In vitro Transient Expression System of Latex C-serum was used for Analysis of Hevein Promoter in Response to Abscisic Acid in Hevea brasiliensis
Supported by the National Natural Science Foundation of China (39960065).
Abstract
Hevein has been found to be an essential element in coagulation of rubber particles in latex of rubber trees. In a previous study, we cloned a 1 241-bp fragment of a 5′ upstream region of the hevein gene by genome walking. This fragment was analyzed by a 5′ end nested deletion method in the present study, fused with a uidA (gus) gene to produce a series of tested constructs, which were transferred into C-serum of latex and the Gus activities were detected. Results showed that the fragment from −749 to −292 was sufficient for expression of gus gene in latex, and the fragment from −292 to −168 was crucial in response to abscisic acid inducement. In a transient transgenic test of rubber leaf with particle bombardment, construct Hev749 conferred gus-specific expression in veins, in which the latex tubes mainly distributed. This implies that the fragment from −749 to −292 was laticiferous-specific.
Natural rubber (cis-1, 4-polyisoprene) is synthesized in at least 2 000 species of plants in the world. The rubber tree (Hevea brasiliensis) is the most important economically viable source of natural rubber mainly because of the abundance of latex in the tree (30–50% by volume of fresh latex), its excellent quality and wide distribution in tropical areas of the world (Fay and Jacob 1989). In H. brasiliensis, rubber is synthesized in specialized organelles (the rubber particles) in latex. Another specialized organelle found in latex is lutoid, which is vacuole-like in structure and function. When centrifuged, the latex is separated into three phases. The upper phase is the rubber particle, the second named C-serum includes cellular organelles, nucleus, mRNA and soluble proteins, and the lower phase named B-serum comprises mainly lutoids, however, other minor organelles are also deposited in the bottom fraction when centrifuged at high speed.
The physiological function of latex has been implicated to play a role in defense (Dussourd and Eisner 1987; Farrell et al. 1991). Kush et al. (1990) found that the transcript level of plant defense- or stress-related genes was 10- to 50-fold higher in latex than in leaves. Hydrolytic enzymes such as β-1,3-glucanase (Chye and Cheung 1995), cellulase, and polygalacturonase (Kush et al. 1990) were also highly expressed in latex. In addition, the in vitro antifungal activity of latex has been demonstrated in several species including Lactuca sativa, Asclepias curassavica, Carica papaya, and Candida albicans (Moulin et al. 1990; Giordani et al. 1991; Giordani et al. 1996; Giordani et al. 1999). Another study showed that HbASRLP1 and HbASRLP2 had high levels of accumulation exclusively in the latex (Ko et al. 2003). Putative proteins coded by these genes have an ABA–WDS domain, which is the characteristic domain of plant proteins induced by water deficit stress (WDS) or abscisic acid (ABA) stress and ripening, suggesting their roles in defense.
Hevein, a single-chain serine protease containing a 43 residue antifungal peptide (Walujono et al. 1975), is a major protein in the lutoids and has been described as an antifungal protein (Lynn Clevette-Radford 1984; van Parijs et al. 1991; Koo et al. 1998). In the current study, hevein is shown to accumulate more than 10-fold in latex compared with in leaves (Ko et al. 2003). Currently, the molecular structure of Hevein protein is clear. It is translated first as a protein total of 204 amino acids, and then is processed into three regions, including the N-terminal region of 17 amino acid signal peptides, the midst region of 43 amino acid mature peptides and the C-terminal region of 144 amino acid polypeptides (Broekaert et al. 1990; Lee et al. 1991). Hevein has the ability to bind chitin, and Xavier Gidrol showed that it is involved in the coagulation of latex by bringing together rubber particles (Gidrol et al. 1994), which, upon tapping, burst open essentially due to the difference in turgor pressure. Rubber particles are the site for natural rubber biosynthesis, which occurs in the cytosol only. Under normal conditions Hevein does not interact with the rubber particles in situ due to its compartmentalization in the lutoids. However in a disease called tapping panel disease (TPD) or bark dryness, bursting of lutoid particles has been reported, suggesting that the bursting of lutoids may allow the rubber particles and Hevein to interact, resulting in situ coagulation. Since Hevein is localized in latex and has been reported with the accumulation of transcripts in response to the inducement of ethylene (Itzhaki et al. 1994) and ABA (Broekaert et al. 1990), there should be a latex-specific expression element (LSE) and ABA or ethylene responsive elements in its 5′ end upstream region.
In a previous study, a 1 241 bp 5′ upstream fragment was cloned by genomic walking (Gene accessible number: AF327518) (Deng et al. 2002). After that, Renand and co-workers cloned five of the hevein homologs and their promoters (Pujade-Renaud et al. 2005). The transgenic test showed that the hev2.1 promoter conferred a high level of expression to the transgene in various tissues of rice. In the present study, we were interested in the latex specific expression element of the hevein promoter, using 5′ nested deletion analysis to localize the promoter elements. As a result, a 457-bp fragment between −749 and −292 of the hevein promoter was found to account for the latex specific expression, and a fragment from −292 to −168 was essential for the inducible expression in response to plant hormone ABA.
Results
The fragment between −749 to −292 was essential for regulating hevein gene-specific expression in latex
Latex is a kind of protoplast, containing rubber particles and all of the components for synthesis of new protein (Gomez 1982; Goncalves et al. 2005), which has the advantage of expressing promoters and foreign genes. Due to the effect of the coagulation of the rubber particles on the analysis of the reporter gene, the latex expression system should be optimized by keeping the rubber particles as few as possible while keeping the organelles for transcription and transformation. Plasmid pBI121, which contains the gus gene, was used to optimize the centrifuge condition. When centrifuging for less than 1 h at 10 000g, there were still considerable rubber particles left in the C-serum. While on the contrary, Gus activities were not detectable when centrifuging for 2 h at 10 000g and 15 000g, implying that the apparatus-like nucleolus and ribosome, which account for protein production, were in the bottom of the tube although rubber particles were completely excluded. Only when centrifuged at 10 000g for 1 h could most of the rubber particles be excluded. The contents that are involved in gene transcription, exporting of mRNA, mRNA splitting and modification, and gene translation were still in the C-serum (Table 1). Therefore, the latex C-serum was used for in vitro gene expression after being centrifuged at 10 000g for 1 h.
| Number | Centrifuge speed and timeb | Concentration of rubber particles in C-serumc | Gus activity detectabled | Evaluation |
|---|---|---|---|---|
| 1 | 10 000g, 20 min | 0.38 g/mL | Yes | Rubber particles will affect the expression of the reporter gene |
| 2 | 10 000g, 40 min | 0.20 g/mL | Yes | Rubber particles will affect the expression of the reporter gene |
| 3 | 10 000g, 1 h | 0.14 g/mL | Yes | Can be used as expression system |
| 4 | 10 000g, 2 h | 0.08 g/mL | No | No |
| 5 | 15 000g, 2 h | 0.02 g/mL | No | No |
- aFresh latex was used for latex C-serum separation; bcentrifuges were at 4 °C; cthe detection of the concentration of rubber particles was repeated three times; deach reaction was comprised of 250 μL C-serum, 250 μL 2.5 Mm X-Gluc, and 0.25 μg plasmid Hev749.
To confirm the effectual concentrations of ABA for the inducement expression of hevein, a series of gradient dilutions of ABA were tested. As Figure 1 shows, the C-serum without Hev749 will reach high Gus activities in 3.8 mM and 19 mM ABA compared with in 0.38 mM ABA. Therefore, 0.38 mM ABA was the ideal concentration for inducement, which was then used for inducement of the reporter gene expression.
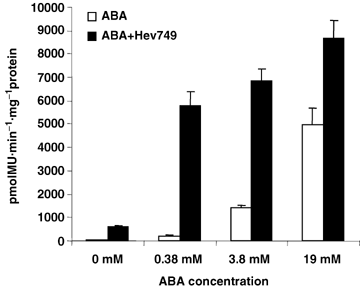
Quantitative measurement of the Gus activities in C-serums with different concentration of abscisic acid (ABA) inducement.After 24 h incubation of construct Hev749 in latex C-serum with different concentrations of ABA, 4-methylumbelliferyl-β-D-glucuronide was added as a substrate. Bars represent average ratio of Gus activity in arbitrary units from five independent measurements.
To localize the LSE in the 5′ upstream region of the hevein gene, we produced a series of 5′ nested deletion constructs including Hev1184, Hev749, Hev292, Hev168 and Hev63 (Figure 2). These constructs contained the 5′ upstream fragment from position −1 184, −749, −292, −168 and −63 to +56, respectively, fusing to the gus gene of pBI121, which then mixed to latex C-serum without rubber particles in vitro for 24 h. The Gus activities had been tested by fluorometric assay with 4-methylumbelliferyl-β-D-glucuronide added as a substrate. Data showed that latex C-serums containing constructs Hev1184 and Hev749 permitted LSE of the gus gene. The Gus activities were 566.7 pmolMU·min−1·mg−1protein and 592.2 pmolMU·min−1·mg−1protein (Figure 3, constructs Hev1184 and Hev749) compared with the latex C serum containing pBI121(133.4 pmolMU·min−1·mg−1protein, Figure 3 pBI121). While latex C-serums containing constructs Hev292 (113.4 pmolMU·min−1·mg−1protein), Hev168 (70.7 pmolMU·min–1·mg–1protein) and Hev63 (58.4 pmolMU·min–1·mg–1protein) (Figure 1B, constructs Hev292, Hev168 and Hev63) resulted in a loss of Gus expression in the tested samples. This showed that LSE of the hevein gene lies between positions of −749 to −292 in 5′ upstream region.
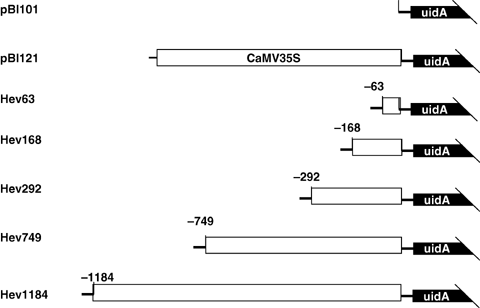
Schematic of different deletion constructs.Fragments from the position −1 184, −749, −292, −168, and −63 to +56 of the hevein 5′ upstream region fused to a uidA (gus) gene to generate tested constructs Hev1184, Hev749, Hev292, Hev168 and Hev63, respectively. These constructs were then added to latex C-serum and the Gus activities were measured.
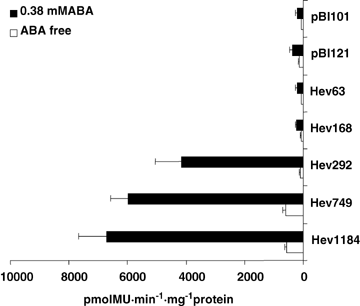
Quantitative measurement of the Gus activities in C-serums containing different constructs.Tested construct expression in latex C-serum with 0.38 mM abscisic acid (ABA) and ABA-free was measured through Gus activities. Gus activities were indicated in pmolMU/min per mg protein. Bars represent average ratio of Gus activity in arbitrary units from five independent measurements. Black bars represent the Gus activities without adding 0.38 mM ABA, while white bars represent the Gus activities without 0.38 M ABA.
The fragment between −292 to −168 was essential for ABA inducible expression
Latex C-serums containing constructs Hev1184 (6715.3 pmol MU·min–1·mg–1protein), Hev749 (5968.1 pmolMU·min–1·mg–1protein) and Hev292 (4141.6 pmolMU·min–1·mg–1protein) showed higher Gus activities in response to the inducement of ABA compared with C-serum containing pBI121 and pBI101 (350.6 and 195.7 pmolMU·min–1·mg–1protein). However, C-serum containing constructs Hev168 (220.9 pmolMU· min–1·mg–1protein) and Hev63 (196.1 pmolMU·min–1·mg–1protein) showed lower Gus activities in response with the inducement of 0.38 mM ABA. (Figure 3). This suggested that the fragments from position −292 to −168 were essential for ABA inducible expression.
Transient expression in rubber leaf
The Hev749-containing LSE element was used to analyze how hevein was expressed in the rubber tree by particle bombardment. Leaf tissues from rubber plantlets growing in the test tubes were used as explants. After bombardment, samples were incubated in Murashige and Skoog (MS) medium for 2 d, and then mixed with Gus assay buffer containing 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc) to analyze their histochemical localization. Veins of the leaves bombarded by particles coated with Hev749 appeared bright blue (Figure 4A), and there were no Gus activities detectable when bombarded by particles coated with promoterless vector pBI101 (Figure 4B). Gus activity presented in parenchyma cells but not in veins with pBI121, which contained a CaMV 35S::uidA gene (Figure 4C). Since latex is located in the latex tube in the vein, it is suggested that construct Hev749 containing the LSE element could drive Gus-specific expression in latex.
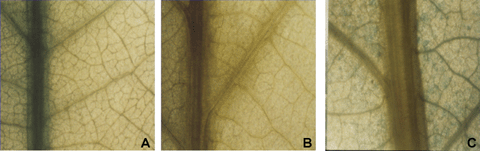
Histochemical localization of Gus activity in rubber leaf.Rubber leaf tissues were bombarded with particles coated with Hev749, pBI101and pBI121, respectively. The Gus activities were tested after samples were incubated in MS plates for 2 d.(A) Leaf tissues bombarded by particles coated with Hev749 could find the GUS expression in veins.(B) Leaf tissues bombarded by particles coated with pBI101 with promoterless uidA gene could not be found in any GUS expression in leaf tissues.(C) Leaf tissues bombarded by particles coated with pBI121 with a CaMV 35S::uidA chimeric gene could be found in GUS expression in parenchyma cells but not in veins.
Discussion
In the present study we identified a latex-specific region between −749 and −292 upstream of the hevein gene by the method of 5′ end nested analysis and using C-serum of latex as an in vitro reaction system. As listed in Table 1, the rubber particle is the main problem in preparing the in vitro reaction mixture. The lower or higher centrifuge speed would leave the rubber particle or some small organelles deposited into the bottom, which directly affects the C-serum as the in vitro reaction system.
A Database of PlantCARE (http://intra.psb.ugent.be:8080/PlantCARE) was used for cis-action element analysis. Motifs of ABA responsive element (ABRE) and ethylene responsive element (ERE) had been found in the hevein promoter region. Among them, a typical ABA response element ABRE (Marcotte et al. 1989; Godoy et al. 1990; Mundy et al. 1990; Marcotte et al. 1992; Pla et al. 1993; Straub et al. 1994) with a ggtACGTgtt motif was found in the position from −1 072 to −1 063. Although construct Hev1184 contained this motif and responded to ABA inducement (Figure 3), two other constructs, Hev749 and Hev292, also responsed to ABA inducement, implying a non typical ABRE in the region from −292 to −168. Moreover, we didn't find any typical ethylene responsive element (GCCGCC) (Sessa et al. 1995; Dongyun et al. 1998;Yamamoto et al. 1999) in the hevein promoter region, because none of the deletion constructs induced by ethylene in vitro expression (data not shown), implying that ethylene response elements should not be in the cloned hevein promoter, most possibly in the far more upstream region.
For histochemical localization of Gus activity in rubber tissue, we used rubber leaf, stem and root as material for bombardment. As latex can be easy to pour out from the latex vessel when stems or roots are cut to slides, we can only select rubber leaf as explants for bombardment. In rubber leaf, latex vessels are mainly distributed in the veins. The results show that construct Hev749 conferred specific expression of Gus in veins (Figure 4), implying that it conferred the expression of Gus in latex. The results have potential commercial use when the foreign gene specifically expressed in latex under the promoter is mediated.
As genetic transformation of the rubber tree is still difficult, successful reports exist of rubber tree genetic transformation by Agrobacterium using the immature anther-derived calli (Jayashree et al. 2003) or embryogenic calli (Montoro et al. 2003; Blanc et al. 2006) to develop transgenic plants. The technology of the transformation is complex and will take more than 5 months from the calli inducement to having transgenic plants in test tubes (Jayashree et al. 2003). The transient expression of test genes in latex C-serum supplies an alternative approach to confirm the genes or promoter functions in rubber trees. Moreover, the Hevein promoter will be useful for the production of recombinant proteins of industrial or medical interest in the extractable latex of the rubber tree, in effect, using the tree as a bio-technological factory (Yeang et al. 1998; Arokiaraj et al. 2002).
Materials and Methods
Plant material
Latex was harvested from a 20-year-old rubber tree (Hevea brasiliensis Muell. Arg. clone RRIM600) in the Chinese Agricultural Academy of Tropical Crops, Danzhou, China. Rubber plantlets derived from somatic embryos growing in test tubes were used as explants for particle bombardment. Plasmid containing the 1241 bp hevein promoter is described in a former article (Deng et al. 2002).
Preparing in vitro reaction mixture
Twenty milliliters of rubber latex was collected with a 50-mL tube on ice, and then centrifuged at different conditions. Conditions were 10 000g for 20 min, 10 000g for 40 min, 10 000g for 1 h, 10 000g for 2 h, and 15 000g for 2 h. After excluding the rubber particles carefully, the middle phase C-serum were collected and transferred to a new tube and used as an in vitro reaction mixture.
Generation of 5′ nested deletion constructs
For generating the 5′ nested deletion constructs of the hevein gene, a reverse primer Hev-BamHI-56R (5′-GTTGGATCCAACTCTTCCCATTTCTTCCCAA-3′) was combined to forward primers Hev-HindIII-1184 (5′-GGTAAGCTTAAAAATATTTTCACGTATTTTGCC-3′), Hev-HindIII-749 (5′GGTAAGCTTTGAGGCAAGAGGGTATTTCTAA-3′), Hev-HindIII-292-(5′GGTAAGCTTCTCTATATTGGGGATGGAGTGG-3′), Hev-HindIII-168 (5′-GGTAAGCTTTCAATATCACACGATCTACTTT- 3′), and Hev-HindIII-63 (5′-GGTAAGCTTACCCTGATGGATTAAAGATGA-3′) to amplify the upstream regions between positions +56 and −1 184, −749, −292, −168 and −63, respectively. These polymerase chain reaction (PCR) products were digested by HindI III and BamHI, and were then inserted into the same double-digested restriction sites of the reporter vector, pBI121 (contains gus gene), to generate chimeric constructs Hev1184, Hev749, Hev292, Hev168 and Hev63.
Fluorometric assay of Gus in latex C-serum
C-serum, the in vitro reaction mixture, the constructs tested here and ABA (if necessary) were added and incubated at room temperature for 24 h. The Gus fluorometric assay was carried out as described by Jefferson et al. (1987). The supernatant of homogenized mixture including C-serum, the tested constructs and ABA was incubated with 4-methylumbelliferyl-β- D-glucuronide (MUG) as a substrate and the amount of the reaction product, 4-methylumbelliferone (4 MU) was measured. Protein concentrations were determined according to Bradford (1976). The GUS activity was described as picomoles of 4 MU per min per mg of protein.
Transient expression of rubber leaf
Leaf tissues from rubber plantlets growing in test tubes derived from the somatic embryo were used as explants. For preparation of microcarriers, 5 μg of tested constructs were mixed with CaCl2, spermidine and 3 mg of gold powders. For each bombardment, the sample chamber was evacuated to 6.77 × 104 Pa, and the gap distance between the rupture disk and macrocarrier was 0.6 cm. Each experiment was repeated three to four times. The histochemical Gus staining was also carried out as described by Jefferson (Jefferson et al. 1987). Fixed tissues were incubated in the Gus assay buffer containing 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc) at 37 °C for 12–16 h. The reactions were stopped with 70–100% ethanol. When necessary, chlorophyll was removed from green tissues with 100% ethanol.
(Handling editor: Xiao-Ya Chen)
Acknowledgements
We would thank Professor Xiong Tin Chen for providing rubber plantlets, and Professor Huali Gao for her useful advice in writing the paper.




