Phosphorylation of Microtubule-associated Protein SB401 from Solanum berthaultii Regulates Its Effect on Microtubules
Supported by the State Key Basic Research and Development Plan of China (2006CB100101) and the National Natural Science Foundation of China (30421002, 30370707 and 30570925) to M.Yuan.
Abstract
We reported previously that the protein SB401 from Solanum berthaultii binds to and bundles both microtubules and F-actin. In the current study, we investigated the regulation of SB401 activity by its phosphorylation. Our experimental results showed that the phosphorylation of SB401 by casein kinase II (CKII) downregulates the activities of SB401, namely the bundling of microtubules and enhancement of the polymerization of tubulin. However, phosphorylation of SB401 had no observable effect on its bundling of F-actin. Further investigation using extract of potato pollen indicated that a CKII-like kinase may exist in potato pollen. Antibodies against CKII alpha recognized specifically a major band from the pollen extract and the pollen extract was able to phosphorylate the SB401 protein in vitro. The CKII-like kinase showed a similar ability to downregulate the bundling of microtubules. Our experiments demonstrated that phosphorylation plays an important role in the regulation of SB401 activity. We propose that this phosphorylation may regulate the effects of SB401 on microtubules and the actin cytoskeleton.
Microtubule-associated proteins (MAPs) have been increasingly identified from plant cells and play important roles in various functions of microtubules (MTs) (Gardiner et al. 2001; Whittington et al. 2001; Hussey et al. 2002; Webb et al. 2002; Sedbrook et al. 2004; Fu et al. 2005; Wang et al. 2007; Huang et al. 2007). The regulation of MAP activities may be an effective way for the MT cytoskeleton to respond to signal transduction and to alter its functions.
It has been reported that the activities of MAPs are regulated by phosphorylation. For example, phosphorylation and dephosphorylation of AtMAP65-1 occurs during the cell cycle, and the phosphorylation reduces the binding and bundling of AtMAP65-1 to MTs (Smertenko et al. 2006). The mitogen-activated protein kinase (MAPK) cascade of tobacco plants is also involved in the phosphorylation of tobacco NtMAP65-1a (Sasabe et al. 2006). Similarly, NRK1/NTF6 phosphorylates the threonine residue at position 579 of NtMAP65-1a and downregulates its MT-bundling activity. This phosphorylation of NtMAP65-1a enhances the destabilization and turnover of MTs at the equator of the phragmoplast and thereby facilitates expansion of the phragmoplast.
The protein SB401 was first identified from the cDNA library of in vitro-germinated pollen from the diploid potato species Solanum berthaultii (Liu et al. 1997). Recently, we reported that SB401 binds to MTs and causes their bundling in vitro. In addition, SB401 binds to and bundles actin filaments. Hence, SB401 may function as a link between actin and the MT cytoskeleton (Huang et al. 2007). Nevertheless, our experiment showed that SB401 binds preferentially to MTs (Huang et al. 2007). The mechanism of regulation of the binding of SB401 to MTs or F-actin is unknown.
In the study reported herein, we obtained experimental results that showed that phosphorylation by casein kinase II (CKII) downregulates the involvement of SB401 in the bundling of MT and the enhancement of the polymerization of tubulin. A CKII-like kinase exists in extracts of potato pollen and causes similar downregulation of the bundling of MTs. These findings indicate that phosphorylation plays an important role in the regulation of SB401 activity. However, the phosphorylation of SB401 has no effect on its involvement in the bundling of F-actin. Therefore, the phosphorylation of SB401 may be involved in the regulation of how SB401 acts on MTs and F-actin.
Results
Casein kinase II phosphorylates SB401
Analysis of the SB401 protein sequence predicts that there are three sites for CKII phosphorylation in the protein domain that contains the VEEKKEE repeats, which are important for the binding of SB401 to MTs (http://www.predictprotein.org/) (Figure 1). This suggests that CKII may be involved in the regulation of SB401 activity.

Prediction of casein kinase II (CKII) phosphorylation sites on SB401.Analysis of the SB401 protein sequence predicted three sites for CKII phosphorylation located in the protein domain containing the VEEKKEE repeats (in gray) (http://www.predictprotein.org/). Asterisks (*) represent the predicted CKII phosphorylation sites.
First, we used a commercial CKII to test whether CKII could phosphorylate SB401. When SB401 was incubated with CKII and [γ-32P] ATP, an apparent signal was observed (Figure 2A, B, lane 2). However, no signal was detected when SB401 was incubated with [γ-32P] ATP in the absence of CKII (Figure 2A, B, lane 1), or with CKII (Figure 2A, lane 3). Bovine serum albumin (BSA), which was used as a negative control, was not phosphorylated by CKII (Figure 2A, B, lane 4). This result demonstrates that CKII is capable of phosphorylating SB401.

casein kinase II (CKII) is capable of phosphorylating SB401.(A) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel.(B) Autoradiography of phosphorylated SB401.Lane 1, SB401 alone; lane 2, SB401 incubated with CKII; lane 3, CKII alone; lane 4, bovine serum albumin (BSA) control. Radioactive ATP and ATP were equal in each reaction system.
Phosphorylation of SB401 downregulates its activity in bundling microtubules and enhancing the polymerization of tubulin
Further experiments were carried out to investigate the effect of phosphorylation of SB401 on its involvement in the bundling of MTs.
The activity of SB401 in influencing the bundling of MTs was tested. The MTs were preformed in the presence of 10 μmol/L taxol. The MTs that had been stabilized by taxol were incubated with or without SB401, or P-SB401 (phosphorylated SB401), respectively, before observation using confocal microscopy. The results showed that taxol-stabilized MTs without the addition of SB401 appeared in a pattern of single filaments (Figure 3A). When 2 μmol/L of SB401 was added, MT bundles formed (Figure 3B). However, no MT bundles were observed when 2 μmol/L of P-SB401 was added to the preformed MTs (Figure 3C). This observation indicates that the phosphorylation of SB401 downregulates its activity with respect to the bundling of MTs.
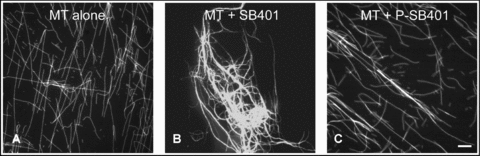
Phosphorylation of SB401 downregulates its microtubule (MT)-bundling activity.(A) MTs alone were scattered as single filaments.(B) Long, thick bundles of MTs were formed when 2 μmol/L of SB401 protein was added.(C) No obvious bundles of MTs were formed when 2 μmol/L of P-SB401 protein was added. Bar (A–C), 10 μm.Samples containing 0.5 μmol/L taxol-stablized MTs were incubated with 2 μmol/L of SB401 protein or 2 μmol/L of P-SB401 (phosphorylated SB401) protein.
The activity of SB401 in the enhancement of tubulin polymerization was also investigated. The tubulins were polymerized in the absence or presence of varying concentrations of SB401. The polymerization was monitored by turbidimetric assay. The results showed that the polymerization of tubulin was enhanced by the addition of SB401 in a concentration-dependent manner (Figure 4). In contrast, when P-SB401 (phosphorylated SB401) was used in the experiments, the polymerization of tubulin was downregulated (Figure 4).
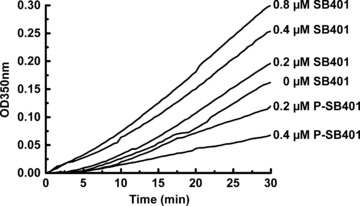
Phosphorylation of SB401 inhibits tubulin polymerization.Tubulin (30 μmol/L) was polymerized at 35 °C in the presence of various concentrations (0.2 or 0.4 μmol/L) of P-SB401 (phosphorylated SB401) protein or of SB401 protein (0.0, 0.2, 0.4, or 0.8 μmol/L).
In conclusion, our tests demonstrated that the effects of SB401 on MTs were altered when SB401 was phosphorylated by CKII.
Phosphorylation of SB401 has no effect on its activity in bundling F-actin
Our previous report provided evidence that SB401 also binds to and bundles F-actin (Huang et al. 2007). Therefore, the effect of SB401 on the bundling of F-actin was also tested. F-actin was preformed in the presence of 0.5 μmol/L phalloidin. Phalloidin-stabilized F-actin appeared in a pattern of single filaments (Figure 5A). When 2 μmol/L of SB401 was added, bundles of F-actin were formed (Figure 5B). This effect was also observed when 2 μmol/L of P-SB401 was added (Figure 5C). Therefore, the phosphorylation of SB401 has no effect on its activity in the bundling of F-actin.
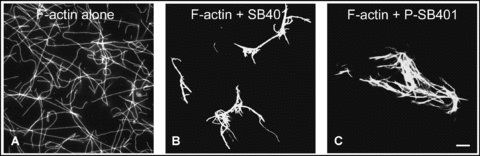
Phosphorylation of SB401 has no effect on its involvement in the bundling of F-actin.(A) F-actin alone.(B) F-actin bundles were induced when 2 μmol/L of SB401 protein was added.(C) F-actin bundles were induced when 2 μmol/L of P-SB401 (phosphorylated SB401) protein was added. Bar (A–C), 10 μm.F-actin (0.5 μmol/L) was incubated with 2 μmol/L of SB401 protein and 2 μmol/L of P-SB401 (phosphorylated SB401) protein at room temperature for 1 h, then labeled with Alexa-488 phalloidin and observed using confocal microscopy.
A CKII-like kinase exists in potato pollen extract
The experimental results described above indicate that phosphorylation of SB401 by CKII downregulated its involvement in the bundling of MTs and enhancement of the polymerization of tubulin. We also wished to investigate whether any CKII-like kinase in cells exerts such an effect. Given that SB401 is expressed in potato pollen and is enriched in extracts of mature pollen grains and in pollen that is germinated in vitro (Liu et al. 1997), we used extracts from potato pollen grains and pollen that was germinated in vitro to test whether any CKII-like kinase was present. In order to identify CKII-like kinase from the potato pollen extract (PE), an anti-CKII alpha antibody was used in Western blotting experiments. A major protein band with a molecular mass of 55 kDa was recognized specifically in the PE by the antibody (Figure 6A, B, lane 2). No signal was detected from BSA, which was used as a control (Figure 6A, B, lane 1). The molecular mass of the protein identified herein is different from those reported for Arabidopsis (45 kDa, Espunya and Martinez 1997), broccoli (48 kDa, Klimczak and Cashmore 1994), and pea (41 kDa, Zhang et al. 1993). This finding suggests that different isoforms of CKII-like kinases exist in plants.
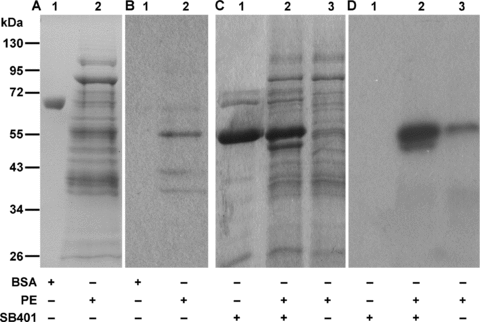
Casein kinase II (CKII)-like kinase is present in potato pollen extract.(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Lane 1, 0.5 μg bovine serum albumin (BSA) control; lane 2, 16 μg protein of potato pollen extract.(B) Western blotting using CKII alpha antibody. The protein molecular mass of the band recognized specifically by the antibody was ∼55 kDa.(C) SDS-PAGE gel.(D) Autoradiography of phosphorylated SB401 (P-SB401).Lane 1, 2.8 μg SB401 alone as control; lane 2, 2.8 μg SB401 incubated with 10 μg PE; lane 3, 10 μg PE alone. Radioactive ATP and ATP were equal in each reaction system.
In addition, a phosphorylation assay was carried out to test whether there is any kinase activity that influences the SB401 protein in PE. When SB401 was incubated with PE and [γ-32P] GTP, an apparent signal was detected (Figure 6C, D, lane 2). However, no signal was detected when SB401 was incubated with [γ-32P] GTP in the absence of PE (Figure 6C, D, lane 1). A signal was also detected in the PE, which suggests that the endogenous SB401 was phosphorylated (Figure 6C, D, lane 3). These observations suggest that CKII-like kinase is present in potato pollen extract.
Potato pollen extract downregulates SB401 involvement in bundling of microtubules
To further elucidate whether phosphorylation of SB401 by pollen extract had an effect on the bundling of MTs similar to that described above, we pre-incubated SB401 with PE and ATP to allow the SB401 protein to be phosphorylated and then tested its effect on the bundling of MTs.
Preformed taxol-stabilized MTs appeared in a pattern of single filaments (Figure 7A). The addition of 3 μmol/L of SB401 caused bundling of the MTs (Figure 7B). The addition of PE (0.2 mg/mL), without or with ATP (50 μmol/L), had no effect on MT bundling (Figure 7C, D). Therefore, PE alone was not involved in the bundling of MTs. When 3 μmol/L of SB401 was added together with PE (0.2 mg/mL), the MTs formed bundles (Figure 7E). The addition of ATP had no effect on this formation of bundles by SB401 (Figure 7F). However, no MT bundles were observed in the presence of SB401, PE, and ATP (Figure 7G), which suggests that PE phosphorylated the SB401 and consequently downregulated its effect on bundling of MTs. To confirm this, the CKII-like kinase was removed from PE by immunodepletion. In the presence of SB401, AbB (PE in which CKII-like-kinase was immunodepleted) and ATP, the MTs formed bundles (Figure 7H). Pretreatment of the PE with protein A-sepharose beads without antibody (ckB) had no effect on MT bundling (Figure 7I). Therefore, the CKII-like kinase that was present in the PE was responsible for the phosphorylation of SB401 and therefore downregulated its involvement in the formation of MT bundles.
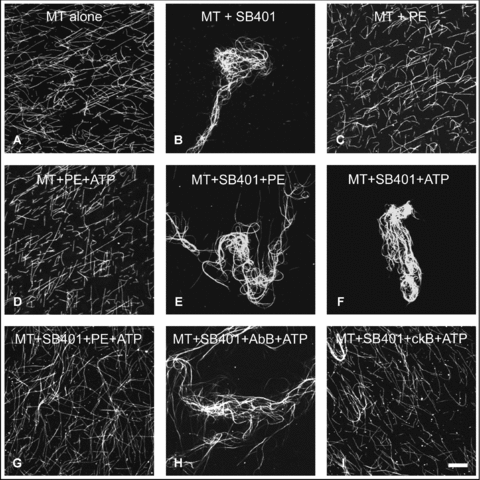
Casein kinase II (CKII)-like kinase in pollen extract downregulates the activity of SB401 in bundling microtubules (MTs).(A) Fluorescence micrographs of 1 μmol/L taxol-stabilized MTs alone (in the absence of SB401) show them scattered as single filaments.(B) MT bundles were formed when 3 μmol/L SB401 protein was added.(C–D) MTs also formed single filaments when incubated with pollen extract (PE) (C), or with both PE and ATP (D).(E–F) Bundled MTs promoted by SB401 were maintained after incubation with 0.2 mg/mL PE (E) or 50 μmol/L ATP (F) alone.(G) No MT-bundling activity was observed when MTs were incubated with both 3 μmol/L SB401 and pollen extract (0.2 mg/mL) in the presence of ATP (50 μmol/L).(H) PE was incubated with CKII antibody then treated with protein A resin beads. A suspension of treated PE after centrifugation was reacted with MT, SB401 and ATP, which resulted in the formation of MT bundles.(I) MTs also show single filaments when the system in (H) was incubated with protein A resin beads alone, without CKII antibody, as a control.Bar (A–I), 20 μm.
Discussion
Phosphorylation of SB401 switches its functional activity between MTs and F-actin
Phosphorylation plays an important role in the regulation of the activities of MAPs. Phosphorylation of XMAP215 reduces its activity in the regulation of MT dynamics (Vasquez et al. 1999). Phosphorylation of Tau at S262 inhibits its activity in bundling of MTs, whereas phosphorylation at S214 inhibits its nucleation activity in the polymerization of tubulin (Yoshida et al. 2004). A number of studies have demonstrated that phosphorylation by various kinases of proteins that bind and stabilize MTs is an important mechanism for the regulation of mitosis (Yarm 2002; Rong et al. 2007). In plant cells, it has also been reported that Arabidopsis AtMAP65-1 and tobacco NtMAP65-1 are phosphorylated during the cell cycle (Sasabe et al. 2006; Smertenko et al. 2006).
We have reported previously that SB401 from S. berthaultii is able to bind to both MTs and F-actin, which suggests that SB401 may function as a link between actin and the MT cytoskeleton (Huang et al. 2007). However, SB401 binds preferentially to MTs. There must be some mechanism to regulate the switch of the function of SB401 between MTs and F-actin. In the study reported herein, we demonstrated that phosphorylation may play a role in such regulation. The phosphorylation of SB401 downregulates its involvement in the bundling of MTs and the enhancement of the polymerization of tubulin. In contrast, such phosphorylation has no effect on the involvement of SB401 in the bundling of F-actin. Therefore, phosphorylated SB401 may function mainly in association with F-actin, whereas dephosphorylated SB401 may act mainly on MTs.
Regulation by phosphorylation is also found in other proteins that bind to both MTs and F-actin. For instance, MAP1B, which also contains VEEKKEE repeats and binds to both MTs and F-actin, has at least one Proline Directed Protein Kinase (PDPK) and two CKII phosphorylation sites. Phosphorylation of purified MAP1B results in the loss of its ability to bind to F-actin, whereas dephosphorylation restores this ability (Pedrotti et al. 1994; Gong et al. 2000; Riedere 2007). It appears that phosphorylation of MAP1B and SB401 by CKII has different effects on F-actin.
Phosphorylation of SB401 regulates its conformation
Our experimental results demonstrated that the phosphorylation of SB401 downregulates its involvement in the formation of MT bundles and in enhancement of the polymerization of tubulin. How is this regulation achieved?
Analysis of the structure predicted by the domain of SB401 protein (http://www.predictprotein.org/), which contains six VEEKKEE repeats and three CKII phosphorylation sites, suggests that this protein sequence has structurally ambivalent sequence fragments. These are able to adopt either helical or sheet conformations, and a limited number of substitutions results in the conversion of a helical protein to a protein of predominantly sheet formation (Kuznetsov and Rackovsky 2003). The structurally ambivalent fragments in the SB401 protein may be the key to the regulation of bundling of MTs. For example, the phosphorylation of Ser63 in Op18/stathmin disrupts the central helix and suppresses its binding to tubulin (Steinmetz et al. 2001). Phosphorylation modifies both the negative charge and the local conformation near the phosphorylation sites in Tau protein (Du et al. 2005). These structural changes suggest that phosphorylation may act as a conformational switch in the binding domain of MAPs. Phosphorylation is one strategy by means of which control of biological activity can be enforced.
Further investigations are required to identify the CKII-like kinase in potato pollen, to elucidate how the phosphorylation of SB401 functions within the cell, and to investigate the physiological role of the phosphorylation of SB401. Nevertheless, the current study has provided evidence to show that CKII-like kinase activity exists in potato pollen, and that phosphorylation of SB401 affects its function with regard to MTs. These results will provide the basis for our future studies.
Materials and Methods
In vitro pollen germination and preparation of pollen extracts
Pollen grains were collected from mature flowers of Solanum berthaultii and stored at −80 °C. The pollen grains were spread in liquid germination medium (20 mmol/L Mes-potassium hydroxide (KOH) pH 6.0, 0.01% (w/v) KNO3, 0.02% MgSO4-7H2O, 0.01% H3BO4, 0.07% Ca(NO3)2-4H2O, 2% sucrose, 15% PEG6000) and germinated as reported previously (Liu et al. 1997).
To prepare the protein extract from the germinated pollen, potato pollen grains (∼0.2 g) were collected by centrifugation at 12 000g for 10 min and homogenized with a mortar and pestle under liquid nitrogen. The mixture was resuspended with 2–3 mL of extraction buffer. After centrifugation at 50 000g for 40 min, the supernatants were transferred into clean tubes and kept in an iced-water bath. The protein concentration of the pollen extract was determined by a Bio-Rad Laboratories (Hercules, CA, USA) protein assay with BSA as the standard. Two extraction buffers were used for the preparation. The extraction buffer for the kinase assay contained 100 mmol/L Hepes (pH 7.5), 5 mmol/L ethylene diamine tetraacetic acid (EDTA, pH 8.0), 5 mmol/L ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA, pH 7.0), 10 mmol/L NaF, 50 mmol/L glycerophosphate (sodium salt), 5% (v/v) glycerol, 10 mmol/L Na3VO4, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mmol/L phenylmethanesulfonyl fluoride (PMSF), and 10 mmol/L dithiothreitol (DTT) (Zhang and Liu 2001).
The extraction buffer for the MT assay contained 100 mmol/L Pipes, 1 mmol/L EGTA (pH 8.0), 1 mmol/L MgCl2, 10 μg/mL aprotinin, and 10 μg/mL leupeptin, at pH 6.9, adjusted with KOH.
Antibodies and protein gel blots
The protein samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes. Blots were probed with a polyclonal antibody to CKII alpha subunit (ab13410, Abcam, Cambridge, UK) at a dilution of 1:500 with TBST (50 mmol/L Tris, 150 mmol/L NaCl, and 0.05% Tween 20, pH 7.5). The secondary antibody was horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA), applied at a dilution of 1:10 000.
Phosphorylation assays
Recombinant SB401 protein was prepared as reported previously (Huang et al. 2007). Phosphorylation assays were prepared using commercial CKII kits (#P6010S, New England Labs, Beverly, MA, USA). Briefly, 3 μg SB401 was phosphorylated in a reaction system (30 μL) that contained CKII reaction buffer (20 mmol/L Tris-HCl, pH 7.5, 50 mmol/L KCl, 10 mmol/L MgCl2, 3 μCi [γ-32P] ATP, and 50 μmol/L ATP) in the presence of 5–10 U commercial CKII at 25 °C for 30 min. The protein samples were separated by SDS-PAGE. The gels were stained using Coomassie brilliant blue, dried, and subjected to autoradiography. BSA (3 μg) was used as the negative control.
To phosphorylate the SB401 protein, 10 μg of pollen protein extract in PE buffer was mixed with 3 μg of SB401 in the reaction system (30 μL) and incubated with 3 μCi [γ-32P] of GTP at 25 °C for 20 min; 50 μmol/L of GTP was then added to the solution and incubated for an additional 60 min. The protein samples were then separated by SDS-PAGE. The gels were stained using Coomassie brilliant blue, dried, and subjected to autoradiography.
Microtubule polymerization assay
Porcine brain tubulin was prepared as reported previously (Huang et al. 2007). To monitor the time course of tubulin polymerization, various concentrations (0.0, 0.2, 0.4, or 0.8 μmol/L) of SB401 protein or phosphorylated SB401 protein (0.2 or 0.4 μmol/L) were added to 50 μmol/L of tubulin in PEM (100 mmol/L PIPES, 1 mmol/L EGTA, 1 mmol/L MgCl2) buffer containing 1 mmol/L of GTP. Microtubule polymerization was monitored spectrophotometrically at OD350 in a 0.4 cm-wide quartz cuvette at 35 °C for 30 min using a DU-640 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). Each experiment was repeated at least two times.
Microtubule and F-actin bundling assays
Fluorescence labeling experiments were carried out in vitro to investigate the bundling of SB401 to MTs and F-actin. NHS-rhodamine (5(and-6)carboxytetra-methyl-rhodamine succinimidyl ester) labeled tubulin was prepared as reported previously (Huang et al. 2007). Purified recombinant SB401 (2 μmol/L) or P-SB401 (2 μmol/L) was incubated with taxol-stabilized, rhodamine-labeled MTs (0.5 μmol/L) in 50 μL PEM buffer at room temperature in a tube by gently pipetting with a cut tip at room temperature for 5 min. One microliter of the mixture was gently pipetted on to the surface of a glass slide, gently covered with a cover slip, and observed using a confocal microscope (Meta 510, Zeiss, Jena, Germany) with a Zeiss 40× magnification oil objective (NA 1.3).
Rabbit muscle actin was prepared as previously reported (Huang et al. 2007). F-actin (0.5 μmol/L) polymerized with 100 nmol/L of Alexa-488 phalloidin was incubated with 2 μmol/L of SB401 protein or 2 μmol/L of P-SB401 protein at room temperature for 5 min. Aliquots (1 μL) of the samples were placed on to a slide and observed using the confocal microscope.
Immunodepletion
The immunodepletion experiment was carried out as described by Lee et al. (1998). Ten milligrams of protein A sepharose CL-4B beads (GE Healthcare, Uppsala, Sweden) were swollen in 1 mL fresh distilled water. After the beads had settled by gravity, the supernatant was drawn off and the medium was resuspended with 1 mL of fresh distilled water. This procedure was repeated six times. The beads were then transferred into PEM buffer and washed with 1 mL PEM buffer five times. The settled medium was used in the immunodepletion experiments. Approximately 200 μg of pollen extract and 5.5 μg of commercial CKII antibody were mixed and incubated overnight at 4 °C. Subsequently, 5 μL of pre-washed protein A-sepharose bead slurry was added to the mixture and incubated for 5 h. Immunodepleted supernatants (AbB) were obtained by centrifugation at 12 000g for 90 s. Those treated with protein A-sepharose bead without antibody were used as a negative control, designated ckB.
(Handling editor: Chun-Ming Liu)
Acknowledgements
We thank Professor Dongtao Ren at the China Agricultural University for critical discussions of the work, and Professor Ziding Zhang for help with the prediction and analysis of casein kinase II (CKII) phosphorylation sites on SB401.




