High-Sensitivity Detection of Fruit Tree Viruses Using Bacterial Magnetic Particles
Abstract
Prunus necrotic ring spot virus (PNRSV) and grapevine fanleaf virus (GFLV) were detected by fluoroimmunoassay using bacterial magnetic particles (BMPs), and a double antibody sandwich enzyme linked immunosorbent assay (DAS-ELISA). For the fluoroimmunoassay, fluorescein isothiocyanate labeled anti-PNRSV antibody or anti-GFLV antibody was conjugated onto BMPs of Magnetospirillum gryphiswaldense MSR-1. With this method, a very low minimum antigen concentration (1 × 106 dilution of the original sample concentration) could be detected. Using DAS-ELISA, the minimum antigen detection concentration was the original sample concentration. Thus, comparing these two methods, a BMP-based method could increase the sensitivity up to six orders of magnitude (106) higher than an ELISA-based method of detection PNRSV and GFLV.
Bacterial magnetic particles (BMPs) are separated from magnetotactic bacteria, and they are nano size (50–100 nm), and enveloped with a stable lipid membrane (Gorby et al. 1988). The main composition of the enveloped crystal is iron oxide magnetite (Fe3O4) (Matsunaga 1991). Purified BMPs can easily be dispersed in aqueous solution (Tanaka and Matsunaga 2000). The small size and intact magnetosome membrane give BMP a large surface to volume ratio. Therefore, it is possible to immobilize a large quantity of a bioactive substance onto the surface membrane of BMPs (Matsunaga 1991), such as antibodies (Nakamura et al. 1991; Nakamura and Matsunaga 1993; Chen et al. 2006) and enzymes (Matsunaga and Kamiya 1987). The surface-protein-modified magnetic particles can be used for various biotechnological applications (Matsunaga et al. 2007). A high immobilization efficiency is necessary to provide the advantage of sensitivity, rapidity, and precision in detection assays. High detection sensitivity was obtained using BMPs for detecting mouse immunoglobulin G (IgG) (in a detectable range of 0.5–100 ng/mL) (Nakamura et al. 1991). For detecting allergen, the detectable range was 0.5–100 pg/mL (Nakamura and Matsunaga 1993), and for Escherichia coli, the detectable range was 102–106 cells/mL (Nakamura et al. 1993).
Although a highly sensitive method in substance detection using BMPs, and the importance of BMPs is recognized by many researchers, use of BMPs has been limited. Applications, however, have varied, for example, detection of substances like IgG (Nakamura et al. 1991), allergen (Nakamura and Matsunaga 1993), and insulin (Tanaka and Matsunaga 2000), removal of Escherichia coli (Nakamura et al. 1993), extraction of DNA (Yoza et al. 2002, 2003), separation of cells (Kuhara et al. 2004), a single nucleotide polymorphism detection (Ota et al. 2003; Nakagawa et al. 2006), and the study of immobilization conditions (Chen et al. 2006). Therefore, BMPs have a high potential in practical use. The characters of BMP can let it be applied in detecting substance in aqueous solution. It is possible to detect an object in a small quantity in some living things.
Fruit trees are perennials, with long commercial lives; and they usually need at least 2–3 years to start bearing. It is practically impossible to remove a virus from an infected nature fruit tree. Virus infections often result in weak growth, delayed maturity, and the formation of smaller fruit that are unacceptable in the marketplace. Therefore, the best approach is to screen for and remove virus infected trees at an early stage in order to avoid further damage and particularly, spread of the infection.
There are many methods for fruit tree virus detection, such as microscopic observation of viruses in plant cells, ELISA (enzyme linked immunosorbent assay) detection, and molecular biological identification. A highly-sensitive, cost-effective diagnosis method to detect fruit tree viruses is quite desirable, and we are not aware of any comparisons of the detection sensitivity of a BMP-based method with a common assay such as ELISA. However, the conjugation conditions have been studied systemically for goat anti-rabbit antibody immobilization onto BMPs (Chen et al. 2006). Consequently, we present a comparison of the sensitivity of a BMP-based method with an ELISA-based method in detecting two fruit tree viruses: prunus necrotic ring spot virus (PNRSV) and grapevine fanleaf virus (GFLV).
Results
SDS-PAGE identification of BMPs-conjugated antibodies
Anti-PNRSV antibody or anti-GFLV antibody was conjugated on BMPs. The conjugated antibodies were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1). Lanes 1 and 3 (protein from BMPs and anti-PNRSV antibody complex) appeared as a 50 kDa band, the same band as standard the anti-PNRSV antibody (lane 4), and lanes 6, 8 and 9 (protein from BMPs and anti-GFLV antibody complex) had the same band as the standard anti-GFLV antibody (lane 5); lanes 2 and 7 (magnetosome membrane protein of BMPs without binding antibodies) lacked the 50 kDa band. These results indicate that the antibody was immobilized onto BMPs.
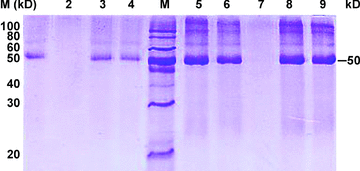
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) identification antibody immobilized on bacterial magnetic particles (BMPs).Lanes 1, 3, protein from BMPs and anti-prunus necrotic ring spot virus (PNRSV) antibody complex; lane 2, membrane protein of BMPs without binding anti-PNRSV antibody; lane 4, standard anti-PNRSV antibody. M, protein marker; lane 5, standard anti-grapevine fanleaf virus (GFLV) antibody; lanes 6, 8, 9, protein from BMPs and anti-GFLV antibody complex; lane 7, membrane protein of BMPs without binding anti-GFLV antibody.
Fluoroimmunoassay using BMPs and DAS-ELISA for detecting the fruit tree viruses of PNRSV and GFLV
The BMPs-Ab complex (anti-PNRSV antibody or anti-GFLV antibody conjugated onto BMPs) was applied for the detection of PNRSV or GFLV, respectively. In fluoroimmunoassay, the fluorescence intensity was determined for each reactant at the excitation wavelength (492 nm) and the emission wavelength (512 nm) with 10 mmol/L Tris-Cl used as a resuspension buffer. Although the two viruses had different relative fluorescence intensities, the tendency was similar, and a linear relationship was obtained between the relative fluorescence intensity and antigen concentration in the range of 1 × 10−6–1 × 10−3 (Figures 2,3). Consequently, the relative fluorescence intensity decreased as the antigen concentration increased. The minimum detectable antigen (PNRSV or GFLV) concentration was a 1 × 106 dilution of the original positive control.
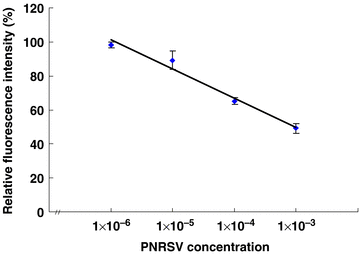
The correlation of relative fluorescence intensity and prunus necrotic ring spot virus (PNRSV) concentration.
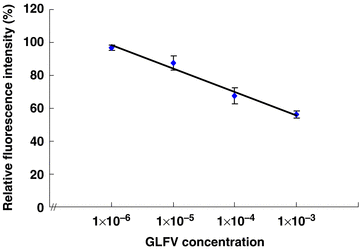
The correlation of relative fluorescence intensity and grapevine fanleaf virus (GFLV) concentration.
Double antibody sandwich enzyme linked immunosorbent assay (DAS-ELISA) for the detection of PNRSV, when the absorbance of a negative control sample at 405 nm was 0.231 3 (OD405 nm= 0.231 3), and with the positive/negative level set at ≥2.1, the positive level would be OD405 nm≥ 0.485 7. Therefore, the original concentration of the PNRSV positive control was detectable. Similarly, for detection of GFLV, the absorbance of a negative control sample at 405 nm was 0.242 1 (OD405 nm= 0.242 1), and with the positive/negative level set at ≥2.1, the positive level would be OD405 nm≥ 0.508 4. Consequently, the antigen detectable concentration with the DAS-ELISA was also the undiluted positive control of GFLV (Table 1).
| Antigen dilution times | OD405nm for PNRSV | OD405nm for GLFV | ||
|---|---|---|---|---|
| 1× | 0.8323 | + | 0.844 9 | + |
| 10× | 0.455 0 | − | 0.404 2 | − |
| 50× | 0.307 8 | − | 0.390 0 | − |
| 100× | 0.274 5 | − | 0.304 1 | − |
| 200× | 0.242 8 | − | 0.300 0 | − |
| 500× | 0.224 5 | − | 0.289 1 | − |
| 1 000× | 0.225 3 | − | 0.281 4 | − |
| 5 000× | 0.221 5 | − | 0.280 2 | − |
| 10 000× | 0.221 3 | − | 0.280 6 | − |
| Negative (N) reading | 0.231 3 | 0.242 1 | ||
| P/N level | ≥2.1 | ≥2.1 | ||
| Positive (P) levels | ≥0.485 7 | ≥0.508 4 | ||
- +, detectable; −, undetectable.
To detect PNRSV or GFLV, the two different methods described above clearly showed different detection sensitivities. The BMP-based method was much more sensitive (106 times) than the ELISA-based method.
Discussion
ELISA is a common method widely used in many fields, for example, as a pesticide residue monitor in food and water, to assess environmental contamination, and for phytosanitary, and pathological diagnoses. Here, however, when PNRSV and GFLV were detected, the detection sensitivity of ELISA was quite inferior to a BMP-based method. High detection sensitivities using BMPs have been also obtained with other substances (Nakamura et al. 1991; Nakamura and Matsunaga 1993; Nakamura et al. 1993; Tanaka and Matsunaga 2000). As described here, the BMP-based detection method could be used in quarantine, to avoid importing pests and diseases, which may cause serious damage for humans, animals or plants, and to avoid the spread of existing viruses or diseases, particularly since the high detection sensitivity of using BMPs should permit discovery of infected material at an early stage.
When BMP is used, not only can it have high antigen detection sensitivity, it also can shorten the detection time because BMP can be controlled by an applied magnetic field where it can accelerate the antibody and antigen reaction in order to finish the reaction in a shorter time (Nakamura et al. 1993). BMPs may therefore be especially useful in quarantine pest detection situations that need to make diagnoses with high sensitivity, reliability, rapidity, and cost-effectiveness. Taking into consideration the financial costs, practicality, and the importance of substance, BMPs may provide a precise high detection sensitivity to plant viruses in the field, early disease diagnosis, and specific site chemotherapy (Matsunaga 1991; Sun et al. 2007).
Materials and Methods
Preparation of BMPs and antibodies
Magnetotactic bacteria (Magnetospirillum gryphiswaldense MSR-1) were cultured for 2–3 d in a BIOFLO 110 fermenter/Bioreactor (New Brunswick Scientific Co., Inc. Edison, NJ, USA) containing 5 L medium at pH 7.5, 30 °C, with the culture conditions of 1% dissolved oxygen, 300 r/min rotor speed. The bacteria were collected by centrifugation at 5.96 × 1 000 g for 10 min, and then resuspended in 10 mmol/L HEPES-buffered saline (HeBS, pH 7.4) with a weight-to-volume ratio of 1:10, and broken by an ultrasonic cell disrupter (Scientz, JY92-II. Xinzhi Bio-techniques Ltd., Ningbo, China). BMPs were collected by a neodymium iron boron magnet (Nd-Fe-B magnet, ϕ29 × 8 mm), and purified by 20 washes with 10 mmol/L HEPES (pH 7.4), and then modified by SPDP (N-succinimidyl 3-(2-pyridyldithio) propionate) (Pierce Chemical Co., Rockford, IL, USA). SPDP-modified BMPs were collected using an Nd-Fe-B magnet, and washed four times in phosphate-buffered saline (PBS) (pH 7.4) for removing unbound SPDP (Nakamura et al. 1991; Nakamura and Matsunaga 1993; Nakamura et al. 1993).
Anti-PNRSV antibody and anti-GFLV antibody (Agdia Inc., Elkhart, IN, USA) was reduced using dithiothreitol (DTT) (Promega Chemical Co., Madison, WI, USA). A mixture of 0.2 mL antibody (2 mg/ml) and 50 μL dithiothreitol (DTT) (0.2 M) was incubated for 2 h at 23 °C. After the incubation, the reduced antibody was desalted by chromatography on a NAP-10 column (Nakamura et al. 1991; Nakamura and Matsunaga 1993; Nakamura et al. 1993).
Conjugating DTT-reduced antibody onto SPDP-modified BMPs
Anti-PNRSV antibody or anti-GFLV antibody 61 μL (containing 4.93 μg) was reduced by DTT. The reduced antibody was added into 50 μg SPDP-modified BMPs in 200 μL Na3PO4 (10 mmol/L, pH 5.6) conjugation buffer. The mixture was suspended in a sonication bath (KQ218 sonication cleaner, Sonication Instrument Co. Ltd, Zhejiang, China). The reactants were then placed on a shaker at 200 r/min, at 23 °C for 1 h. The BMPs-antibody (BMPs-Ab) was collected by Nd-Fe-B magnet, and then washed four times in PBS (pH 7.4) for removing free antibody.
SDS-PAGE identification of immobilized antibody
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (through a 5% stacking gel and a 12% separating gel) was carried out for identification of antibodies (anti-PNRSV antibody and anti-GFLV antibody), which were bound on the BMPs. Prior to loading, buffer (25 μL) was added to the BMPs-Ab complex and standard antibody, respectively, boiled for 5 min, and the reactants were then centrifuged for 5 min at 2.71 × 1 000g to remove sediments. Sample supernatant (20 μL) was loaded on the gel, and then the gel was stained by Coomassie Brilliant Blue R250.
FITC labeling the immobilized antibody
Bacterial magnetic particle-antibody (50 μg) was suspended in 0.5 mL borate buffer (0.5 M, pH 9.2). Fluorescein isothiocyanate (FITC) (25 μg) was added and the resultant mixture was incubated on a shaker for 2 h at 260 r/min, at room temperature. After incubation, FITC-conjugated BMPs-Ab was collected using an Nd-Fe-B magnet, and washed four times in PBS (pH 7.4) to remove free FITC.
Antibody and antigen reaction
Prunus necrotic ring spot virus and grapevine fanleaf virus-positive controls (antigens) (Agdia Incorporaed, Elkhart, IN, USA) were dissolved and centrifuged according the manufacturer's instructions. The supernatant contained the original concentration of the positive control, and was then diluted with PBS. One hundred microliters of each dilution was added to the appropriate test tube containing 50 μg FITC-labeled BMPs-Ab complexes, and suspended using a sonication bath, with three replicates of each dilution. The reactants were incubated on an Nd-Fe-B magnet, at 37 °C for 15 min (Nakamura et al. 1991; Nakamura and Matsunaga 1993; Nakamura et al. 1993).
PNRSV and GFLV detection
Before carrying out the fluoroimmunoassay, the reactant volume was brought to 1 mL with 10 mmol/L Tris-Cl (pH 8.0), resuspended several times, transferred to a 10 × 10 mm quarts curette, and left to stand for 15 min at room temperature. The reactants were then determined by fluorescence intensity at an excitation wavelength of 492 nm, and an emission wavelength of 513 nm. The relative fluorescence intensity (the percentage of fluorescence intensity with antigen and without antigen) was calculated after the determination.
The same lot of PNRSV and GFLV positive solution used in the BMP-based method was used in ELISA, following the manufacturer's instructions to detect PNRSV and GFLV by DAS-ELISA.
(Handling editor: Xiaoquan Qi)
Acknowledgements
The authors are grateful to Professor Wayne H. Loescher at Michigan State University for his critical review of the manuscript.




