Involvement of Polyamine Oxidase in Abscisic Acid-induced Cytosolic Antioxidant Defense in Leaves of Maize
Supported by the Science Foundation for New Teachers of Doctoral Subject Point of the Ministry of Education of China (20070307018).
Abstract
Using pharmacological and biochemical approaches, the role of maize polyamine oxidase (MPAO) in abscisic acid (ABA)-induced antioxidant defense in leaves of maize (Zea mays L.) plants was investigated. Exogenous ABA treatment enhanced the expression of the MPAO gene and the activities of apoplastic MPAO. Pretreatment with two different inhibitors for apoplastic MPAO partly reduced hydrogen peroxide (H2O2) accumulation induced by ABA and blocked the ABA-induced expression of the antioxidant genes superoxide dismutase 4 and cytosolic ascorbate peroxidase and the activities of the cytosolic antioxidant enzymes. Treatment with spermidine, the optimum substrate of MPAO, also induced the expression and the activities of the antioxidant enzymes, and the upregulation of the antioxidant enzymes was prevented by two inhibitors of MPAO and two scavengers of H2O2. These results suggest that MPAO contributes to ABA-induced cytosolic antioxidant defense through H2O2, a Spd catabolic product.
The phytohormone abscisic acid (ABA) controls many important aspects of plant growth and development and regulates plant adaptive responses to various adverse environmental conditions (Zhu 2002). An increasing body of evidence indicates that one mode of the actions of ABA is related to oxidative stress in plant cells. It has been documented that ABA can induce the expression of antioxidant genes encoding superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) (Guan and Scandalios 1998; Guan et al. 2000; Park et al. 2004), and enhance the activities of these antioxidant enzymes in plant tissues (Bueno et al. 1998; Bellaire et al. 2000; Jiang and Zhang 2001, 2002a,b, 2003). ABA-induced hydrogen peroxide (H2O2) production originating from NADPH oxidase plays an important role in the ABA signal transduction pathway leading to the induction of antioxidant defense systems (Jiang and Zhang 2002a,b, 2003; Hu et al. 2005). A recent study showed that H2O2 accumulation induced by ABA only occurred in apoplast in leaves of maize plants, and the accumulation of apoplastic H2O2 is involved in the induction of the cytosolic antioxidant enzymes (Hu et al. 2005). Zhu et al. (2006) reported that exogenous ABA treatment can lead to significant increases in apoplastic H2O2 accumulation through upregulating the activities of the plasma-membrane NADPH oxidase, cell wall peroxidase (POD) and apoplastic polyamine oxidase (PAO). In addition to NADPH oxidase, however, whether other apoplastic sources of H2O2 are involved in the ABA-induced antioxidant defense remains to be determined.
Polyamine oxidase is highly expressed, particularly in monocots. PAO bears a non-covalently bound molecule of flavorprotein (FAD) as a cofactor and catalyses the oxidation of spermine (Spm), spermidine (Spd) and/or their acetylated derivatives at the secondary amino groups (Cona et al. 2006). The PAO from maize seedlings (MPAO) is the most studied member of this enzyme class. In plants, the production of hydrogen peroxide (H2O2) deriving from polyamine oxidation has been correlated with cell wall maturation and lignification during development as well as with wound-healing and cell wall reinforcement during pathogen invasion (Cona et al. 2005; Angelini et al. 2008). As a signal molecule, H2O2 derived from polyamine oxidation mediates cell death, the hypersensitive response and the expression of defense genes (Yoda et al. 2006). However, the specific contribution of MPAO to ABA-induced H2O2 accumulation and the effect of MPAO to subcellular compartment antioxidant defense are not yet clear.
In this study, an effort was made to elucidate the contribution of MPAO in ABA-induced H2O2 production and ABA-induced cytosolic antioxidant defense. The results of the present study showed that MPAO contributes to ABA-induced cytosolic antioxidant defense through H2O2, a product of Spd oxidation in leaves of maize seedlings.
Results
MPAO contributes to ABA-induced H2O2 generation in maize leaves
Abscisic acid treatment resulted in an enhancement in the transcript level of MPAO. The time-course analysis of MPAO gene expression showed that the transcript level of MPAO reached the maximum value after 20 min of ABA treatment, and returned to the control level after 30 min (Figure 1A). On the other hand, ABA treatment also resulted in the enhancement in the activity of apoplastic MPAO, which reached the maximum value after 0.5 h of ABA treatment (Figure 1B).
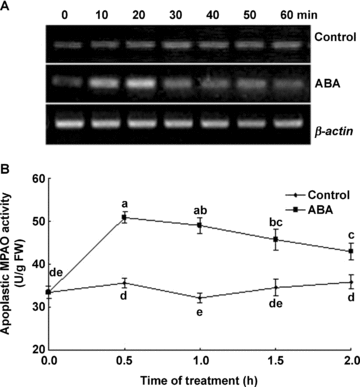
Time course of changes in the expression of MPAO gene and the activities of apoplastic maize polyamine oxidase (MPAO) in leaves of detached maize plants exposed to 100 μM abscisic acid (ABA) treatment.(A) The transcript levels of MPAO gene analyzed by reverse transcription-polymerase chain reaction (RT-PCR).(B) The activities of apoplastic MPAO.In (A), experiments were repeated at least three times with similar results. In (B), values are means ±SE (n= 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Previous studies showed that ABA only induced the apoplastic H2O2 accumulation in maize leaves (Hu et al. 2005, 2006). To test a possible effect of MPAO on H2O2 accumulation in the leaves of maize plants exposed to ABA, a histochemical method for H2O2 detection was used. Under control conditions, no visible H2O2 accumulation was observed (Figure 2A). ABA treatment led to an obvious accumulation of H2O2 (Figure 2B). Pretreatment with the MPAO inhibitors guazatine (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L) and 1, 12-diaminododecane (DADD) (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L), partly blocked the accumulation of H2O2 induced by ABA (Figure 2C,D).
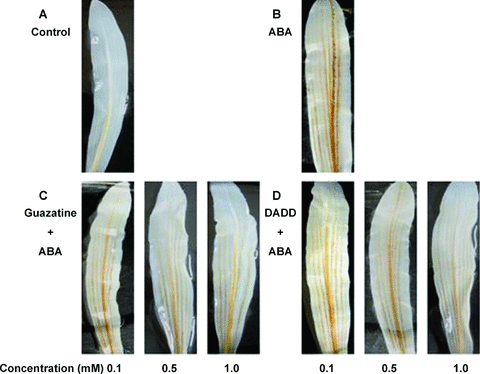
Histochemical detection of H2O2 with 3,3′-diaminobenzidine tetrachloride (DAB) staining in maize leaves.(A) The detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control.(B–D) The detached plants were pretreated with distilled water, guazatine (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L) or 1, 12-diaminododecane (DADD) (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L) for 8 h, respectively, and then exposed to 100 μM abscisic acid (ABA) treatment for 2 h. All experiments were repeated at least five times with similar results.
The role of MPAO in the accumulation of H2O2 induced by ABA was further examined by a cytochemical technique with cerium chloride (CeCl3), which reacts with H2O2 to produce electron-dense deposits of cerium perhydroxides (Bestwick et al. 1997). In the control leaves, no CeCl3 deposits, indicative of the accumulation of H2O2, were observed in the mesophyll cells (Figure 3A). Treatment with ABA led to H2O2 accumulation in apoplast of the mesophyll cells, and the greatest accumulation of H2O2 was observed in the cell walls facing intercellular spaces (Figure 3B). Pretreatment with the MPAO inhibitors guazatine (1 mmol/L) and DADD (1 mmol/L) significantly abolished the H2O2 accumulation detected with the CeCl3 staining (Figure 3C,D). Moreover, treatments with guazatine and DADD alone did not affect H2O2 production in control leaves (data not shown).
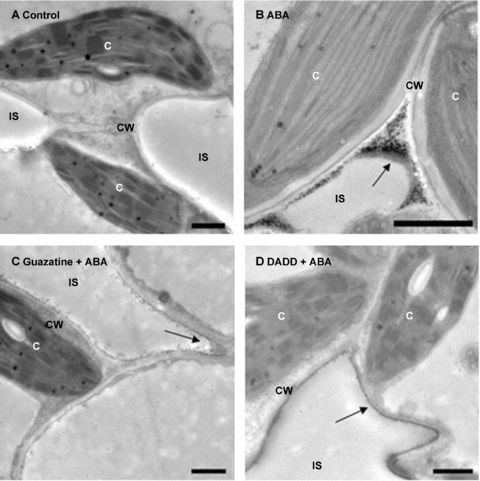
Cytochemical localization of H2O2 accumulation in mesophyll cells of maize leaves by transmission electron microscopy after CeCl3-staining.(A) The detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control.(B–D) The detached plants were pretreated with distilled water, 1 mmol/L guazatine or 1 mmol/L 1, 12-diaminododecane (DADD) for 8 h, respectively, and then exposed to 100 μM abscisic acid (ABA) treatment for 2 h. All experiments were repeated at least three times with similar results. Arrows indicate CeCl3 precipitates. C, chloroplast; CW, cell wall; IS, intercellular space. Bar, 1 μm.
MPAO is involved in ABA-induced cytosolic antioxidant defense in maize leaves
Previous study has shown that ABA can increase the activities of cytosolic antioxidant enzymes such as SOD and APX (Hu et al. 2005). In order to determine whether MPAO contributes to ABA-induced cytosolic antioxidant defense, the MPAO inhibitors guazatine and DADD were used. Pretreatments with two inhibitors partly blocked increases in the transcript levels of the antioxidant genes cytosolic ascorbate peroxidase (cAPX), encoding a cytosolic isoform of ascorbate peroxidase, and superoxide dismutase 4 (SOD4), encoding a cytosolic isoform of superoxide dismutase (Figure 4A), and the activities of cytosolic APX (Figure 4B) and SOD (Figure 4C) induced by ABA treatment in the leaves of maize seedlings, and the inhibition of these parameters exhibited a dose-dependent manner, but guazatine and DADD alone had no effect (data not shown), suggesting that MPAO is involved in ABA-induced cytosolic antioxidant defense in maize leaves.
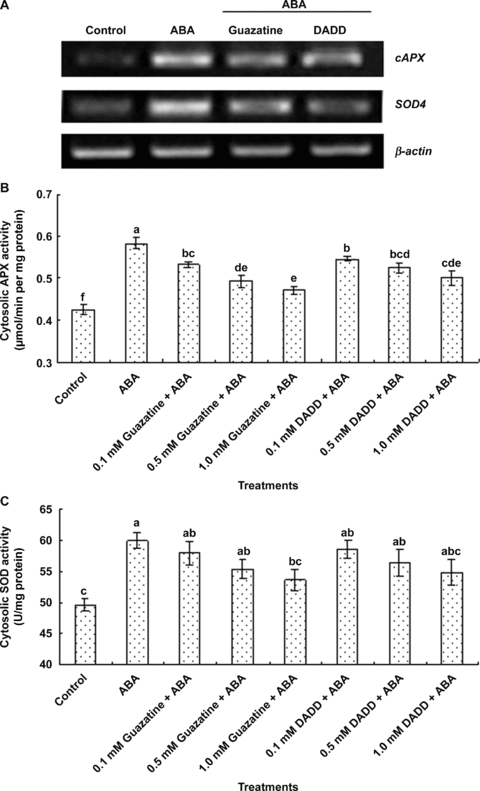
Effects of pretreatments with the polyamine oxidase (PAO) inhibitors guazatine and 1, 12-diaminododecane (DADD) on abscisic acid (ABA)-induced expression and activities of cytosolic antioxidant enzymes in maize leaves.(A) The transcript levels of antioxidant genes cAPX and SOD4 analyzed by reverse transcription-polymerase chain reaction (RT-PCR).(B–C) The activities of the cytosolic antioxidant enzymes ascorbate peroxidase (APX) and superoxide dismutase (SOD), respectively.In (A), the detached maize plants were pretreated with 1 mmol/L guazatine and 1 mmol/L DADD for 8 h, and then exposed to ABA treatment for 8 h. In (B) and (C), the detached maize plants were pretreated with different concentrations of guazatine and DADD for 8 h, and then exposed to ABA treatment for 12 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control. In (A), experiments were repeated at least three times with similar results. In (B) and (C), the values are means ±SE (n= 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Exogenous Spd increases cytosolic antioxidant defense in maize leaves
To further identify the effect of MPAO on the cytosolic antioxidant defense in maize leaves, the detached plants were treated with Spd, the optimum substrate of MPAO, and then the leaves were analyzed after 4, 8, 12 and 24 h of treatment. As shown in Figure 5A, the transcript of cAPX started to accumulate after 4 h of Spd treatment and reached the highest levels at 12 h, and the Sod4 transcript increased in response to Spd within 4 h and reached its maximum value after 8 h of Spd treatment. Moreover, Spd treatment led to significant changes in the activities of antioxidant enzymes APX and SOD in cytosol. A marked increase in the activities of SOD (Figure 5C) and APX (Figure 5B) occurred within the 4 or 12 h of Spd treatment, respectively. Treatment with Spd for 12 h enhanced the activities of cytosolic APX and SOD by 28% and 21%, compared with the control values.
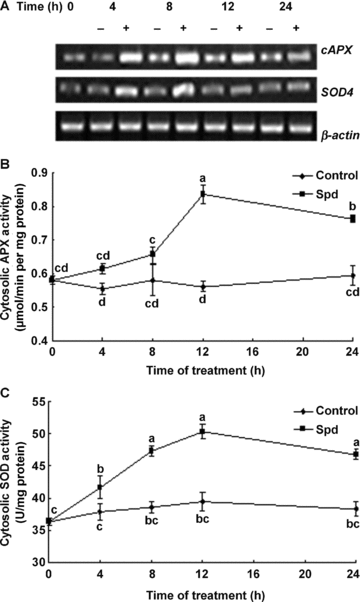
Time course of changes in the expression and the activities of the cytosolic antioxidant enzymes in leaves of detached maize plants exposed to 1 mmol/L spermidine (Spd) treatment.(A) The transcript levels of antioxidant genes cAPX and SOD4 analyzed by reverse transcription-polymerase chain reaction (RT-PCR).(B–C) The activities of the cytosolic antioxidant enzymes ascorbate peroxidase (APX) and superoxide dismutase (SOD), respectively. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control.In (A), experiments were repeated at least three times with similar results. In (B) and (C) values are means ±SE (n= 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
In order to determine whether Spd-induced increases in the activities of cytosolic antioxidant enzymes result from MPAO-catalyzed H2O2 production, the detached plants were pretreated with the MPAO inhibitors guazatine and DADD and the H2O2 scavengers CAT and dimethylthiourea (DMTU), respectively, and then exposed to Spd treatment. Pretreatment with guazatine, DADD, CAT and DMTU significantly blocked the increases in the transcript levels of cAPX and Sod4 (Figure 6A) and the activities of cytosolic APX (Figure 6B) and SOD (Figure 6C) induced by Spd treatment, and the inhibition exhibited a dose-dependent manner, but these inhibitors or scavengers alone had no effect on the transcript levels and the activities of antioxidant enzymes (data not shown), suggesting that MPAO induces cytosolic antioxidant defense through H2O2, a Spd catabolic product in maize leaves.
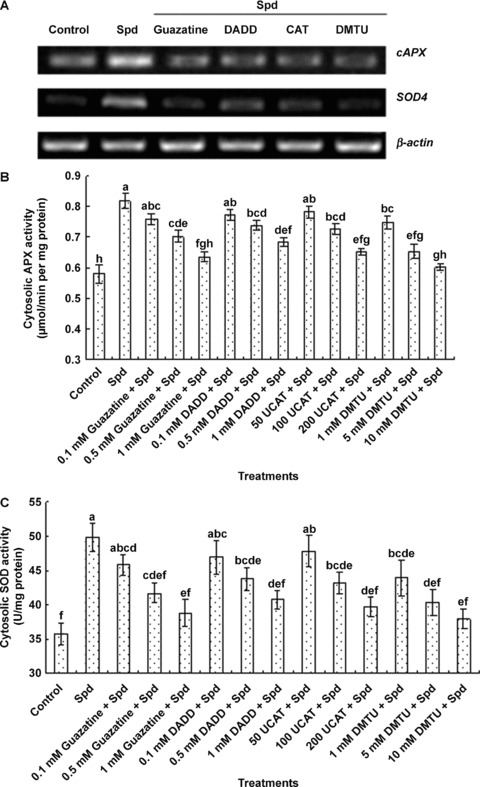
Effects of pretreatments with the polyamine oxidase (PAO) inhibitors guazatine and 1, 12-diaminododecane (DADD) and the H2O2 scavengers dimethylthiourea (DMTU)and catalase (CAT) on spermidine (Spd)-induced expression and activities of cytosolic antioxidant enzymes in maize leaves.(A) The transcript levels of antioxidant genes cAPX and SOD4 analyzed by reverse transcription-polymerase chain reaction (RT-PCR).(B–C) The activities of the cytosolic antioxidant enzymes ascorbate peroxidase (APX) and superoxide dismutase (SOD), respectively.In (A), the detached maize plants were pretreated with 1 mmol/L guazatine, 1 mmol/L DADD, 200 U CAT and 10 mmol/L DMTU for 8 h, respectively, and then exposed to Spd treatment for 8 h. In (B) and (C), the detached maize plants were pretreated with different concentrations of guazatine, DADD, CAT and DMTU for 8 h, respectively, and then exposed to Spd treatment for 12 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control. In (A), experiments were repeated at least three times with similar results. In (B) and (C) values are means ±SE (n= 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Discussion
There are many potential sources of reactive oxygen species (ROS), including intracellular sources such as chloroplasts, mitochondria and peroxisomes, plasma membrane NADPH oxidases, cell wall peroxidases, germine-like oxalate oxidases and amine oxidases in plant cells (Mittler 2002; Neill et al. 2002; Mittler et al. 2004). It has been well documented that ABA can cause the increased generation of H2O2, which only occurred in apoplast in leaves of maize seedlings (Jiang and Zhang 2001; Hu et al. 2005, 2006). NADPH oxidases have been shown to be a major source of H2O2 production in ABA signaling (Jiang and Zhang 2002a, 2003; Kwak et al. 2003; Hu et al. 2005). A recent study suggests that exogenous ABA treatment can lead to significant increases in H2O2 accumulation in apoplast through upregulating the activities of the plasma-membrane NADPH oxidase, cell wall POD and apoplastic PAO (Zhu et al. 2006). In the present study, our results showed that pretreatments with the MPAO inhibitors guazatine and DADD had a marked effect on ABA-induced H2O2 accumulation. The MPAO inhibitors significantly reduced the ABA-induced accumulation of H2O2 detected by 3,3-diamino-benzidine (DAB) (Figure 2) and CeCl3 staining (Figure 3). Moreover, exogenous ABA supplied to maize seedlings resulted in not only the enhancement in the activity of apoplastic MPAO but also the enhancement in the transcript level of MPAO (Figure 1). These results suggest that MPAO has an important contribution to ABA-induced H2O2 production in leaves of maize plants.
Superoxide dismutase and APX are two key enzymes of the removal of ROS. These antioxidant enzymes have many isoenzymes, and are located in different subcellular compartments (Mittler 2002; Mittler et al. 2004). Previous studies have demonstrated that ABA induces the total activities of antioxidant enzymes such as SOD and APX and the expression of antioxidant enzyme genes, and the enhancement in the expression and the activities of these antioxidant enzymes is associated with the increased production of ROS in leaves of maize plants exposed to ABA treatment (Guan and Scandalios 1998; Jiang and Zhang 2002a,b; Zhang et al. 2006). Moreover, ABA treatment induced apoplastic H2O2 production, then significant increases in the expression of antioxidant enzyme genes cAPX and SOD4, and the activities of the cytosolic antioxidant enzymes SOD and APX (Hu et al. 2007). However, it is not clear whether MPAO regulates the subcellular activities of antioxidant enzymes. In the present study, we selected the whole leaf cytosol as a model to investigate the effects of MPAO on the activities of antioxidant enzymes in the cellular compartments. Pretreatments with the MPAO inhibitors guazatine and DADD partly blocked increases in the transcript levels of cAPX and SOD4 and the activities of cytosolic APX and SOD induced by ABA (Figure 4). These results suggest that MPAO is involved in ABA-induced cytosolic antioxidant defense in maize leaves.
Plant amine oxidases contribute to important physiological processes through its reaction products H2O2. Exogenous Spm treatment induces the expression of alternative oxidases (AOXs) genes, causes mitochondrial dysfunction via a signaling pathway in which ROS is involved in tobacco leaves (Takahashi 2003). Agazio and Zacchini (2001) reported that treatment with Spd caused a strong increase of APX activity and gene expression, which was induced to a lesser extent by removing Spd-genetated H2O2 by DMTU in maize roots. Our results confirmed the findings of previous studies. Spd treatment resulted in an enhancement in transcript levels of two antioxidant genes cAPX and SOD4, and the activities of cytosolic APX and SOD (Figure 5). Pretreatments with the scavengers for H2O2 blocked the above-mentioned change (Figure 6). These indicated that MPAO induces cytosolic antioxidant defense, at least in part, through H2O2, a Spd catabolic product.
In conclusion, our data provides evidence for the involvement of MPAO in ABA-induced apoplastic H2O2 production and cytosolic antioxidant defense. Moreover, MPAO contributes to ABA-induced cytosolic antioxidant defense through H2O2, a Spd catabolic product.
Materials and Methods
Plant material and treatments
Seeds of maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 22–28 °C, with a photosynthetic active radiation (PAR) of 200 μmol/m2 per s and a light : dark cycle of 14:10 h, and watered daily. When the second leaf was fully expanded, the plants were collected and used for all investigations.
The plants were excised at the base of the stem, and placed in the distilled water for 1 h to eliminate the wound stress. After the treatment, the cut ends of the stems were placed in the beakers wrapped with aluminum foil containing 100 μM ABA solution up to 12 h and 1 mmol/L Spd up to 24 h at 22 °C with a continuous light intensity of 200 μmol/m2 per s. In order to study the effects of inhibitors and scavengers, the detached plants were pretreated with guazatine (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L), DADD (0.1 mmol/L, 0.5 mmol/L, 1 mmol/L), CAT (50 U, 100 U, 200 U), DMTU (1 mmol/L, 5 mmol/L, 10 mmol/L) for 8 h, respectively, and then exposed to ABA or Spd treatment under the same conditions as described above. After treatments of detached maize plants, the second leaves were sampled and immediately frozen under liquid N2.
Preparation of the apoplastic fraction
Apoplastic fraction was isolated according to the method as described by Hernandez et al. (2001). Leaf segments (10 g) were soaked in deionized water, and subsequently vacuum infiltrated for 5 min at 1.0 kPa and 4 °C with 50 mmol/L K-phosphate buffer (pH 6.5) containing 0.2 M KCl and 0.1 mmol/L CaCl2. Leaves were then quickly dried and centrifuged at 1 000g for 5 min at 4 °C in a 25 mL-syringe barrel placed in a centrifuge tube.
Preparation of cytosolic fraction
Cytosolic fraction was isolated according to the method as described by Yang and Komatsu (2000). Leaf segments (0.5 g) were homogenized in a mortar and pestle in 5 mL homogenization buffer containing 50 mmol/L phosphate buffer (pH 7.5), 0.25 M sucrose, 10 mmol/L ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mmol/L dithiothreitol (DTT) and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged at 10 000g for 10 min. The supernatants were centrifuged at 100 000g for 1 h. The resulting supernatant is referred to as the cytosolic fraction. On the basis of the content of chlorophyll, the contamination of chloroplasts was about 3%.
Enzyme assays
The activities of MPAO in the apoplast were determined as described by Liu and Liu (2004). Reaction solution (3.0 mL) contained 2.5 mL 0.1 mol/L sodium phosphate buffer (pH 6.5), 0.1 mL crude enzyme extracts, 0.1 mL peroxidase (250 U/mL), and 0.2 mL 4-aminoantipyrine/N,N′-dimethylaniline. The reaction was initiated by the addition of 0.1 mL 20 mmol/L Spd for the determination of PAO activity. A 0.01 change in the absorbance value at 555 nm was regarded as one enzyme activity unit.
The activities of antioxidant enzymes in the cytosol were determined as previously described (Jiang and Zhang 2001). SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of nitro blue tetrazolium as monitored at 560 nm. APX activity was measured by monitoring the decrease in absorbance at 290 nm as ascorbate was oxidized.
Protein content was measured following the method of Bradford (1976), with bovine serum albumin (BSA) as a standard.
Histochemical detection of H2O2
H2O2 was visually detected in the leaves of plants by using DAB as substrate (Orozco-Cardenas and Ryan 1999). Briefly, plants were excised at the base of stems with a razor blade and supplied through the cut stems with a 1 mg/mL solution of DAB (pH 3.8) for 8 h under light at 25 °C, and then exposed to distilled water, guazatine or DADD plus ABA treatments. After these treatments, the second leaves were decolorized by immersion of leaves in boiling ethanol (96%) for 10 min. This treatment decolorized the leaves except for the deep brown polymerization product produced by the reaction of DAB with H2O2. After cooling, the leaves were extracted at room temperature with fresh ethanol and photographed.
Cytochemical detection of H2O2
H2O2 was visualized at the subcellular level using CeCl3 for localization (Bestwick et al. 1997). Electron-dense CeCl3 deposits are formed in the presence of H2O2 and are visible by transmission electron microscopy. Tissue pieces (approximately 1–2 mm2) were excised from the treated and untreated leaves and incubated in freshly prepared 5 mmol/L CeCl3 in 50 mmol/L 3-(N-morpholino) propanesulfonic acid (Mops) at pH 7.2 for 1 h. The leaf sections were then fixed in 1.25% (v/v) glutaraldehyde and 1.25% (v/v) paraformaldehyde in 50 mmol/L sodium cacodylate buffer, pH 7.2, for 1 h. After fixation, tissues were washed twice for 10 min in the same buffer and postfixed for 45 min in 1% (v/v) osmium tetroxide, and then dehydrated in a graded ethanol series (30%–100%; v/v) and embedded in Eponaraldite (Agar Aids, Bishop's Stortford, UK). After 12 h in pure resin, followed by a change of fresh resin for 4 h, the samples were polymerized at 60 °C for 48 h. Blocks were sectioned (70–90 nm) on a Reichert-Ultracut E microtome, and mounted on uncoated copper grids (300 mesh). Sections were examined using a transmission electron microscope at an accelerating voltage of 75 kV.
Isolation of total RNA and reverse transcription- polymerase chain reaction
Total RNA was isolated from leaves using the RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the instructions supplied by the manufacturer. Approximately 3 μg of total RNA was reverse-transcribed using an oligo (dT) primer and SuperScript Reverse Transcriptase (Invitrogen, Carlesbad, CA, USA). cDNA was amplified by polymerase chain reaction (PCR) using the following primers: cAPX: forward 5′-TCACACCCTGGGAAGATG-3′ and reverse 5′-GCTTCATATCAAACCTTCTCC-3′; SOD4: forward 5′-GGGCACAATCTTCTTCACC-3′ and reverse 5′-GTCCGATGATCCCACAAG-3′; MPAO: forward 5′-AGCAGACCAAGGCGGAGAT-3′ and reverse 5′-TGATACCTGAAAGATAGGCTCCAT-3′; β-actin: forward 5′-GCGAACAACTGGTATTGTG-3′ and reverse 5′-CATCTGCTGCTGAAAAGTG-3′. To standardize the results, the relative abundance of β-actin was also determined as the internal standard.
The cycle number of the PCR reactions was adjusted for each gene to obtain barely visible bands in agarose gels. Aliquots of the PCR reactions were loaded on agarose gels and stained with ethidium bromide.
Statistical analysis
The results presented were the mean of three replicates in the enzyme assays. Means were compared by one-way anova and Duncan's multiple range test at 5% level of significance.
(Handling editor: Jianhua Zhang)




