterosis. Stuber et al. (1992) analyzed the genetic factors contributing to heterosis in a hybrid from two elite maize inbred lines and found that heterozygotes of almost all of the QTLs for yield had higher phenotypic values than the respective homozygotes. They suggested that both over-dominance and QTLs detected by single-locus analysis played a significant role in heterosis. By mapping analysis for a triple testcross design with recombinant inbred lines, Frascaroli et al. (2007) obtained similar results. Xiao et al. (1994) conducted an inheritance study of quantitative traits in an intersubspecific cross of rice and revealed that dominance may be the genetic basis of heterosis in rice. However, other studies suggested that epistasis may play an important role in heterosis. Li et al. (1997a,b) tested the F4 progeny from an intersubspecific rice cross using restriction fragment length polymorphism (RFLP) markers, and indicated that hybrid breakdown may be largely due to incompatibilities between alleles at many epistatic loci. Yu et al. (1997) reported over-dominance at several main-effect QTLs and pronounced additive epistasis affecting grain yield and its components in F2:3 population derived from a highly heterotic rice cross. Li et al. (2001) and Luo et al. (2001) investigated the main-effect and epistatic QTL associated with inbreeding depression and heterosis for grain yield and biomass in five related mapping populations. They suggested that epistasis and over-dominance were the major genetic basis of inbreeding depression and heterosis in rice. Hua et al. (2003) studied the genetic basis of heterosis of an elite rice hybrid by using an immortalized F2 population produced by randomly permutated intermating of a set of recombinant inbred lines from a cross between the parents of Shanyou 63, they suggested that heterotic effects at the single-locus level and dominance by dominance interaction at the two-locus level could adequately explain the genetic basis of heterosis in Shanyou 63. Mei et al. (2003, 2005) compared QTLs mapped in recombinant inbred lines (RILs) and their testcross and backcross F1 populations, and indicated that over-dominance resulting from epistatic loci is the primary genetic basis of inbreeding depression and heterosis in rice. Furthermore, Semel et al. (2006) carried out quantitative genetic and phenotypic analysis on a population of tomato (Solanum lycopersicum) introgression lines (ILs), and revealed that over-dominant QTLs are more prevalent for yield and fitness in tomato in the absence of epistasis. Użarowska et al. (2007) compared expression profiling in meristems of inbred-hybrid triplets of maize based on morphological investigations of heterosis for plant height, and revealed that more than 50% of genes showed overdominance.
In the study, we constructed a recombinant inbred (RI) population from the cross between rice cultivars 93-11 and DT713, and developed two backcross F1 (BCF1) populations by backcrossing RILs to both parents. QTL analysis in these three populations was conducted for both trait performance and heterosis to detect main-effect loci and digenic epistasis. Gene actions were explored again for such aspects as relative importance between additive and dominant or over-dominant effects, and between main-effect and epistasis effect.
Results
Construction of linkage map using the RI population
A total of 122 (29.0%) markers showed polymorphism between the parents 93-11 and DT713. The linkage map with 122 markers spanned 1 469.1 cM, and covered 12 rice chromosomes with an average interval of 13.4 cM between two flanking markers (Figure 1). There were 29 (23.8%) simple sequence repeat (SSR) markers showed segregation distortion, largely clustered in terminal regions chromosomes 1, 2, 3 and 12. On average, 93-11 alleles accounted for 49.1%± 8.3% of the genome, ranging from 13.7% to 77.0%.
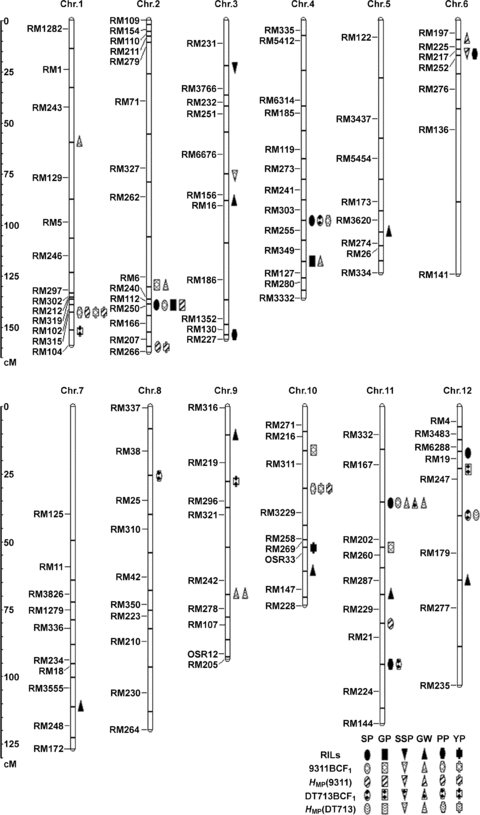
Genomic locations of main-effect quantitative trait loci (QTLs) influencing yield-related traits in 93-11/DT713 RI and two related backcross populations.
Performance of the RILs and mid-parental heterosis of their BCF1s
Table 1 shows the summary of statistics for the yield components of the parents, F1 (93-11×DT713), RILs, two BCF1 populations, as well as the estimated hybrid breakdown (HB) and the HMP of the BCF1s. The performance of the two parents was significantly different for most traits measured. Compared with 93-11, DT713 had significantly greater values for spikelets per panicle (SP), lower values for seed set percent (SSP), 1 000-grain weight (GW), and similar values for panicles per plant (PP) in both environments. However, for filled grains per panicle (GP) and grain yield per plant (YP), 93-11 had greater values in the environment of the Anhui Academy of Agricultural Sciences (AAAS), and lower values in the environment of the China Agricultural University (CAU) Experiment Station compared with DT713. The SP, GP, PP and YP values of the (93-11×DT713) F1s were higher than both parents, but the SSP and GW values were between the parents in both environments.
| Trait values | ||||||
|---|---|---|---|---|---|---|
| SP | GP | SSP | GW | PP | YP | |
| The CAU environment | ||||||
| 93-11 | 162.3 ± 15.6 | 129.2 ± 13.9 | 79.6 ± 3.4 | 24.3 ± 0.5 | 6.8 ± 1.4 | 21.8 ± 3.2 |
| DT713 | 239.0 ± 16.8 | 173.7 ± 14.7 | 72.7 ± 3.6 | 20.1 ± 0.3 | 7.0 ± 1.2 | 22.9 ± 4.6 |
| (93-11×DT713)F1 | 248.6 ± 17.1 | 196.9 ± 16.2 | 79.2 ± 5.6 | 23.9 ± 0.3 | 10.0 ± 1.4 | 46.3 ± 4.3 |
| (93-11×DT713)HMPa | 53.0*** | 45.5*** | 3.1** | 1.7* | 3.1*** | 23.9*** |
| CK (Liangyoupeijiu) | 187.0 ± 17.1 | 128.6 ± 14.5 | 68.6 ± 3.8 | 20.6 ± 0.4 | 10.1 ± 1.5 | 27.7 ± 3.8 |
| RILs: Mean ±SD | 182.7 ± 49.6 | 119.3 ± 44.9 | 65.3 ± 20.5 | 19.5 ± 3.9 | 5.7 ± 1.2 | 14.5 ± 5.3 |
| Range | 90 − 354.7 | 46.9 − 223.7 | 28.2 − 96.7 | 11.9 − 28.1 | 3.4 − 9.8 | 6.1 − 26.9 |
| Skewness | 0.9 | 0.3 | −0.6 | 0.1 | 0.6 | 0.1 |
| Kurtosis | 1.2 | −0.3 | 0.0 | −0.7 | 0.5 | −0.4 |
| HB (RIL-MP)b | −18.0*** | −32.2*** | −10.9*** | −2.7*** | −1.2* | −7.9*** |
| (RIL/93-11)F1: Mean ±SD | 206.6 ± 38.6 | 161.8 ± 34.3 | 78.7 ± 10.9 | 24.3 ± 3.3 | 8.1 ± 2.1 | 33.4 ± 10.5 |
| Range | 138.4 − 328.3 | 74.1 − 279.3 | 49.9 − 96.6 | 14.5 − 32.5 | 2.0 − 16.0 | 5.5 − 85.7 |
| Skewness | 1.1 | 0.6 | −0.6 | −0.6 | 1.0 | 1.0 |
| Kurtosis | 1.3 | 1.1 | −0.3 | 0.2 | 1.6 | 2.3 |
| (RIL/93-11)HMPa | 34.1 ± 31.9 | 37.6 ± 31.9 | 6.3 ± 10.7 | 2.8 ± 2.8 | 1.7 ± 1.7 | 14.3 ± 9.4 |
| −44.4 − 165.5 | −45.0 − 159.8 | −31.1 − 27.2 | −7.4 − 8.6 | −3.2 − 7.6 | −13.2 − 36.0 | |
| (RIL/DT713)F1: Mean ±SD | 236.1 ± 88.1 | 171.7 ± 61.9 | 73.1 ± 25.7 | 21.3 ± 7.6 | 6.9 ± 1.8 | 27.1 ± 10.2 |
| Range | 112.7 − 352.7 | 43.6 − 245.5 | 35.6 − 95.0 | 12.8 − 28.4 | 4.1 − 15.0 | 10.4 − 82.3 |
| Skewness | 0.2 | −0.8 | −1.1 | −0.2 | 1.2 | 1.8 |
| Kurtosis | 1.6 | 2.0 | 2.3 | 0.3 | 2.2 | 2.8 |
| (RIL/DT713)HMPa | 25.3 ± 31.1 | 25.8 ± 29.2 | 4.1 ± 9.8 | 3.3 ± 2.2 | 0.4 ± 1.4 | 7.6 ± 7.2 |
| −95.9 − 128.5 | −59.2 − 133.7 | −20.2 − 34.5 | −3.6 − 9.2 | −1.8 − 6.2 | −4.7 − 40.0 | |
| The AAAS environment | ||||||
| 93-11 | 159.3 ± 10.6 | 133.6 ± 11.3 | 83.9 ± 4.9 | 29.1 ± 0.5 | 8.5 ± 1.3 | 36.4 ± 3.6 |
| DT713 | 204.6 ± 12.6 | 123.2 ± 13.2 | 60.6 ± 6.2 | 21.6 ± 0.4 | 8.2 ± 1.4 | 25.3 ± 2.9 |
| (93-11×DT713)F1 | 263.5 ± 13.1 | 188 ± 12.3 | 79.4 ± 6.5 | 27.5 ± 0.6 | 12.5 ± 1.7 | 64.6 ± 3.4 |
| (93-11×DT713)HMPa | 81.6*** | 59.6*** | 7.2*** | 2.2** | 4.2*** | 33.8*** |
| CK (Liangyoupeijiu) | 164.5 ± 11.7 | 128.5 ± 10.9 | 78.1 ± 5.8 | 26.1 ± 0.6 | 8.8 ± 1.8 | 31.2 ± 2.7 |
| RILs: Mean ±SD | 171.2 ± 41.5 | 115.3 ± 34.0 | 67.3 ± 12.9 | 24.9 ± 3.2 | 7.0 ± 1.5 | 24.2 ± 5.2 |
| Range | 72.8 − 315.3 | 46.6 − 247.4 | 23.3 − 92.1 | 17.5 − 35.6 | 4.3 − 13.8 | 9.2 − 46.7 |
| Skewness | 0.1 | 0.3 | −1.0 | 0.6 | 1.4 | 0.7 |
| Kurtosis | 0.2 | 0.3 | 1.0 | 1.0 | 2.2 | 1.8 |
| HB (RIL-MP)b | −10.8*** | −13.1*** | −5.0*** | −1.5* | −1.4*** | −6.7*** |
| (RIL/93-11)F1: Mean ±SD | 208.9 ± 29.2 | 178.2 ± 27.5 | 85.4 ± 7.4 | 27.7 ± 2.0 | 9.8 ± 2.3 | 49.4 ± 13.4 |
| Range | 131.4 − 275.2 | 86.3 − 242.2 | 57.0 − 96.6 | 22.6 − 32.9 | 5.3 − 18.3 | 24.0 − 85.6 |
| Skewness | 0.1 | −0.5 | −1.0 | −0.1 | 1.0 | 0.5 |
| Kurtosis | −0.2 | 0.7 | 2.1 | −0.1 | 1.7 | 0.1 |
| (RIL/93-11) HMPa | 43.7 ± 22.9 | 53.8 ± 23.8 | 9.8 ± 8.5 | 0.7 ± 1.6 | 2.0 ± 2.2 | 19.3 ± 13.6 |
| −7.1 − 105.6 | −35.5 − 100.8 | −21.9 − 30.7 | −4.7 − 2.8 | −5.7 − 8.3 | −13.6 − 56.6 | |
| The AAAS environment | ||||||
| (RIL/DT713)F1: Mean ±SD | 232.3 ± 35.8 | 165.4 ± 33.3 | 71.7 ± 11.2 | 24.9 ± 1.8 | 9.1 ± 2.5 | 41.3 ± 14.9 |
| Range | 112.2 − 348.2 | 66.3 − 295.5 | 22.6 − 91.9 | 20.2 − 30.3 | 4.0 − 19.0 | 17.0 − 98.6 |
| Skewness | 0.0 | 0.6 | −1.4 | 0.5 | 1.3 | 1.5 |
| Kurtosis | 1.3 | 2.1 | 2.6 | 0.5 | 2.2 | 2.3 |
| (RIL/DT713)HMPa | 44.4 ± 30.5 | 46.2 ± 32.4 | 7.8 ± 10.4 | 1.6 ± 1.5 | 1.6 ± 2.4 | 16.5 ± 15.4 |
| −26.5 − 137.4 | −39.7 − 140.2 | −29.8 − 32.1 | −3.5 − 6.2 | −2.9 − 11.0 | −10.4 − 75.7 | |
- *P < 0.05; **P < 0.01; ***P < 0.001 (based on t-tests). aThe mid-parental heterosis, HMP= F1– MP, where MP were the mid-parental trait values (93-11+DT713)/2 for the 93-11/DT713F1, (RIL +93-11)/2 for 93-11BCF1s, (RIL +DT713)/2 for DT713BCF1s, respectively. bHB = RIL–MP, where HB is hybrid breakdown and MP = (93-11+DT713)/2. AAAS, Anhui Academy of Agricultural Sciences; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
The mean values of RILs were obviously lower than the mid-parental values, and significantly HMP was observed in 93-11×DT713 F1s for all traits in both environments.
Heterosis average levels of the BCF1s showed similar trends for all traits in both environments (Table 1), with significantly positive HMP, but were lower than the HMP of the 93-11×DT713 F1 except SSP and GW.
Wide ranges of variations were observed for each trait in the RILs, BCF1s and HMP (data not shown). The RIL population showed transgressive distribution (Table 1). Extreme individuals in the RIL and both BCF1 populations showed trait values exceeding those of the F1 between two parents. Consequently, many lines showed higher HMP in the BCF1s than the 93-11× DT713 F1 (Table 1).
Relationships between the mean trait values of RILs, HMP, and BCF1 performance
Table 2 shows the correlation coefficients between the mean values of BCF1s, their mid-parent heterosis, and the mean values of their material RILs for the yield components. In 93-11BCF1s and DT713BCF1s, high positive correlations between HMP and F1 performance were found for most traits in both environments (Table 2). The average R2 (determination coefficient) was 0.645 in 93-11BCF1s and 0.650 in DT713BCF1s. There was a general positive but lower correlation between the trait value of the RILs and that of their BCF1s in the CAU environment, the average R2 was 0.164 in 93-11BCF1s and 0.180 in DT713BCF1s. In the AAAS environment, this was not the case for YP, the average R2 was 0.209 in 93-11BCF1s and 0.171 in DT713BCF1s. There were negative correlations between trait values of RILs and HMP except PP in both environments. In the CAU environment, the average R2 value was of 0.071 in 93-11BCF1s and 0.145 in DT713BCF1s, while in the AAAS environment, for the same populations, the values were of 0.075 and 0.046, respectively.
| Traits | Between RILs and F1 | Between HMP and F1 | Between RILs and HMP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9311BCF1s | DT713BCF1s | 9311BCF1s | DT713BCF1s | 9311BCF1s | DT713BCF1s | |||||||
| r | R 2 | r | R 2 | r | R 2 | r | R 2 | r | R 2 | r | R 2 | |
| The CAU environment | ||||||||||||
| SP | 0.518 | 0.268 | 0.544 | 0.296 | 0.742 | 0.551 | 0.722 | 0.521 | −0.190 | 0.036 | −0.189 | 0.036 |
| GP | 0.387 | 0.150 | 0.293 | 0.086 | 0.786 | 0.618 | 0.672 | 0.452 | −0.266 | 0.071 | −0.514 | 0.264 |
| SSP | 0.356 | 0.127 | 0.404 | 0.163 | 0.586 | 0.343 | 0.579 | 0.335 | −0.549 | 0.301 | −0.697 | 0.486 |
| GW | 0.544 | 0.296 | 0.533 | 0.284 | 0.788 | 0.621 | 0.656 | 0.430 | −0.088 | 0.008 | −0.287 | 0.082 |
| PP | 0.354 | 0.125 | 0.405 | 0.164 | 0.957 | 0.916 | 0.925 | 0.856 | 0.068 | 0.005 | 0.024 | 0.001 |
| YP | 0.138 | 0.019 | 0.296 | 0.088 | 0.976 | 0.953 | 0.947 | 0.897 | −0.080 | 0.006 | −0.029 | 0.001 |
| Mean | 0.383 | 0.164 | 0.413 | 0.180 | 0.806 | 0.667 | 0.750 | 0.582 | −0.184 | 0.071 | −0.282 | 0.145 |
| The AAAS environment | ||||||||||||
| SP | 0.632 | 0.399 | 0.529 | 0.280 | 0.685 | 0.469 | 0.818 | 0.669 | −0.132 | 0.017 | −0.055 | 0.003 |
| GP | 0.519 | 0.269 | 0.318 | 0.101 | 0.769 | 0.591 | 0.858 | 0.736 | −0.147 | 0.022 | −0.214 | 0.046 |
| SSP | 0.225 | 0.051 | 0.405 | 0.164 | 0.702 | 0.493 | 0.822 | 0.676 | −0.536 | 0.287 | −0.188 | 0.035 |
| GW | 0.617 | 0.381 | 0.646 | 0.417 | 0.569 | 0.324 | 0.586 | 0.343 | −0.297 | 0.088 | −0.332 | 0.110 |
| PP | 0.392 | 0.154 | 0.230 | 0.053 | 0.943 | 0.889 | 0.956 | 0.914 | 0.064 | 0.004 | −0.066 | 0.004 |
| YP | −0.013 | 0.000 | −0.103 | 0.011 | 0.985 | 0.970 | 0.984 | 0.968 | −0.184 | 0.034 | −0.277 | 0.077 |
| Mean | 0.395 | 0.209 | 0.338 | 0.171 | 0.776 | 0.623 | 0.837 | 0.718 | −0.205 | 0.075 | −0.189 | 0.046 |
- AAAS, Anhui Academy of Agricultural Sciences; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
Main-effect QTLs detected in the RILs, BCF1 performance and HMP of BCF1s
A total of 42 main-effect QTLs (M-QTLs) were identified for trait performance of the RILs or BCF1s and mid-parental heterosis in the two environments (Table 3), and only seven of them were detected in both environments, and the remaining 35 M-QTLs were detected only in one environment. These M-QTLs were mapped to all of the rice chromosomes (Figure 1).
| Trait | QTL | Chr. | Marker interval | RILs | BCF1(93-11) | H MP(93-11) | BCF1(DT713) | H MP(DT713) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | a | R 2 | LOD | a+d | R 2 | LOD | d | R 2 | LOD | a+d | R 2 | LOD | d | R 2 | ||||
| SP | QSp2 | 2 | RM112 −RM250 | 2.47 | −8.2 | 4.0 | 4.09 | −27.7 | 16.8 | |||||||||
| QSp4 | 4 | RM303 −RM255 | 4.74 | 14.8 | 13.5 | |||||||||||||
| QSp11 | 11 | RM167 −RM202 | 4.98 | 14.3 | 12.6 | 2.92 | −25.0 | 12.6 | ||||||||||
| QSp12 | 12 | RM247 −RM179 | 3.26 | 31.5 | 15.3 | 4.00 | 23.0 | 14.2 | ||||||||||
| QSp2a′ | 2 | RM112 −RM250 | 3.07 | −10.4 | 7.1 | 3.89 | −21.3 | 14.9 | ||||||||||
| QSp2b′ | 2 | RM207 −RM266 | 2.19 | 15.0 | 11.3 | |||||||||||||
| QSp4′ | 4 | RM303 −RM255 | 5.65 | 15.8 | 16.5 | 4.05 | −27.7 | 14.6 | ||||||||||
| QSp11′ | 11 | RM229 −RM21 | 2.21 | 15.6 | 13.9 | |||||||||||||
| QSp12′ | 12 | RM6288 −RM19 | 2.39 | −11.3 | 8.4 | |||||||||||||
| GP | QGp2 | 2 | RM6 −RM240 | 4.75 | −28.8 | 13.1 | ||||||||||||
| QGp4 | 4 | RM349 −RM127 | 6.41 | 14.1 | 13.2 | |||||||||||||
| QGp11 | 11 | RM202 −RM260 | 2.14 | −18.8 | 13.2 | |||||||||||||
| QGp12 | 12 | RM19 −RM247 | 2.50 | 21.9 | 13.9 | |||||||||||||
| QGp2′ | 2 | RM112 −RM250 | 7.29 | −12.1 | 12.6 | 3.09 | 21.7 | 16.7 | ||||||||||
| QGp10′ | 10 | RM216 −RM311 | 3.05 | 22.8 | 11.6 | |||||||||||||
| SSP | QSsp3a | 3 | RM231 −RM3766 | 3.72 | 7.2 | 14.7 | ||||||||||||
| QSsp3b | 3 | RM6676 −RM156 | 2.23 | 7.7 | 14.6 | |||||||||||||
| QSsp6′ | 6 | RM217 −RM252 | 2.25 | 7.0 | 10.8 | |||||||||||||
| GW | QGw5 | 5 | RM3620 −RM274 | 6.74 | 1.4 | 11.1 | ||||||||||||
| QGw7 | 7 | RM3555 −RM248 | 3.80 | −1.1 | 8.7 | |||||||||||||
| QGw9a | 9 | RM316 −RM219 | 2.56 | −0.8 | 5.3 | |||||||||||||
| QGw9b | 9 | RM242 −RM278 | 4.38 | 2.5 | 11.1 | 2.32 | 1.5 | 11.1 | ||||||||||
| QGw10 | 10 | OSR33 −RM147 | 3.83 | −1.2 | 12.7 | |||||||||||||
| QGw11 | 11 | RM167 −RM202 | 2.84 | −2.1 | 12.6 | 3.55 | 1.7 | 12.6 | 2.95 | 0.8 | 13.6 | |||||||
| QGw12 | 12 | RM179 −RM277 | 3.16 | −0.9 | 6.0 | |||||||||||||
| QGw1′ | 1 | RM243 −RM129 | 2.63 | −1.4 | 11.6 | |||||||||||||
| QGw2′ | 2 | RM6 −RM240 | 2.21 | 0.9 | 9.7 | |||||||||||||
| QGw3′ | 3 | RM156 −RM16 | 4.63 | −1.0 | 9.2 | |||||||||||||
| QGw4′ | 4 | RM349 −RM127 | 2.42 | −1.3 | 9.1 | |||||||||||||
| QGw5′ | 5 | RM3620 −RM274 | 5.86 | 1.4 | 13.2 | |||||||||||||
| QGw6′ | 6 | RM197 −RM225 | 3.68 | 1.3 | 13.8 | |||||||||||||
| QGw10′ | 10 | OSR33 −RM147 | 4.69 | −1.1 | 11.0 | |||||||||||||
| QGw11a′ | 11 | RM167 −RM202 | 2.90 | 1.3 | 9.6 | 3.67 | 0.6 | 8.2 | ||||||||||
| QGw11b′ | 11 | RM287 −RM229 | 5.13 | −1.1 | 8.6 | |||||||||||||
| PP | QPp3 | 3 | RM130 −RM227 | 2.81 | 0.4 | 8.8 | ||||||||||||
| QPp4 | 4 | RM303 −RM255 | 2.90 | −1.4 | 9.1 | |||||||||||||
| QPp6 | 6 | RM217 −RM252 | 2.90 | −0.3 | 6.8 | |||||||||||||
| QPp10 | 10 | RM311 −RM3229 | 4.01 | 1.5 | 14.2 | |||||||||||||
| QPp11 | 11 | RM21 −RM224 | 5.24 | 0.5 | 15.2 | |||||||||||||
| QPp1′ | 1 | RM102 −RM315 | 2.83 | 1.7 | 8.7 | 3.97 | 1.9 | 16.1 | ||||||||||
| QPp6′ | 6 | RM217 −RM252 | 3.83 | −0.6 | 13.4 | |||||||||||||
| QPp8′ | 8 | RM38 −RM25 | 2.93 | 1.6 | 14.2 | |||||||||||||
| QPp11′ | 11 | RM21 −RM224 | 4.25 | 0.4 | 13.2 | 3.12 | 1.4 | 10.1 | ||||||||||
| YP | QYp1 | 1 | RM315 −RM104 | 2.94 | 4.7 | 13.8 | ||||||||||||
| QYp9 | 9 | RM219 −RM296 | 4.19 | 3.7 | 10.9 | |||||||||||||
| QYp10 | 10 | RM311 −RM3229 | 2.94 | 3.8 | 14.6 | 2.38 | 3.2 | 11.3 | ||||||||||
| QYp1′ | 1 | RM102 −RM315 | 3.27 | 5.9 | 13.4 | 2.98 | 5.3 | 12.9 | ||||||||||
| QYp2′ | 2 | RM207 −RM266 | 2.00 | 4.3 | 9.1 | |||||||||||||
| QYp10′ | 10 | RM269 −OSR33 | 5.23 | −1.8 | 14.6 | |||||||||||||
- a H MP is the mid-parental heterosis of the BCF1s calculated from HMP= BCF1– MP, where MP = (female RIL +93-11 or DT713)/2. In the RILs, QTL effects were associated with the DT713 allele (due to replacement of the 93-11 allele by the DT713 allele). In the BC populations, QTL effects for F1 and HMP were estimated by the heterozygotes – the homozygotes. The genetic expectation of a QTL effect obtained is the additive gene effect (a) when estimated from the RILs, the additive and dominance effects (a+d) from the F1 mean values, and the dominance effect (d) from the HMP values. ′ QTL was detectable in the AAAS environment. AAAS, Anhui Academy of Agricultural Sciences; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
For spikelets per panicle, seven M-QTLs were detected in the two environments, explaining 41.6%, 53.7% (25.2% for HMP) and 44.1% (14.2% for HMP) of the total variances in the RILs, 93-11BCF1s and DT713BCF1s, respectively. Interestingly, two additive M-QTLs (QSp2 and QSp4b) that showed opposite effects were consistently detected in two environments, QSp2 (or QSp2a′) decreased SP by 8.1–10.4 grains in RILs, and by 21.3–27.7 grains in 93-11BCF1s. Contrarily, QSp4b (or QSp4’) increased SP by 14.8–15.8 grains in RILs. A locus, QSp12, detected between DT713BCF1 performance and DT713HMP, showed over-dominant effect of increasing 23.0 grains. QSp2b′ and QSp11′ also appeared to be over-dominant loci for they were only detectable in HMP but not in F1s and RILs. The two loci had an effect causing increased SP by 15.0 and 15.6 grains in 93-11BCF1s, respectively. The remaining two M-QTLs were all additive loci only detected in RILs or/and F1 performance. QSp11 had a positive effect of 14.3 grains in RILs, while it had a negative effect of 25.0 grains in 93-11BCF1s. QSp12′ had positive effect of 11.4–31.5 grains in RILs.
For filled grains per panicle, a total of six M-QTLs were detected in two environments, explaining 25.8%, 54.6% (16.7% for HMP) and 13.9% of the total variances in the RILs, 93-11BCF1s and DT713BCF1s, respectively. Of six QTLs, five QTLs appeared to be additive as they were detectable only in RILs or BCF1 performance: QGp4, QGp12 and QGp10′ increased GP by 14.1, 21.9 and 22.8 grains, respectively, while QGp2 and QGp11 decreased GP by 28.8 and 18.8 grains, respectively. The remaining one M-QTL (QGp2′) appeared to be an over-dominant locus, this locus decreased GP by12.1 grains in the RILs, while 20.1 grains of positive effects was detected for HMP in 93-11BCF1s.
For seed setting percent, only three M-QTLs were detected in two environments, explaining 14.7% and 25.4% (10.8% for HMP) of the total variances in the RILs and 93-11BCF1s, respectively. QSsp3a and QSsp3b appeared to be additive loci, with a significant positive effect detected in RILs and 93-11BCF1s, respectively. An additional over-dominant QTL was located on chromosome 6 (QSsp6′) that had an effect increasing SSP for 7.0% in 93-11BCF1s. No M-QTL was identified in DT713BCF1s.
For 1 000-grain weight, a total of 13 M-QTLs were detected in the two environments, explaining 61.3%, 58.2% (13.8% for HMP) and 42.8% (31.7% for HMP) of the total variances in the RILs, 93-11BCF1s and DT713BCF1s, respectively. Of 13 QTLs, only three (QGw5, QGw10 and QGw11) were detected in both environments. QGw5 (or QGw5′) showed a positive additive effect of average 1.4 g in RILs, while QGw10 (or QGw10′) showed a negative additive effect of average 1.2 g in RILs. QGw11 (or QGw11a) was detected in both BCF1s and appeared to be dominant with 2d < a+d, the locus had a positive effect of 1.5 g and 0.7 g on GW in DT713BCF1 performance and DT713HMP, respectively, but had a negative effect of 2.1 g in 93-11BCF1 performance. Furthermore, three loci (QGw9b, QGw2′ and QGw6′) appeared to be over-dominance. QGw9b was identified in both 93-11BCF1 performance and DT713HMP and showed positive effect. QGw2′ and QGw6′ were detected in DT713HMP and 93-11HMP, respectively, and also showed positive effect. The remaining seven QTLs appeared to have an additive effect and were only detected in RILs or BCF1s.
For panicles per plant, seven M-QTLs were detected in the two environments, explaining 33.1%, 48.1% (16.1% for HMP) and 24.3% of the total variances in the RILs, 93-11BCF1s and DT713BCF1s, respectively. Six loci (QPp3, QPp4, QPp6, QPp10, QPp11 and QPp8′) appeared to be additive loci, with a significant effect detected in RILs or BCF1 performance. Of the six loci, two loci, QPp6 (or QPp6′) and QPp11 (or QPp11′), were identified in both environments, and QPp6 (or QPp6′) showed a significant negative effect, while QPp11 (or QPp11′) showed a significant positive effect. The remaining one locus, QPp1′, acted as over-dominant QTLs for increasing PP by 1.9 panicles in the 93-11BCF1 performance and by 1.7 panicles in the 93-11HMP.
For grain yield per plant, six M-QTLs were detected in both environments, explaining 14.6%, 61.3% (33.3% for HMP) and 24.7% of the total variances in the RILs, 93-11BCF1s and DT713BCF1s, respectively. Of the six QTLs, three, QYp1, QYp9 and QYp10′, acted as additive QTLs that were detected only in RILs or BCF1 performance: the former two (QYp1 and QYp) increased YP by 4.7 g and 3.7 g in DT713BCF1s, respectively, while the third (QYp10′) decreased by 1.8 g in RILs. Furthermore, QYp10 and QYp1′ were detected in both 93-11BCF1 performance and 93-11HMP and appeared to have over-dominance with 2d > a+d. The two loci had significant positive effects of 3.1 g and 5.9 g for increasing YP in 93-11BCF1 performance, and 3.2 g and 5.3 g in 93-11HMP, respectively. The remaining QYp2′ was identified only in 93-11HMP and showed an over-dominant effect of 4.3 g for increasing YP.
Epistatic QTLs detected in the RILs and BC populations
A total of 41 epistatic QTL (E-QTL) pairs were identified in the RILs in the two environments and only one pair were identified in both environments (Table 4). These epistatic QTLs included eight pairs accounting for 52.1% of the total variation in SP, eight pairs explaining 62.1% of the total variation in GP, eight pairs explaining 68.4% of the total variation in SSP, five pairs explaining 34.2% of the total variation in GW, four pairs explaining 28.6% of the total variation in PP and eight pairs explaining 74.2% of the total variation in PP. Of these E-QTLs, only one pair was detected in both intervals having significant additive effects, and 14 pairs were detected between an interval having significant additive effect and other loci. The remaining interactions occurred between two complementary loci.
| Trait | Environment | Chr. | Marker intervala | Chr. | Marker intervala | LOD | Ai b | Aj b | AAij b | R 2 c |
|---|---|---|---|---|---|---|---|---|---|---|
| SP | CAU | 1 | RM319 −RM102 | 2 | RM112 −RM250 | 6.39 | −9.5** | −13.7 | 5.5 | |
| CAU | 2 | RM109 −RM154 | 9 | RM296 −RM321 | 3.67 | 11.4 | 3.8 | |||
| CAU | 2 | RM110 −RM211 | 10 | RM147 −RM228 | 3.25 | 13.2 | 5.2 | |||
| CAU | 4 | RM303 −RM255 | 10 | RM269 −OSR33 | 6.38 | 13.4*** | 10.0 | 3.0 | ||
| CAU | 9 | RM296 −RM321 | 12 | RM277 −RM235 | 5.36 | 22.3 | 14.7 | |||
| CAU | 10 | RM258 −RM269 | 12 | RM4 −RM3483 | 3.53 | −16.9 | 8.5 | |||
| AAAS | 2 | RM109 −RM154 | 12 | RM6288 −RM19 | 3.56 | −10.1** | 6.6 | 3.0 | ||
| AAAS | 6 | RM276 −RM136 | 11 | RM167 −RM202 | 3.24 | −10.9 | 8.4 | |||
| GP | CAU | 1 | RM1282 −RM1 | 3 | RM6676 −RM156 | 6.30 | −19.9 | 14.6 | ||
| CAU | 2 | RM279 −RM71 | 11 | RM167 −RM202 | 7.91 | 16.6 | 10.2 | |||
| CAU | 4 | RM119 −RM273 | 9 | RM219 −RM296 | 3.90 | 13.8 | 7.0 | |||
| CAU | 6 | RM136 −RM141 | 9 | RM296 −RM321 | 4.68 | −5.6* | −13.9 | 7.1 | ||
| CAU | 9 | RM321 −RM242 | 9 | OSR12 −RM205 | 3.69 | 13.3 | 6.5 | |||
| AAAS | 1 | RM212 −RM319 | 8 | RM230 −RM264 | 5.50 | −10.2 | 6.7 | |||
| AAAS | 2 | RM112 −RM250 | 11 | RM287 −RM229 | 8.54 | −11.2*** | −9.1 | 5.3 | ||
| AAAS | 6 | RM276 −RM136 | 8 | RM350 −RM223 | 5.23 | 4.0* | 8.5 | 4.7 | ||
| SSP | CAU | 1 | RM315 −RM104 | 10 | RM258 −RM269 | 3.61 | 7.6 | 10.6 | ||
| CAU | 3 | RM231 −RM3766 | 7 | RM125 −rm11 | 5.19 | 8.8*** | 7.0 | 9.1 | ||
| CAU | 4 | RM119 −RM273 | 9 | RM316 −RM219 | 5.18 | 7.4 | 7.1 | |||
| CAU | 7 | rm11 −RM3826 | 8 | RM310 −RM42 | 4.71 | −6.3 | 7.4 | |||
| AAAS | 1 | RM319 −RM102 | 8 | RM42 −RM350 | 3.59 | 3.8 | 7.3 | |||
| AAAS | 2 | RM207 −RM266 | 6 | RM252 −RM276 | 3.70 | −4.5 | 7.0 | |||
| AAAS | 3 | RM231 −RM3766 | 3 | RM6676 −RM156 | 5.69 | 6.4 | 10.7 | |||
| AAAS | 6 | RM217 −RM252 | 8 | RM42 −RM350 | 3.37 | −4.3 | 9.2 | |||
| GW | CAU | 1 | RM315 −RM104 | 7 | RM3555 −RM248 | 5.84 | −1.3 | 9.5 | ||
| CAU | 4 | RM119 −RM273 | 11 | RM167 −RM202 | 4.79 | 1.3 | 9.0 | |||
| CAU | 7 | RM3555 −RM248 | 8 | RM42 −RM350 | 4.93 | −1.2 | 7.6 | |||
| AAAS | 1 | RM315 −RM104 | 7 | RM3555 −RM248 | 7.08 | −0.5 | 7.0 | |||
| AAAS | 2 | RM327 −RM262 | 3 | RM156 −RM16 | 5.51 | −1.0*** | −0.4 | 5.3 | ||
| AAAS | 5 | RM122 −RM3437 | 6 | RM136 −RM141 | 6.93 | 0.6* | 0.5 | 4.1 | ||
| PP | CAU | 2 | RM110 −RM211 | 11 | RM167 −RM202 | 5.07 | −0.2* | −0.3 | 6.9 | |
| CAU | 4 | RM241 −RM303 | 11 | RM21 −RM224 | 6.55 | 0.4*** | −0.2 | 5.0 | ||
| AAAS | 1 | RM315 −RM104 | 12 | RM6288 −RM19 | 4.06 | 0.6 | 7.8 | |||
| AAAS | 6 | RM217 −RM252 | 12 | RM179 −RM277 | 5.08 | −0.7*** | −0.3 | 8.6 | ||
| YP | CAU | 2 | RM207 −RM266 | 7 | RM3555 −RM248 | 3.36 | 1.2 | 5.6 | ||
| CAU | 3 | RM3766 −RM232 | 3 | RM6676 −RM156 | 4.99 | 1.9 | 13.3 | |||
| CAU | 6 | RM217 −RM252 | 9 | RM107 −OSR12 | 3.51 | −1.7 | 10.4 | |||
| CAU | 9 | RM316 −RM219 | 12 | RM19 −RM247 | 3.40 | 1.4 | 7.0 | |||
| AAAS | 1 | RM1282 −RM1 | 7 | RM336 −RM234 | 4.34 | −0.9* | 1.7 | 9.4 | ||
| AAAS | 1 | RM243 −RM129 | 9 | RM107 −OSR12 | 4.52 | 0.7* | −1.6 | 7.9 | ||
| AAAS | 7 | RM125 −rm11 | 10 | RM147 −RM228 | 3.54 | 1.7 | 9.2 | |||
| AAAS | 10 | RM269 −OSR33 | 11 | RM287 −RM229 | 8.29 | −1.8*** | 0.7* | −1.9 | 11.4 |
- *P < 0.05; **P < 0.001; ***P < 0.0001. aMarkers indicated in bold are those flanking M-QTLs identified in Table 3. bAi and Aj are the main effects of the loci i and j, arising from by the substitution of the 93-11 allele by the DT713 allele. AAij is the epistatic effect between loci i and j, as defined by Mather and Jinks (1982), which were all significant at P < 0.001. cR2 is the proportion of the total phenotypic variation explained by the AAij. AAAS, Anhui Academy of Agricultural Sciences; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
A total of 38 E-QTL pairs were detected in 93-11BCF1s in the two environments and only one pair was detected in both environments (Table 5). There were six QTL pairs detected for SP, totally explaining 51.6% and 19.4% of the total variation in F1 performance and HMP, respectively; six QTL pairs for GP, explaining 32.6% and 19.2% of the total variation for F1 performance and HMP, respectively; seven QTL pairs for SSP, explaining 40.5% and 50.3% of the total variation for F1 performance and HMP, respectively; six QTL pairs for GW, explaining 41.8% and 32.3% of the total variation for F1 performance and HMP, respectively; six QTL pairs for PP, explaining 39.4% and 32.3% of the total variation for F1 performance and HMP, respectively; and seven QTL pairs for YP, explaining 34.6% and 40.9% of the total variation for F1 performance and HMP, respectively. Of these E-QTLs, 10 pairs were detected in both intervals having significant additive effects, and 23 pairs were detected between an interval having significant additive effect and other loci. The remaining five interactions occurred between two complementary loci.
| Trait | Environment | Chr. | Marker intervala | Chr. | Marker intervala | BCF1(93-11) | BCHMP(93-11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | Ai b | Aj b | AAij b | R 2 c | LOD | Ai b | Aj b | AAij b | R 2 c | ||||||
| sp | CAU | 2 | RM240 −RM112 | 9 | RM242 −RM278 | 3.84 | −20.2* | 26.1 | 18.3 | ||||||
| CAU | 9 | RM219 −RM296 | 11 | RM202 −RM260 | 3.55 | −16.1** | −24.9 | 8.6 | |||||||
| AAAS | 1 | RM1 −RM243 | 3 | RM156 −RM16 | 3.16 | 12.5* | 18.9 | 12.1 | |||||||
| AAAS | 2 | RM279 −RM71 | 4 | RM349 −RM127 | 3.79 | 15.1* | 9.7* | 17.5 | 9.2 | ||||||
| AAAS | 9 | RM296 −RM321 | 11 | RM229 −RM21 | 3.11 | 11.8* | −19.2 | 10.2 | |||||||
| AAAS | 11 | RM224 −RM144 | 12 | RM6288 −RM19 | 2.83 | −11.0* | 19.3 | 12.6 | |||||||
| gp | CAU | 2 | RM6 −RM240 | 10 | RM269 −OSR33 | 7.14 | −19.3*** | 15.1* | 12.6 | 6.6 | |||||
| CAU | 6 | RM276 −RM136 | 10 | RM269 −OSR33 | 4.32 | 11.9* | 14.6* | −13.8 | 8.5 | ||||||
| AAAS | 1 | RM212 −RM319 | 4 | RM255 −RM349 | 3.69 | 11.0* | 13.0* | 2.0 | 12.3 | ||||||
| AAAS | 2 | RM166 −RM207 | 10 | OSR33 −RM147 | 4.17 | 12.3* | 12.4* | 8.1 | 6.9 | ||||||
| AAAS | 3 | RM6676 −RM156 | 7 | RM125 −RM11 | 4.45 | 16.5* | 24.6 | 17.5 | |||||||
| AAAS | 9 | RM296 −RM321 | 10 | RM216 −RM311 | 3.38 | 12.7** | 13.1 | 12.4 | 4.04 | 13.2* | 13.4* | 2.4 | 15.2 | ||
| ssp | CAU | 2 | RM109 −RM154 | 3 | RM6676 −RM156 | 2.93 | 8.2** | 10.2 | 6.3 | ||||||
| CAU | 4 | RM119 −RM273 | 12 | RM277 −RM235 | 5.18 | −5.8* | −12.7 | 16.9 | |||||||
| CAU | 4 | RM349 −RM127 | 7 | RM11 −RM3826 | 4.05 | 10.3 | 9.7 | ||||||||
| AAAS | 3 | RM231 −RM3766 | 4 | RM127 −RM280 | 3.97 | 8.3 | 8.5 | ||||||||
| AAAS | 3 | RM6676 −RM156 | 6 | RM217 −RM252 | 3.27 | 5.2* | 10.3 | 15.9 | |||||||
| AAAS | 3 | RM130 −RM227 | 12 | RM4 −RM3483 | 3.18 | −11.0 | 11.3 | ||||||||
| AAAS | 5 | RM26 −RM334 | 10 | RM147 −RM228 | 4.53 | 5.7* | 6.2* | 16.9 | 12.1 | ||||||
| gw | CAU | 1 | RM212 −RM319 | 9 | RM107 −OSR12 | 4.58 | 1.6*** | 0.6 | 8.5 | ||||||
| CAU | 3 | RM6676 −RM156 | 11 | RM202 −RM260 | 3.08 | 1.9* | 3.7 | 20.4 | |||||||
| CAU | 7 | RM3555 −RM248 | 11 | RM167 −RM202 | 5.66 | −1.3* | −1.1** | −2.8 | 7.9 | ||||||
| AAAS | 1 | RM302 −RM212 | 6 | RM252 −RM276 | 3.60 | −0.9* | −3.0 | 12.2 | |||||||
| AAAS | 3 | RM231 −RM3766 | 3 | RM130 −RM227 | 5.28 | −0.8* | −1.4** | −3.0 | 13.2 | ||||||
| AAAS | 3 | RM6676 −RM156 | 11 | RM202 −RM260 | 3.02 | 0.7* | 1.6 | 10.2 | |||||||
| AAAS | 7 | RM3826 −RM1279 | 9 | OSR12 −RM205 | 3.53 | 0.6* | 1.5 | 9.9 | |||||||
| pp | CAU | 2 | RM327 −RM262 | 10 | RM271 −RM216 | 3.97 | 0.8* | 1.7 | 12.5 | ||||||
| CAU | 3 | RM1352 −RM130 | 9 | RM107 −OSR12 | 4.17 | −0.8* | −2.9 | 17.2 | |||||||
| CAU | 4 | RM255 −RM349 | 5 | RM3437 −RM5454 | 2.99 | −0.8* | −0.7* | −2.0 | 8.6 | ||||||
| AAAS | 2 | RM71 −RM327 | 7 | RM3555 −RM248 | 3.39 | −1.0* | −3.0 | 13.6 | |||||||
| AAAS | 5 | RM122 −RM3437 | 11 | RM224 −RM144 | 3.01 | 2.5 | 5.6 | ||||||||
| AAAS | 7 | RM1279 −RM336 | 9 | RM321 −RM242 | 4.34 | −1.2* | −3.4 | 14.2 | |||||||
| yp | CAU | 2 | RM262 −RM6 | 10 | RM311 −RM3229 | 4.09 | 3.0** | 4.1 | 9.5 | ||||||
| CAU | 2 | RM250 −RM166 | 10 | RM3229 −RM258 | 3.84 | 6.5 | 16.2 | ||||||||
| CAU | 5 | RM122 −RM3437 | 11 | RM224 −RM144 | 3.82 | 4.3 | 8.8 | ||||||||
| CAU | 9 | RM321 −RM242 | 10 | RM311 −RM3229 | 2.87 | −4.1 | 8.5 | ||||||||
| AAAS | 1 | RM129 −RM5 | 2 | RM262 −RM6 | 3.60 | 3.8* | 5.7 | 16.3 | |||||||
| AAAS | 1 | RM102 −RM315 | 4 | RM127 −RM280 | 3.05 | 3.6* | −3.8 | 5.9 | |||||||
| AAAS | 2 | RM262 −RM6 | 2 | RM207 −RM266 | 3.17 | 3.1* | −4.9 | 10.3 | |||||||
- *P < 0.05; **P < 0.001; ***P < 0.0001. aMarkers indicated in bold are those flanking M-QTLs identified in Table 3. bAi and Aj are the main effects of the loci i and j, were estimated by the heterozygotes (93-11/DT713) – the homozygotes (93-11/93-11) using the mean values of F1 and HMP. AAij is the epistatic effect between loci i and j, as defined by Mather and Jinks (1982), which were all significant at P < 0.001. cR2 is the proportion of the total phenotypic variation explained by the AAij. AAAS, Anhui Academy of Agricultural Sciences; BCF1, backcross F1; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
A total of 30 E-QTL pairs were detected in DT713BCF1s in both environments (Table 6). There were six QTL pairs detected for SP, explaining 51.0% and 31.7% of the total variation for F1 performance and HMP, respectively; four QTL pairs for GP, explaining 43.2% and 16.2% of the total variation for F1 performance and HMP, respectively; seven QTL pairs for SSP, explaining 44.2% and 21.8% of the total variation for F1 performance and HMP, respectively; six QTL pairs for GW, explaining 52.1% and 22.5% of the total variation for F1 performance and HMP, respectively; four QTL pairs for PP, explaining 21.3% and 18.1% of the total variation for F1 performance and HMP, respectively; and three QTL pairs for YP, explaining 28.0% and 17.4% of the total variation for F1 performance and HMP, respectively. Of these E-QTLs, six pairs were detected in both intervals having significant additive effects, and 15 pairs were detected between an interval having significant additive effect and other loci. The remaining nine interactions occurred between two complementary loci.
| Trait | Environment | Chr. | Marker intervala | Chr. | Marker intervala | BCF1(DT713) | BCHMP(DT713) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | Ai b | Aj b | AAij b | R 2 c | LOD | Ai b | Aj b | AAij b | R 2 c | ||||||
| SP | CAU | 2 | RM109 −RM154 | 2 | RM250 −RM166 | 3.66 | −20.8 | 15.2 | |||||||
| CAU | 4 | RM303 −RM255 | 11 | RM167 −RM202 | 3.07 | −9.9* | −17.3 | 9.6 | |||||||
| AAAS | 1 | RM5 −RM246 | 12 | RM4 −RM3483 | 3.19 | −9.3* | −23.5 | 11.6 | |||||||
| AAAS | 2 | RM250 −RM166 | 8 | RM230 −RM264 | 3.51 | 28.0 | 13.6 | ||||||||
| AAAS | 4 | RM119 −RM273 | 10 | RM271 −RM216 | 3.96 | −11.3* | −23.0 | 16.5 | |||||||
| AAAS | 4 | RM255 −RM349 | 8 | RM223 −RM210 | 8.90 | −17.3*** | −31.0 | 16.2 | |||||||
| GP | CAU | 7 | RM3555 −RM248 | 12 | RM19 −RM247 | 4.94 | −14.3** | 15.9*** | −24.1 | 14.5 | |||||
| AAAS | 1 | RM102 −RM315 | 7 | RM125 −RM11 | 4.40 | −9.4* | −25.8 | 12.8 | |||||||
| AAAS | 3 | RM231 −RM3766 | 4 | RM255 −RM349 | 3.84 | −29.8 | 15.9 | ||||||||
| AAAS | 4 | RM127 −RM280 | 9 | RM296 −RM321 | 3.04 | −10.8* | −12.9* | −16.0 | 16.2 | ||||||
| SSP | CAU | 1 | RM1282 −RM1 | 1 | RM315 −RM104 | 3.21 | −9.5 | 9.5 | |||||||
| CAU | 1 | RM129 −RM5 | 11 | RM21 −RM224 | 3.06 | 4.2* | 11.2 | 12.3 | |||||||
| CAU | 3 | RM3766 −RM232 | 7 | RM125 −RM11 | 4.28 | −9.6 | 10.3 | ||||||||
| CAU | 4 | RM273 −RM241 | 11 | RM21 −RM224 | 4.15 | 4.1* | 13.7 | 13.5 | |||||||
| CAU | 6 | RM310 −RM42 | 9 | RM316 −RM219 | 4.66 | 4.2** | 9.1 | 8.1 | |||||||
| AAAS | 1 | RM1282 −RM1 | 9 | RM278 −RM107 | 3.75 | 5.3* | 6.9* | 8.3 | 6.4 | ||||||
| AAAS | 5 | RM173 −RM3620 | 6 | RM136 −RM141 | 2.91 | 11.7 | 5.9 | ||||||||
| GW | CAU | 1 | RM243 −RM129 | 6 | RM136 −RM141 | 5.56 | 1.1* | 2.6 | 10.5 | ||||||
| CAU | 3 | RM251 −RM6676 | 7 | RM125 −RM11 | 3.84 | −0.9* | −3.2 | 12.6 | |||||||
| CAU | 6 | RM136 −RM141 | 8 | RM337 −RM38 | 7.63 | 1.3* | 1.8* | 3.5 | 12.9 | ||||||
| CAU | 8 | RM210 −RM230 | 11 | RM224 −RM144 | 3.05 | 0.9* | 2.4 | 13.2 | |||||||
| AAAS | 8 | RM350 −RM223 | 9 | RM321 −RM242 | 3.72 | −1.3* | −1.4* | −3.9 | 16.1 | ||||||
| AAAS | 9 | RM296 −RM321 | 11 | RM202 −RM260 | 4.01 | 1.3*** | −0.8 | 9.3 | |||||||
| PP | CAU | 1 | RM243 −RM129 | 6 | RM225 −RM217 | 3.55 | 1.8 | 13.5 | |||||||
| CAU | 6 | RM276 −RM136 | 8 | RM230 −RM264 | 3.08 | −0.6* | −1.4 | 9.4 | |||||||
| AAAS | 6 | RM136 −RM141 | 12 | RM247 −RM179 | 2.94 | 1.2* | 2.4 | 8.7 | |||||||
| AAAS | 10 | RM271 −RM216 | 10 | OSR33 −RM147 | 2.88 | 2.2 | 7.8 | ||||||||
| YP | CAU | 2 | RM154 −RM110 | 4 | RM127 −RM280 | 2.82 | 4.5 | 11.8 | |||||||
| CAU | 8 | RM42 −RM350 | 11 | RM224 −RM144 | 4.71 | 3.9* | 5.2 | 16.2 | |||||||
| AAAS | 8 | RM350 −RM223 | 9 | RM321 −RM242 | 3.84 | −1.6* | −2.1* | −6.1 | 17.4 | ||||||
- *P < 0.05; **P < 0.001; ***P < 0.0001. aMarkers indicated in bold are those flanking M-QTLs identified in Table 3. bAi and Aj are the main effects of the loci i and j, were estimated by the heterozygotes (93-11/DT713) – the homozygotes (DT713/ DT713) using the mean values of F1 and HMP. AAij is the epistatic effect between loci i and j, as defined by Mather and Jinks (1982), which were all significant at P < 0.001. cR2 is the proportion of the total phenotypic variation explained by the AAij. AAAS, Anhui Academy of Agricultural Sciences; BCF1, backcross F1; CAU, China Agricultural University; GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
Discussion
Inbreeding depression in RI population and heterosis in BCF1 population
Inbreeding depression has been intensively investigated in outcrossing species. However, in self-pollinated plant species like rice, inbreeding depression has received less attention. Li et al. (2001) investigated a Lemont/Teqing RI population and found that there was 22.1–43.9% reduction of the RIL from the mid-parental values of Lemont/Teqing. In our study, the 7.0–16.8% reduction of the 93-11/DT713 RI population from the mid-parental values was significant for five yield-related traits, particularly when the reduction of YP reached 28.3%. While at the same time, a high heterosis was observed in the two BCF1s. For instance, there was an average of 19.5% for SP, 29.5% for GP, 9.6% for SSP, 53.5% for YP, 9.1% for GW and 18.4% for PP mid-parental heterosis. The correlation between the RILs, and the F1 performance, and the mid-parent heterosis of their BCF1s showed two general characteristics. The first was the deviation of the RIL performance value from the mid-parental value due to additive gene action, that caused inbreeding depression (Oka 1988; Li et al. 1997a,b, 2001; Luo et al. 2001). Genes of this type were detected directly in the RILs but confounded in the BCF1s. The other is the deviation of the F1 performance value from the mid-parental value, which was due to a non-additive gene action, which contributed to heterosis in the BCF1s but is not directly detectable in the RILs (Li et al. 2001; Luo et al. 2001). Otherwise, in our mapping populations, there was less genetic overlap between inbreeding depression and heterosis. For instance, only one locus was identified in both RILs and HMP. This also provided an explanation for the observation that the mean performance of the female RILs was less correlated with the mean performance of their BCF1s.
Usefulness of the RIL and BCF1 population
The unique advantage of a RILs set and their testcross F1s for QTL mapping allowed us to measure directly heterosis and resolve gene action (Liu et al. 1996). However, the shortcoming of the testcross population was obvious, with some uncertainty about the genotype with respect to the reliability of the QTL analysis and gene action dissection, mainly as a result of the unknown homology and dominant/recessive relationship between alleles from the RIL and tester (Mei et al. 2003). Our experiments using related RI, BCF1 populations were specifically designed to allow simultaneous, mapping of loci contributing inbreeding depression and heterosis. Furthermore, the genotypes of backcross hybrids can be clearly deduced from marker information of parental RILs. Using this method, both additive and non-additive gene actions at the detected loci were more accurately resolved. It was well known that the RILs mostly carried homologous alleles for varied ranges from two parents, theoretically near 50% from each parent without skewing. So the BCF1 population had fewer heterozygous loci than the hybrids between the two parents (93-11 × DT713). The reduction in the proportion of heterozygous loci in the BCF1 population probably caused the reduced average level of heterosis in the BCF1 population compared with the 93-11 × DT713 F1 hybrids. However, the difference of average mid-parental hetetosis was not significant between BCF1s and 93-11 × DT713 F1s. Extremely, heterosis of the former exceeded that of the later for SSP and PP (Table 1). Wide variations were also observed in F1 performance and mid-parental hetetosis in the BCF1 populations. Furthermore, the F1 performance and HMP of some BCF1 hybrids were stronger than that of the 93-11 × DT713 F1, while some other lines expressed an HMP in the opposite direction. With similar design, Mei et al. (2005) also obtained similar results. Taken together, it can be concluded that high heterosis came from heterozygosity at certain loci and not from genome-wide heterozygosity (Zhang et al. 1995; Yu et al. 1997; Li et al. 2001; Mei et al. 2005). The fact that heterosis of the BCF1s derived from the RIL exceeded that of the parental F1 can be explained by the elimination of deleterious heterozygosity.
Epistasis QTLs explain a much greater portion of the total variation than the main-effect QTL for yield related traits
By comparing the genetic effects detected in the RILs, F1 performance and HMP, we were able to summarize the relative importance of M-QTLs and E-QTLs, additive and dominant or over-dominant gene actions (Table 7). In the RI population, there were an average of 3.0 M-QTLs with 31.9% contribution rates and an average of 6.8 E-QTL pairs with 53.2% contribution rates, respectively. In the 93-11BCF1 population, there were an average of 2.0 additive M-QTLs with 24.8% contribution rates and an average of 3.5 additive E-QTL pairs with 40.1% contribution rates; there were average of 1.5 over-dominant M-QTLs with 25.4% contribution rate and an average of 2.7 over-dominant E-QTL pairs with 31.2% contribution rates; no dominant M-QTL was detected, while an average of 0.2 dominant E-QTL pairs with 4.6% contribution rates was detected in the population. In the DT713BCF1 population, there were an average of 1.0 additive M-QTLs with 12.9% contribution rates and an average of 3.3 additive E-QTL pairs with 40.0% contribution rates; there were an average of 0.5 over-dominant M-QTLs with 8.4% contribution rates and average of 1.7 over-dominant E-QTL pairs with 21.3% contribution rates; there was an average of 0.2 dominant M-QTLs with 3.7% contribution rates, while no dominant E-QTLs were detected in the population. Clearly, the number and contribution rate of E-QTLs was much larger than that of the M-QTLs for yield trait and five components in RILs and BCF1 populations, revealed that epistasis was a common feature of most loci associated with the performance and heterosis of rice.
| Traita | RILs | 93-11BC population | DT713BC population | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R 2 | Additive | Complete or partial dominance | Over-dominance | Additive | Complete or partial dominance | Over-dominance | |||||||||||
| No. | R 2(F1) | No. | R 2(F1) | R 2(HMP) | No. | R 2(F1) | R 2(HMP) | No. | R 2(F1) | No. | R 2(F1) | R 2(HMP) | No. | R 2(F1) | R 2(HMP) | |||
| Main-effect QTLs (M-QTLs) | ||||||||||||||||||
| SP | 4 | 41.6 | 2 | 28.5 | 0 | 0 | 0 | 2 | 0 | 25.2 | 1 | 14.6 | 0 | 0 | 0 | 1 | 15.3 | 14.2 |
| GP | 2 | 25.8 | 3 | 37.9 | 0 | 0 | 0 | 1 | 0 | 16.7 | 1 | 13.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| SSP | 1 | 14.7 | 1 | 14.6 | 0 | 0 | 0 | 1 | 0 | 10.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GW | 7 | 61.3 | 4 | 44.4 | 0 | 0 | 0 | 1 | 0 | 13.8 | 0 | 0 | 1 | 11.1 | 10.9 | 2 | 0 | 20.8 |
| PP | 3 | 33.1 | 2 | 23.3 | 0 | 0 | 0 | 1 | 8.7 | 16.1 | 2 | 24.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| YP | 1 | 14.6 | 0 | 0 | 0 | 0 | 0 | 3 | 28 | 33.3 | 2 | 24.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 3 | 31.9 | 2 | 24.8 | 0 | 0 | 0 | 1.5 | 6.1 | 19.3 | 1.0 | 12.9 | 0.2 | 1.9 | 1.8 | 0.5 | 2.6 | 5.8 |
| Epistatic effect QTLs (E-QTLs) | ||||||||||||||||||
| SP | 8 | 52.1 | 4 | 51.6 | 0 | 0 | 0 | 2 | 0 | 19.4 | 4 | 51 | 0 | 0 | 0 | 2 | 0 | 31.7 |
| GP | 8 | 62.1 | 3 | 32.6 | 1 | 12.4 | 15.2 | 2 | 0 | 19.2 | 3 | 43.2 | 0 | 0 | 0 | 1 | 0 | 16.2 |
| SSP | 8 | 68.4 | 4 | 40.4 | 0 | 0 | 0 | 3 | 0 | 50.3 | 5 | 44.2 | 0 | 0 | 0 | 2 | 0 | 21.8 |
| GW | 5 | 34.2 | 4 | 41.8 | 0 | 0 | 0 | 2 | 0 | 25.2 | 4 | 52.1 | 0 | 0 | 0 | 2 | 0 | 22.5 |
| PP | 4 | 28.3 | 3 | 39.4 | 0 | 0 | 0 | 3 | 0 | 32.3 | 2 | 21.3 | 0 | 0 | 0 | 2 | 0 | 18.1 |
| YP | 8 | 74.2 | 3 | 34.6 | 0 | 0 | 0 | 4 | 0 | 40.9 | 2 | 28 | 0 | 0 | 0 | 1 | 0 | 17.4 |
| Mean | 6.8 | 53.2 | 3.5 | 40.1 | 0.2 | 2.1 | 2.5 | 2.7 | 0.0 | 31.2 | 3.3 | 40.0 | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 | 21.3 |
- R 2 (in %) was the proportion of total trait phenotypic variances explained. GP, filled grains per panicle; GW, 1 000-grain weight; PP, panicles per plant; SP, spikelets per panicle; SSP, seed set percent; YP, grain yield per plant.
Historically, a considerable body of classical evidence has strongly suggested the prevalence of epistatic effects on quantitative traits in genetic populations (Spickett and Thoday 1966; Allard 1988; Lark et al. 1995; Maughan et al. 1996; Yu et al. 1997; Kusterer et al. 2007a,b; Melchinger et al. 2007). Interaction analysis of rice yield and its components revealed evidence for the presence of epistasis between QTLs (Li et al. 1997a, 1998). In a similar experimental design, Li et al. (2001) and Luo et al. (2001) identified more E-QTLs explaining 42.5–59.0% phenotypic variation while there were fewer M-QTLs only explaining 9.6–30.4% phenotypic variation for grain yield and biomass in RILs and BCF1 populations, respectively. Furthermore, Mei et al. (2005) also obtained similar results for seven agricultural traits. While epistasis was not adequately evaluated in maize, with a similar experimental design, Stuber et al. (1992) reported six and eight M-QTLs responsible for 60% of the phenotypic variation of GY in two BCF1s. These and other data suggest that M-QTLs tended to explain a greater portion of phenotypic variation for GY in maize than in rice (Stuber et al. 1992; Lin et al. 1996; Li et al. 1997a,b, 2001; Yu et al. 1997; Luo et al. 2001; Mei et al. 2003, 2005). Epistasis for complex traits appears to be more pronounced in self-pollinated crop species than in outcrossing species (Li et al. 2001; Luo et al. 2001), because co-adapted gene complexes generated by epistasis between or among unlinked loci can be more easily maintained in self-pollinated crop species than in outcrossing species (Allard 1988).
Li et al. (1998) suggested the possible presence of three types of epistasis affecting complex traits. Further, Li et al. (2001) revealed that the most interactions occurred between alleles of complementary loci. In this study, a mass of interaction pairs and different interaction effects were detected in the RILs, BCF1s and HMP. A total of 109 interaction pairs were detected in three related populations, and all involved three types of interaction including between main effective loci, between main effective loci and other loci and between complementary loci. In the RI population, the most interactions (63.4%) occurred between alleles of complementary loci. These results are consistent with the findings observed by Li et al. (2001). Nevertheless in the two BCF1 populations, these interaction pairs involved larger numbers of main effective loci. For instance, 54 interaction pairs (79.4%) out of 68 involved at least one main effective locus. Of course, these results require further confirmation by using nearly isogenic lines covering epistatic pairs.
Additive and over-dominance were important properties of most QTLs associated with heterosis for yield-related traits
An important result in the present study was the presence of two predominant types of identified QTLs, the additive QTLs and the over-dominant QTLs, with only a few exceptional QTLs showing complete or partial dominance. In BCF1 populations, the total number of the identified additive QTLs was 18 (58.1%) for M-QTLs and 40 (58.8%) for E-QTLs, respectively, and the total number of the identified over-dominant QTLs was 12 (38.7%) for M-QTLs and 27 (39.7%) for E-QTLs, respectively. Only one M-QTL and one E-QTL appeared to be dominant. In a similar experimental design, Mei et al. (2005) identified 17 (40.5%) of 42 additive M-QTLs and 12 (20.0%) of 65 additive E-QTL pairs and 20 (47.6%) of 42 over-dominant M-QTLs and 45 (68.2%) of 65 over-dominant E-QTL pairs in two reciprocal BCF1 populations. The results above adequately accounted for the importance of additive and over-dominant effects in heterosis of rice.
In numerous classic quantitative genetic or breeding studies using backcrosses, additive gene action was shown to be important to the mean performance of F1 hybrids in rice and other crop species (Simmonds 1979; Virmani 1994; Li et al. 2001). In this study, additive gene action explained 60% and 55% performance varieties in 93-11BCF1s and DT713BCF1s, respectively. This is not surprising since the materials used in most classical studies of backcrosses had been more or less subjected to selection for improved performance (additive gene action). Thus, selection for improved performance of those inbred lines in breeding might have eliminated most hybrid breakdown genes or gene combinations observed in our base RI mapping population. This conclusion was supported by the phenomenon that some recombinant inbred lines were observed whose phenotypes exceeded that of the 93-11 × DT713 F1.
Li et al. (2001) and Luo et al. (2001) suggested that over-dominance is associated with most loci contributing to heterosis in rice. Hua et al. (2003) reported that single-locus heterotic effects with a high degree of over-dominance and dominance by dominance interactions adequately explained the genetic basis of heterosis in an elite rice hybrid. Pronounced over-dominance at M-QTL for yield trait and its components was reported also in maize (Stuber et al. 1992). In this study, over-dominant effects explained 50.1% and 27.1% phenotypic variations in 93-11BC population and DT713BC population, respectively, revealing that over-dominance played an important role for heterosis of rice.
These analyses suggested that additive and over-dominance were more important than complete or partial dominance for M-QTLs and E-QTLs, and that the E-QTLs collectively explained a larger portion of the total phenotypic variation than the M-QTLs. We therefore concluded that additive and over-dominance resulting from episatic QTLs were major genetic bases of heterosis in rice.
Matderials and Methods
Plant materials
Three related mapping populations were used in the study including a set of 148 RILs (F9) derived by single-seed descent from a cross between vars. 93-11 (indica, the male parent of the super hybrid rice, Liangyoupeijiu) and DT713 (japonica, an excellent restorer line) and two BCF1 populations. The first BCF1 population consisted of 138 BCF1 (93-11BCF1s) hybrids from crosses between the RILs (used as female) and one parent, 93-11 and the second one consisted of 143 BCF1 (DT713BCF1s) hybrids from crosses between the RILs and the other parent, DT713. The parents (93-11 and DT713), their F1 (93-11×DT713) and Liangyoupeijiu (LYPJ, a widely grown super rice hybrid cultivar in China) were used as checks.
Phenotypic experiments
Two separate experiments were conducted at two locations, the Experiment Station of China Agricultural University (CAU), Beijing (116°28′E, 39°54′N), and the Experiment Station of Anhui Academy of Agricultural Sciences (AAAS), Hefei (117°18′E, 31°51′N), Anhui Province, China, in 2004. In the CAU environments, the RILs, parents, F1, and the two BCF1 populations (93-11BCF1s and DT713BCF1s), and check hybrid, Liangyoupeijiu, were sowed in the seedling nursery on 4 May 2004. The 25-d-old seedlings were transplanted into nine-row plots, each consisting of three rows of the female RIL and two BCF1 hybrids (the RIL×93-11 and DT713). The plots were arranged in a randomized complete block design with two replications. Each row within a plot consisted of 12 plants with a spacing of 13.3 cm between plants within each row and 26.7 cm between rows. Four check plots consisted of 93-11, DT713, F1 (93-11×DT713), and Liangyoupeijiu were randomly arranged in each replication. In the AAAS environment, all of the materials were sowed on 4 June 2004 and transplanted into the field after 25 d from sowing, and the field arrangement was the same as the CAU environment.
At the maturity stage, 10 plants in the middle of each plot were harvested and evaluated for the following traits: spikelets per panicle (SP), filled grains per panicle (GP), 1 000-grain weight (GW), panicles per plant (PP), and grain yield per plant (YP). One derived trait, seed set percent (SSP = 100·GP/SP), was calculated. For subsequent analyses, the phenotypic values of the means of two replications for six yield-related traits that were evaluated were used.
Genotyping and data analysis
Genomic DNA of the RILs and two parental lines was extracted from freshly harvested leaves of 60-d-old plants grown in the experimental farm of the China Agricultural University by the cetyltrimethyl ammonium bromide (CTAB) method (Rogers and Bendich 1988). A subset of 420 SSR markers was selected from the rice high-density molecular map (McCouch et al. 2002), and the polymorphic SSR markers were analyzed using the RILs. The linkage map was constructed using MAPMARKER/EXP version 3.0 (Lincoln et al. 1992) under LOD > 3.0.
Statistics analysis system (SAS) procedures (SAS Institute 1996) were used to test the differences among the RILs and BCF1s and to obtain the basic statistics of the traits. The hybrid breakdown value (HB) which is a component of inbreeding depression (Li et al. 1997a,b, 2001) was calculated as follows: HB = RIL − MP, where MP = (93-11+DT713)/2 was the mid-parental value of two parents. Mid-parental heterosis (HMP) of each BCF1 was calculated as follows: HMP= F1− MP, where F1 was the mean trait value of the BCF1 and MP = (RIL + recurrent parent)/2 was the mid-parental trait values of the corresponding female RIL and the recurrent parent.
The genotype for each BCF1 was deduced on the basis of the RILs genotype used as the parent for cross. QTL analysis was carried out separately for the RI and BCF1 populations. For the RI population, the mean trait values from two replications were used as raw data. For each of the BCF1 populations, the mean trait values and mid-parental heterosis (HMP) of the BCF1s were used as raw data. Analysis of main-effect QTLs (M-QTLs) and digenic epistatic QTLs (E-QTLs) were conducted in each mapping population by interval mapping using the mixed linear approach and computer software QTLMAPPER ver.1.0 (Wang et al. 1999). A threshold of LOD ≥ 2.0 was used to declare M-QTLs and E-QTLs in the RILs, BCF1 performance and HMP.
The genetic effects in BCF1s were defined as follows: a= (P1P1− P2P2)/2; HMP=d= (BCF1− (P1P1+ P2P2)/2) and BCF1= (a+d) (when P1 is the recurrent parent) or (a+d+ P2P2) (when P2 is the recurrent parent, where P2P2 was constant for all BCF1s). Subsequently, QTL actions can be inferred by comparison between mapping results in RIL and BCF1 performance and mid-parental heterosis. Additive QTLs were detectable only in RIL or BCF1 performance values without a significant dominant effect. QTLs with d/a ≤ 1 were referred to as being complete or partial dominant loci and were expected to have an estimate in BCF1 performance (a+d) equal to or higher than twice the HMP (d). QTLs with d/a > 1, i.e. 2d (2 ×HMP) > a+d (BCF1), or only detectable for HMP were referred to as over-dominant loci (Melchinger et al. 1998).
(Handling editor: Yongbiao Xue)
Acknowledgements
We thank Dr. Hongwei Cai for his assistance in editing this manuscript.




