Excessive Copper Induces the Production of Reactive Oxygen Species, which is Mediated by Phospholipase D, Nicotinamide Adenine Dinucleotide Phosphate Oxidase and Antioxidant Systems
Supported by the State Key Basic Research and Development Plan of China (2003CB114300 and 2006CB100100), the National Natural Science Foundation of China (30170088 and 30370120), and the Doctoral Program Foundation of the Educational Ministry of China (20020019030).
Abstract
Tobacco BY-2 suspension cells were used to study the chemical damage and its associated mechanisms caused by Cu2+. Treatment with 100 μmol/L Cu2+ generated a large amount of H2O2 and thiobarbituric acid-reactive substances (TBARS) in cells. Using phospholipase D (PLD) specific inhibitor (1-butanol) or phosphatidic acid (PA), we demonstrated that PLD plays an important role in the generation of H2O2 and TBARS. Semi-quantitative reverse-transcriptase polymerase chain reaction and enzyme activity assays with wild type and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-overexpressing BY-2 cells revealed that PLD and PA are the key factors leading to NADPH oxidase activation, which is responsible for H2O2 and TBARS production induced by Cu2+. Moreover, the content of ascorbic acid (AsA), an effective antioxidant, was sharply reduced in BY-2 cells exposed to excessive Cu2+. Furthermore, a significant downregulation of the enzymes of AsA biosynthesis and the antioxidant system was found. This evidence suggests that excessive Cu2+-elevated reactive oxygen species (ROS) production is caused by upregulated PLD that elevates the activity of NADPH oxidase and its collapsed antioxidant systems that scavenges ROS.
Copper (Cu), an essential trace element for all higher plants, has several roles in plant metabolic processes (Maksymiec 1997). However, excessive Cu2+, like many other heavy metals, is a potential hazard for plants due to its ability to catalyze the formation of harmful free radicals or lipid peroxidation (Halliwell and Gutteridge 1989; Murphy and Taiz 1995). Production of reactive oxygen species (ROS) by autoxidation and Fenton reaction accelerated by copper (Schützendübel and Polle 2002) is the main resource of harmful free radicals. These ROS possibly result in lipid peroxidation. It has been found that three different mechanisms are involved in lipid peroxidation: autoxidation, photooxidation, and enzyme catalysis via lipo- or cyclo-oxygenases (Halliwell and Gutteridge 1989; Aro et al. 1993; Feussner and Wasternack 2002). However, Quartacci et al. (2001) found that membrane-bound lipoxygenase is not activated in wheat root after Cu2+ treatment and has a minor role in the observed peroxidation of plasma membrane (PM) fatty acids. Thus, Cu2+-induced lipid peroxidation quite possibly results from ROS-caused damage.
Phosphatidic acid (PA), a catalytic product of phospholipase D (PLD) is involved in ROS productions (Zhang et al. 2003). Existing data shows that the level of PA is regulated by Cu2+ in plant chloroplast (Quartacci et al. 2000) and plasma membrane (Quartacci et al. 2001). Furthermore, Zhang et al. (2003) observed that PLD and its derived PA play critical roles in plant responses to H2O2 induced oxidative stresses. PLD is regulated by pH changes, membrane perturbation, Ca2+, polyphosphoinositides and G proteins (Pappan and Wang 1999). It has been proposed that the activation of PLD is an early and crucial step in stress-induced phospholipid hydrolysis (Lee et al. 1997; Wang et al. 2000). Recently, membrane perturbation was observed in wheat root under excessive copper levels (Quartacci et al. 2001).
Plant nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a protein homologous to mammalian gp91phox, is responsible for ROS productions in plant responses to abiotic stress, pathogen attack or elicitor stimulation (Mehdy et al. 1996). PLD possibly mediates superoxide production through elevating NADPH oxidase activity in plants (Pignocchi et al. 2003). In addition, a recent study indicated that NADPH oxidase is involved in Cu2+-induced ROS production in wheat root (Quartacci et al. 2001). Therefore, PLD and PA may participate in the generation of ROS by regulating the activity of NADPH oxidase in plants under excessive Cu2+ stress.
Antioxidant systems play an important role in regulating plant tolerance to biotic and abiotic stresses. Ascorbic acid (AsA) has been recognized not only as an efficient free radical scavenger (Pauling 1989), but also functions as a pro-oxidant under certain conditions, such as at high concentration of AsA in the presence of transition metals or dissolved oxygen (Halliwell and Gutteridge 1984). Cu2+ also regulates the activity of many antioxidant enzymes involved in the glutathione cycle and AsA metabolism, as well as the content of antioxidants (Schützendübel and Polle 2002). At high AsA concentrations, Cu (II) is rapidly reduced to Cu (I) with a concomitant formation of oxidized AsA, dehydro-(6)-ascorbic acid (DAsA). These facts indicate that the AsA and AsA antioxidant systems may play important roles in plant response to excessive copper stress.
In the present study, we investigated the regulation of PLD and NADPH oxidase in BY-2 cells under Cu2+ stress. Our results indicate that PLD is involved in Cu2+-induced ROS production through regulating NADPH oxidase activity, but PLD has no effect on AsA antioxidant systems. Furthermore, excessive Cu2+ led to the collapse of the AsA biosynthesis and AsA antioxidant systems.
Results
H2O2 and TBARS are induced by excessive Cu2+, which elevates medium pH correspondingly
After treatment with 100 μmol/L Cu2+, the amount of H2O2 in BY-2 cells was analyzed by 3,3-Diaminobenzidine (DAB) staining. The staining color became progressively darker as treatment lasted, indicating that Cu2+ treatment induced H2O2 accumulation in BY-2 cells. The level of H2O2 reached the highest level at 45 min after treatment (Figure 1A). These data was confirmed by another H2O2 assay (H2O2-TiCl4 method[0]). H2O2 production began to increase rapidly at 60 min after Cu2+-treatment. It reached to the peak between 60 and 120 min, and kept at this high level till 360 min after Cu2+-treatment, while a marginal change was observed in the control BY-2 cells (Figure 1B).
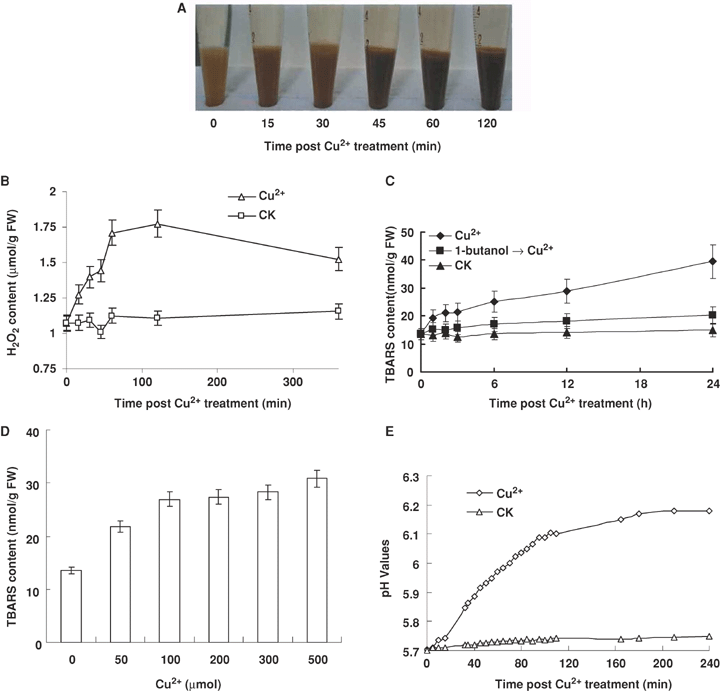
The effects of Cu2+ treatment on the contents of hydrogen peroxide (H2O2) and thiobarbituric acid-reactive substances (TBARS), and the medium pH value in BY-2 cells.(A) The time course of H2O2 generation detected by 3,3-diaminobenzidine (DAB) staining. BY-2 cells were collected and stained with DAB at 0, 15, 30, 45, 60, and 120 min after treatment with 100 μmol/L Cu2+.(B) The time course of H2O2 production assayed by the method of TiCl4. After pretreatment with catalase, cell samples were collected and analyzed at 0, 15, 30, 45, 60, 120, and 360 min after treatment with 100 μmol/L Cu2+. Each value represents the mean ± SD of three independent measurements.(C) The time course of TBARS production assayed by thiobarbituric acid (TBA) test. Cell samples were pretreated with 1-butanol (v/v, 0.2%) for 20 min, then treated with 100 μmol/L Cu2+. The suspension cells were collected and tested at 0, 1, 2, 3, 6, 12, and 24 h after treatment with Cu2+. Each value represents the mean ± SD of ten independent measurements.(D) Dose-dependent TBARS productions in BY-2 suspension cells at 6 h after treatment with Cu2+ at concentrations of 0, 50, 100, 200, 300, 500 μmol/L. Each value represents the mean ± SD of ten independent measurements.(E) Medium alkalinization in BY-2 suspension culture exposed to 100 μmol/L Cu2+. The pH value of cell culture medium was monitored every 5 min within the first 110 min, and then at 165, 180, 210 and 240 min. Each value represents the mean ± SD of ten independent measurements.
To assess lipid peroxidation resulted from H2O2 production, thiobarbituric acid-reactive substances (TBARS) content was monitored in BY-2 cells following 100 μmol/L Cu2+ treatment (Figure 1C). The level of TBARS was a rapid increase at 2 h and had no significant change from 2 to 6 h. After 24 h treatment with 100 μmol/L Cu2+, the level of TBARS was increased 2.4-fold (Figure 1C). We also observed that TBARS induction was correlated with Cu2+concentration. TBARS content increased about 50% in BY-2 cells when BY-2 cells were exposed to 50 μmol/L Cu2+, however it increased about 100% in BY-2 cells exposed to 100 μmol/L Cu2+ (Figure 1D). However, when Cu2+ concentration was higher than 100 μmol/L, it had no further effect on TRARS induction (Figure 1D).
The medium pH of BY-2 cell culture was tested to estimate cell membrane perturbation caused by Cu2+ stress. The pH of the suspension culture medium exhibited a rapid increase from 15 min to 45 min after Cu2+ treatment (Figure 1E). The rate of pH increase declined during the time period between 45 min to 120 min.
PLD is activated and involved in H2O2 and TBARS production under excessive Cu2+ level
The mRNA levels of NtPLDδ rapidly increased in Cu2+-treated BY-2 cells (Figure 2A). The abundance of NtPLDδ mRNA elevated approximately one-fold at 1 h after treatment with 100 μmol/L Cu2+, and it was even higher at 6 h after treatment. These results indicated that the NtPLDδ gene was upregulated at the transcriptional level by excessive Cu2+. 1-butanol, a PLD inhibitor, was used to block the production of PA from PLD catalysis to study the function of PLD (John et al. 2003). Treatment with the addition of 0.2% (v/v) 1-butanol to the suspension cells 30 min prior to Cu2+ treatment caused the H2O2 production to be decreased by 13% (Figure 2B). When the suspension cells were treated with exogenous PA (100 μmol/L), H2O2 production increased 40% compared to the control (Figure 2B), indicating an involvement of PLD and its product PA in Cu2+-induced H2O2 production.
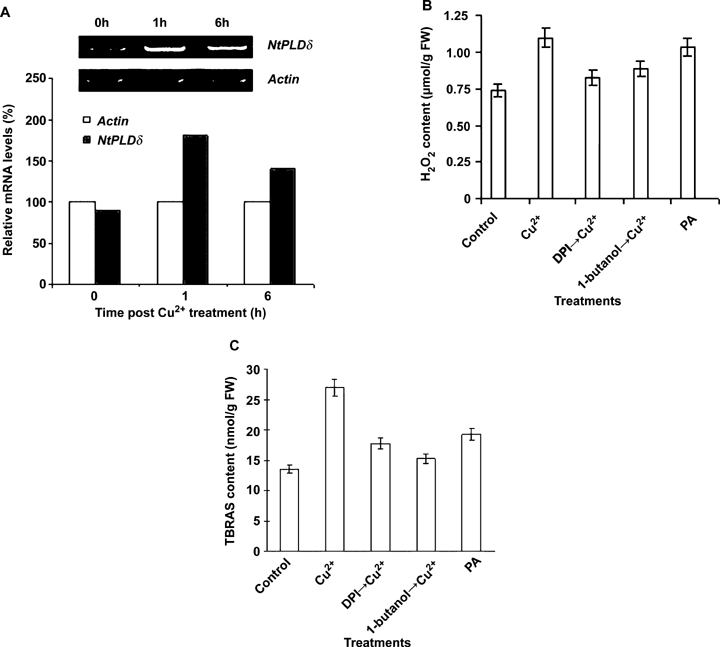
The involvement of phospholipase D (PLD) in the Cu2+-induced H2O2 and thiobarbituric acid-reactive substances (TBARS), production in BY-2 cells.(A)NtPLDδ transcript expression was analyzed by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). Cell samples were collected at 0, 1 and 6 h after treatment with 100 μmol/L Cu2+. Actin expression level was used as the internal control. Relative mRNA levels of NtPLDδ expression were quantified using actin mRNA abundance without copper treatment as 100%.(B) The regulatory effects of 1-butanol (v/v, 0.2%), diphenylene iodonium (DPI) (10 μmol/L), and phosphatidic acid (PA) (100 μmol/L) on the Cu2+-induced H2O2 production. After pretreatment with each chemical for 20 min, respectively, BY-2 cells were treated with or without 100 μmol/L Cu2+, and cell samples were collected after 6 h treatment for the detection of the generation of H2O2 by the method of TiCl4. Each value represents the mean ±SD of three independent measurements.(C) The regulatory effects of 1-butanol (v/v, 0.2%), DPI (10 μmol/L) and PA (100 μmol/L) on the Cu2+-induced TBARS production. After pretreatment with each chemical for 20 min, BY-2 cells were treated with or without 100 μmol/L Cu2+. Cells were collected at 6 h after treatment for the detection of TBARS levels. Each value represents the mean ±SD of ten independent measurements.
1-butanol also displayed a similar inhibitory effect on the Cu2+-induced TBARS production. The level of TBARS dropped approximately 32% in the BY-2 cells pre-treated with 0.2% (v/v) 1-butanol compared to those treated with Cu2+ only (Figure 2C). There was a significant inhibitory effect at each time point. Furthermore, exogenous PA (100 μmol/L) treatment resulted in a 40% increase of TBARS content in suspension cells compared to the control (Figure 2C). These data suggested that PLD and PA mediated the Cu2+-induced TBARS production.
NADPH oxidase plays a role in the elevation of H2O2 and TBARS induced by Cu2+
Diphenylene iodonium (DPI), a specific inhibitor of NADPH oxidase, is used to investigate the role of NADPH oxidase in BY-2 cells under Cu2+ stress (Sagi and Fluhr 2001). The pretreatment with DPI (10 μmol/L) inhibited Cu2+-induced productions of H2O2 by 30% and TBARS by 40%, respectively (Figure 2B,C), demonstrating that NADPH oxidase contributes significantly to the Cu2+-induced productions of H2O2 and TBARS.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the transcript abundance of the tobacco NtrbohD gene encoding a NADPH oxidase in BY-2 cells. The expression of NtrbohD at mRNA level was increased about four-fold at 1 h after Cu2+ treatment, and at 6 h after treatment it dropped rapidly, but still remained 50% higher than that at 0 h (the control) (Figure 3A). The NADPH oxidase activity increased approximately 70% at 1 h after Cu2+ treatment and dropped to a lower level than the control sample (Figure 3B).
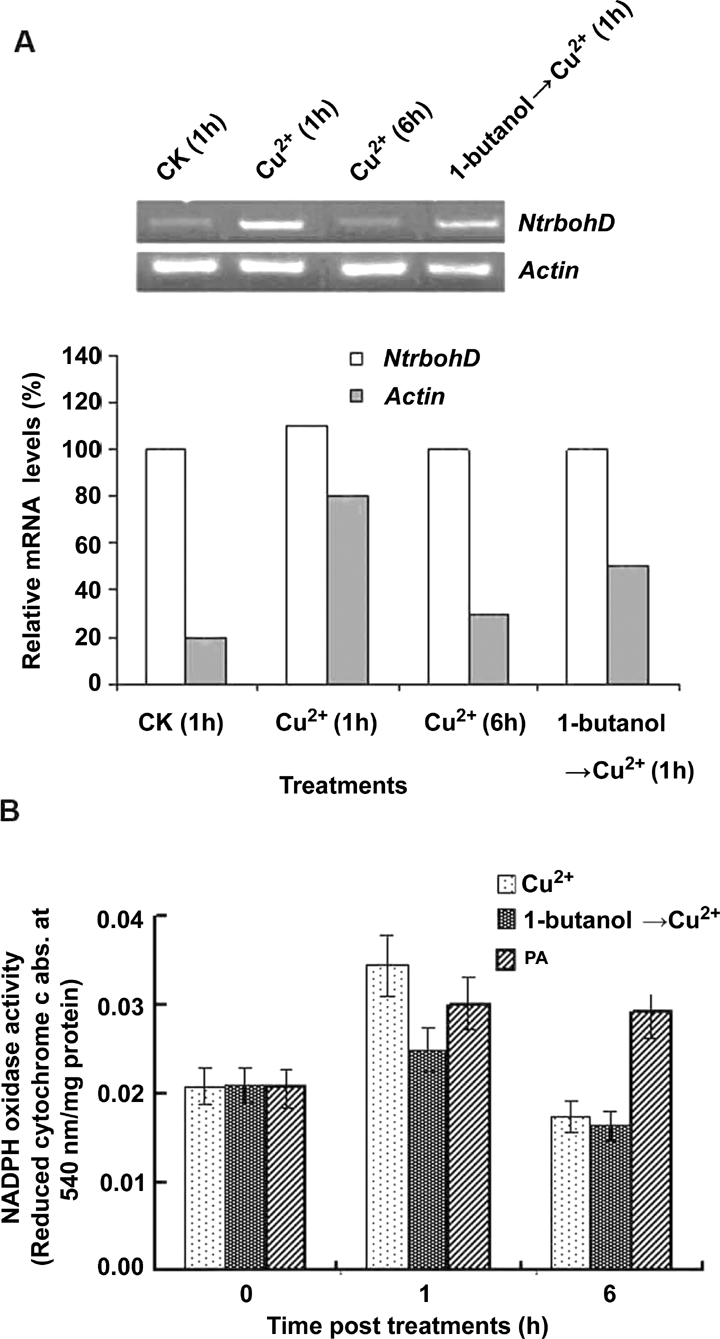
Expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in BY-2 cells under Cu2+ stress.(A) The abundance of NtrbohD mRNA analyzed by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). Cell samples were collected at 0, 1 and 6 h after treatment with 100 μmol/L Cu2+. One set of cell samples was pre-treated by 1-butanol (v/v, 0.2%) for 20 min. Actin expression level was used as the internal control. Relative mRNA levels of the NtrbohD expression were quantified with Actin mRNA abundance as 100% without copper treatment.(B) Activity of plasma membrane NADPH oxidase regulated by Cu2+, 1-butanol and PA. Plasma membrane was isolated from BY-2 suspension cells at 0, 1, and 6 h after treatment with 100 μmol/L Cu2+, 100 μmol/L Cu2+/(v:v, 20%)1-butanol, or 100 μmol/L PA, respectively. The enzymatic activity was analyzed by measuring the catalytic product levels of the reduced cytochrome c (abs. at 454 nm). Each value represents the mean ± SD of three independent measurements.
To further show the role of NADPH oxidase in Cu2+ stress, the NtrbohD, encoding NADPH oxidase, was overexpressed in BY-2 cells. The overexpression of NtrbohD in BY-2 cell lines was confirmed at mRNA level by semi-quantitative RT-PCR (Figure 4A) and at the protein level by Western blot analysis (Figure 4B). Further, the activity of NADPH oxidase in transgenic cells was about 45% higher than that in wild BY-2 cells (Figure 4C), which was accompanied by a higher TBARS content in transgenic lines (Figure 4D). The Cu2+ (100 μmol/L) treatment also resulted in increasing TBARS production in transgenic cells (Figure 4D). These data showed that NADPH oxidase is an important factor resulting in the Cu2+-induced productions of H2O2 and TBARS.
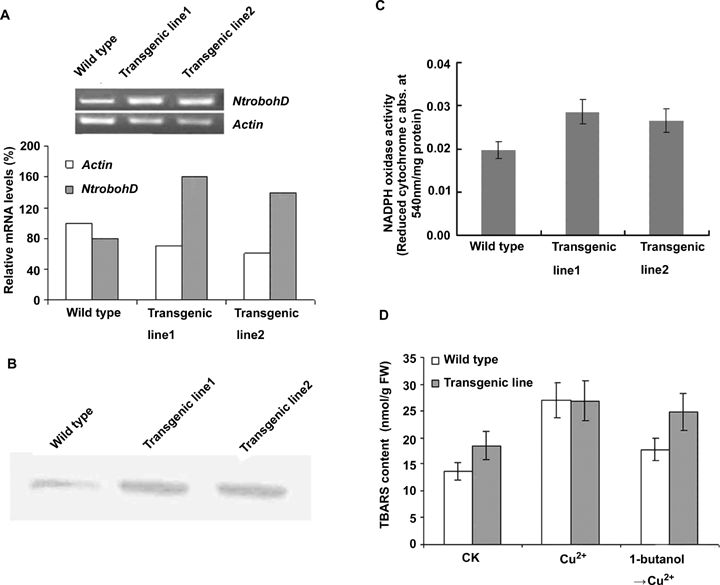
Overexpression of the CaMV 35 S-NtrbohD fusion gene in transgenic BY-2 cell lines.(A) The NtrbohD mRNA levels analyzed by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). Actin expression level was used as the internal control. Relative mRNA levels of the NtrbohD expression were quantified with Actin mRNA abundance in wild type cells as 100%.(B) The NtrbohD protein expression analyzed by Western blot analysis. Sample containing 20 μg of proteins were loaded in each lane. Cells were grown in darkness in hydroponic culture with continuous aeration in a growth chamber at 25°C for 5 days and then collected for analysis.(C) Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in transgenic BY-2 cells. The enzymatic activity was analyzed by testing the catalytic product levels of the reduced cytochrome c (abs. at 454 nm). Cells were grown in darkness in hydroponic culture with continuous aeration in a growth chamber at 25°C for 5 days and then collected for analysis. Each value represents the mean ± SD of three independent measurements.(D) Cu2+-induced TBARS productions in the transgenic BY-2 cells. Cells were collected at 0 and 6 h after treatment with100 μmol/L Cu2+ or 100 μmol/L Cu2+/0.2% (v/v) 1-butanol (pretreated for 20 min). Each value represents the mean ±SD of ten independent measurements.
NADPH oxidase is regulated by PLD and PA
The Cu2+ induced expression of NtrbohD at mRNA level was decreased about 40% when the cells were pre-treated with 1-butanol (Figure 3A). It suggested that PLD might regulate NtrbohD expression at the transcriptional level. Furthermore, the activity of NADPH oxidase was inhibited approximately 30% and 8% at 1 h and 6 h after Cu2+ treatment[0] by1-butanol (0.2%, v/v) (Figure 3B). The influence of exogenous PA on NADPH oxidase activity was tested to further clarify the relationship between PLD and NADPH oxidase in cells. As shown in Figure 3B, 100 μmol/L PA increased NADPH oxidase activity by 40%, indicating a positive effect of PLD on NADPH oxidase activity. However, for overexpressing NtrbohD cells, a pretreatment of 1-butanol showed no significant effect on the Cu2+-induced TBARS production (Figure 4D). These data showed that NADPH oxidase is upregulated by PLD and its product PA.
AsA antioxidant system collapses in BY-2 cells under excessive Cu2+ level
The pre-treatment of the BY-2 cells with vitamin E (VE) reduced the level of TBARS by 19% at 6 h compared to that of treatment with Cu2+ only (Figure 5A). In contrast, the pre-treatment with AsA induced a further increase of TBARS content (Figure 5A). Moreover, we observed that most suspension cells died at 6 h following treatment with both Cu2+ and AsA (added 20 min before Cu2+) (data not shown), suggesting a possible collapse of AsA antioxidant systems in BY-2 cells. The content of AsA decreased about 50% by Cu2+ treatment, and the pre-treatment with 1-butanol did not show any additional effect (Figure 5B).
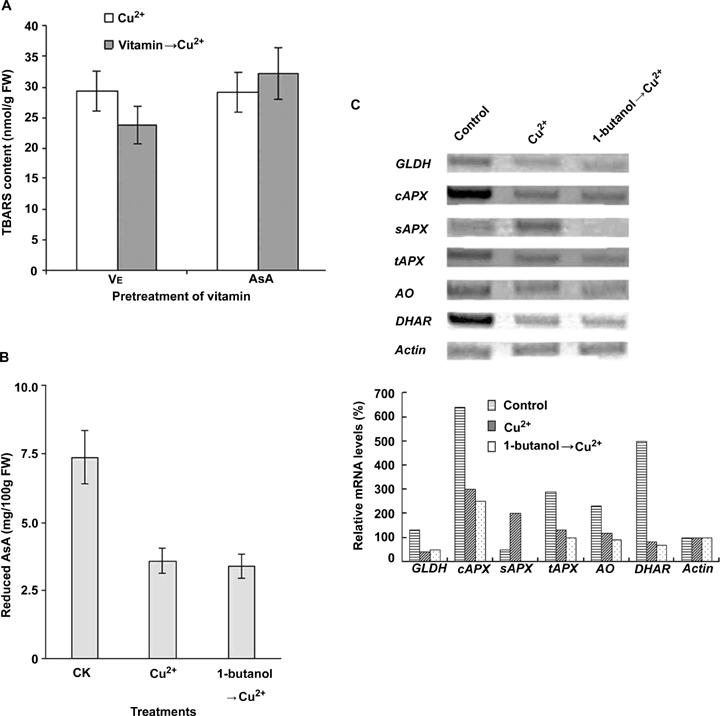
The effects of Cu2+ treatment on the ascorbic acid (AsA) antioxidant system in BY-2 cells under Cu2+ stress.(A) The effect of vitamin E pre-treatment on Cu2+-induced thiobarbituric acid-reactive substances (TBARS) production. After 20 min pre-treatments with vitamin E (VE, 6 mmol/L) and AsA (10 mmol/L) respectively, cells were collected for TBARS detection at 6 h after treatment of 100 μmol/L Cu2+. Each value represents the mean ±SD of ten independent measurements.(B) The content of reduced AsA in BY-2 suspension cells treated with Cu2+ and 1-butanol. Cells were collected for the detection of reduced AsA at 6 h after treatment with 100 μmol/L Cu2+ with or without 1-butanol (v/v, 0.2%) pre-treatment. Each value represents the mean ± SD of three independent measurements.(C) Cu2+ or Cu2+/1-butanol affect the transcription of genes related to AsA metabolism. Cells were harvested at 6 h after treatment with 100 μmol/L Cu2+ with or without pretreatment of 0.2% (v/v) 1-butanol. Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) was carried out using Actin as the internal control. Relative mRNA levels of each gene were quantified with Actin mRNA abundance as 100% in cells without copper treatment. AO, ascorbate oxidase; DHAR, dehydro-(6)-ascorbate reductase; FW, fresh weight; GLDH, l-galactono-γ-lactone dehydrogenase; cAPX, cytosol ascorbate peroxidase; sAPX, stromal ascorbate peroxidase; tAPX, thylakoidal ascorbate peroxidase.
The mRNA abundance of enzymes involved in the AsA biosynthesis and AsA antioxidant system suffered a dramatic reduction except for stromal ascorbate peroxidase (sAPX) (Figure 5C). The expression of l-galactono-γ-lactone dehydrogenase (GLDH), the key enzyme in the AsA biosynthesis, was considerably reduced (Figure 5C). In the AsA antioxidant system, the transcripts of ascorbate oxidase (AO), cytosol ascorbate peroxidase (cAPX), thylakoidal ascorbate peroxidase (tAPX) and dehydro-(6)-ascorbate reductase (DHAR) were also downregulated remarkably (Figure 5C). The pre-treatment with 1-butanol showed no obvious effect on the level of mRNA, which was downregulated by Cu2+ for all of the above enzymes, except for the mRNA of sAPX which was non-detectable (Figure 5C), suggesting that PLD is not involved in the regulation of AsA antioxidant system.
Discussion
Cell suspension culture such as tobacco BY-2 provides a good model for studying cellular responses in plant cells under stresses (Nagata et al. 1992). In this study, suspension cells of Nicotiana tabacum L. cv. BY-2 were used to investigate the production of ROS and TBARS induced by excessive copper.
Phospholipase D hydrolyzes phospholipids to generate PA, a second messenger activating PtdIns-specific phospholipase C and protein kinase C in animal cells (English 1996). Our results revealed that the NtPLDδ mRNA level was upregulated by the treatment with 100 μmol/L Cu2+ (Figure 2A), indicating that PLD expression is induced by Cu2+. We found that the medium pH level was raised rapidly when BY-2 cells were treated with Cu2+ (Figure 1E), implying that membrane perturbation occurred in BY-2 cells exposed to excessive Cu2+. These results were consistent with previous studies showing that the activation of PLD is regulated by pH change and membrane perturbation (Pappan and Wang 1999). Excessive Cu2+ also affects PA production on the chloroplast membrane (Quartacci et al. 2000) and plasma membrane (Quartacci et al. 2001). These data support that PLD is activated by excessive Cu2+. PLD activation is supposed to be an important and early step in stress-induced phospholipid hydrolysis (Lee et al. 1997; Wang et al. 2000). As the product of PLD catalysis, PA level is also increased under various biotic and abiotic stresses, including pathogen attack, elicitation, wounding, freezing, hyper-osmotic stress, and water deficit (Halliwell and Gutteridge 1989; Lee et al. 1997; van der Luit et al. 2000).
Phospholipase D and PA have been known to induce leaf cell death and to elevate the ROS level in whole leaves and single cells (Sang et al. 2001; Pignocchi et al. 2003). Our study showed that PLD and PA are involved in Cu2+-induced ROS productions. 1-butanol, which is used to inhibit PA production, suppresses the Cu2+-induced H2O2 elevation in BY-2 cells (Figure 2B). Meanwhile, exogenous PA (100 μmol/L) induces H2O2 production in BY-2 cells (Figure 2B). Further study is necessary to investigate the mechanism underlying PLD activation under excessive Cu2+ stress.
The ROS production in plants may result from the activity of redox enzymes binding to or associating with the PM of the cell, as well as those associated with the electron transport systems. NADPH oxidase, a mammalian NADPH oxidase homolog, is believed to be responsible for stress-induced ROS production in plants (Mehdy et al. 1996; Zhang et al. 2001). We observed that DPI, a specific inhibitor of NADPH oxidase, inhibited the ROS production in BY-2 cells under excessive Cu2+ stress (Figure 2B), similar to the report in wheat root (Quartacci et al. 2001). Excessive Cu2+ leads to an increased activity of NADPH oxidase in wheat root (Quartacci et al. 2001). Overexpression of Nt-rbothD results in enhanced TBARS production in transgenic BY-2 cell lines (Figure 4D). These data demonstrate that plant NADPH oxidase is possibly involved in the Cu2+-induced ROS production and lipid peroxidation.
Plant NAPDH oxidase is regulated by many factors, including calcium (Sagi and Fluhr 2001), Ca2+-dependent and calmodulin-independent protein kinase (CDPK) (Xing et al. 1997; Olmos et al. 2003), and small GTPase (Suharsono et al. 2002). In mammalian, PA induces NADPH oxidase activity (Palicz et al. 2001). A recent study also implied that the NADPH oxidase activity is regulated by PA (Pignocchi et al. 2003). Quartacci et al. (2001) found that excessive Cu2+ induces the elevation of both PA content and NADPH oxidase activity in wheat root. The formation of PA from PLD can lead to the production of other lipid messengers such as diacylglycerol, free polyunsaturated fatty acids, phosphatidylinositol-4, 5-bisphosphate, and jasmonic acid (Wang et al. 2000). It has been proposed that PLD and PA are involved in NADPH oxidase-mediated ROS production (Jacob et al. 1999). NADPH oxidase activity is enhanced by PA or suppressed by 1-butanol in BY-2 cells (Figure 3B), which provides pharmacological evidence that PLD possibly regulates the activity of NADPH oxidase. Meanwhile, 1-butanol has little inhibitory effect on Cu2+-induced TBARS production in transgenic BY-2 cells overexpressing NADPH oxidase (Figure 4D), suggesting that PLD possibly acts as an upstream component of NADPH oxidase in signal transduction pathway initiated by Cu2+. There is no difference in TBARS levels between transgenic suspension cell line overexpressing NtrbohD and wild type BY-2 cells after excessive Cu2+ treatment (Figure 4D). Further, 1-butanol has no notable effect on the Cu2+-induced TBARS production in these transgenic lines (Figure 4D). These observations suggest that PLD and PA possibly regulate NADPH oxidase on the transcription level.
Ascorbate-glutathione cycle plays a vital role in protecting plants from oxidative stress. However, at high concentrations of AsA, copper (II) is rapidly reduced to copper (I) with the formation of the oxidized form of AsA (DAsA). Our data showed a dramatic reduction in the level of reduced AsA in BY-2 cells exposed to excessive Cu2+ (Figure 5B). Meanwhile, GLDH, the key enzyme for the in vivo production of AsA, is also greatly inhibited at the mRNA level (Figure 5C). The mRNAs abundance of enzymes involved in AsA antioxidant systems except, for sAPX show the similar downregulation in BY-2 cells (Figure 5C). These data suggest a very low redox status of the antioxidant pool in BY-2 cells treated with excessive Cu2+. The low cellular redox status has been reported to lead to the accumulation of O2− radicals in plant cells (Polle 2001). A collapse of the glutathione cycle has also been found in a previous study (Schützendübel and Polle 2002). Altogether, these results imply that Cu2+ may destroy antioxidative defenses in plant cells.
In contrast to the effect on NADPH oxidase, 1-butanol has no significant effect on the reduced AsA content, the AsA biosynthesis and AsA antioxidant system in BY-2 cells treated with excessive Cu2+ (Figure 5B,C), which suggest that the regulation of antioxidant systems is independent of PLD in plant cells under Cu2+ treatment.
In conclusion, Cu2+ induces the activity of PLD and NADPH oxidase and abates the AsA antioxidant system in BY-2 cells. Excessive Cu2+-elevated ROS production is caused by its upregulated PLD that elevates the activity of NADPH oxidase and its collapsed antioxidant system that scavenges ROS. The rapid increase of ROS level results in TBARS production (Figure 6). Further studies on PLD enzyme activity, as well as PA changes in cells may reveal more details about their functions in the Cu2+-induced ROS production.
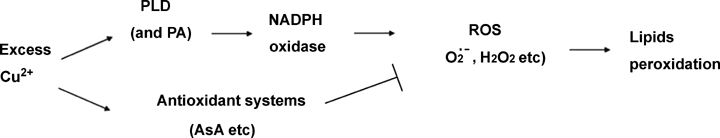
Roles of phospholipase D (PLD), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and ascorbic acid (AsA) antioxidant system in the Cu2+-induced H2O2 and thiobarbituric acid-reactive substances (TBARS) production. ROS, reactive oxygen species.
Materials and Methods
Tobacco cell suspension culture
Tobacco (Nicotiana tabacum) BY-2 cell suspensions were maintained by weekly subculture as described by Nagata et al. (1992). The cells were grown in darkness in hydroponic culture with continuous aeration in a growth chamber at 25°C. Fe-ethylenediaminetetraacetic acid (EDTA) was replaced with FeSO4 and tartaric acid to avoid the formation of non-toxic Cu-EDTA complexes in the Hoagland's solution (Quartacci et al. 2001). On the fifth day after being transferred into new subculture, the cells that were in the logarithmic phase of culture growth course were used for all treatments. The density of cell suspensions used for all treatments was 0.1 g fresh weight (FW)/mL. Inhibitor and other reagents were added into the culture liquid with cells 30 min prior to Cu2+ treatment. Cell samples were harvested at different time points, frozen in liquid N2, and stored at −80°C until analysis. Each treatment was repeated three times for independent experiments.
H2O2 detection
Two methods were used to detect the H2O2 content in Cu2+-treated BY-2 cells. For hydrogen peroxide staining, cells were vacuum-infiltrated with 0.1 mg/mL 3,3-diaminobenzidine (DAB) in 50 mmol/L Tris-acetate buffer (pH 5.0). The samples were incubated in darkness at 22°C for 24 h (Lee et al. 2002), and then the procedure of Lee et al. (2002) was followed for hydrogen peroxide staining.
A modified H2O2-TiCl4 method was also used to detect H2O2 content as described by Brennan and Frenkel (1977). Cells (∼1 g FW) were homogenized in 5 mL of cold acetone in a mortar, and then centrifuged at 1 250 g for 5 min. 0.5 mL 20% (v/v) TiCl4 in concentrated HCl was added to the supernatant, and then 3.5 mL of one-fifth strength NH4OH was added drop-wisely with thorough mixing by shaking. The samples were centrifuged (1 250 g; 5 min) again and the precipitates were washed repeatedly with 5 mL acetone until the supernatant was colorless. The precipitates were dissolved with 10 to 15 mL 2 N H2SO4 to the final volume of 20 to 25 mL, and filtered prior to the absorbance measurement at 415 nm. H2O2, at the concentrations of 0.00, 0.10, 0.25, 0.50, 0.75, 1.00 mmol/L, was reacted with TiCl4 to establish a standard curve. To eliminate any potential factors interfering with H2O2 detection by this method, another set of cells was pretreated with catalase for 2 h, and then detected by TiCl4. H2O2 content was calculated based on the difference between the TiCl4 values of the cells with or without the pretreatment by catalase.
Determination of TBARS content
For the measurement of lipid peroxidation, the thiobarbituric acid (TBA) test was used to determine the content of TBARS, an end product of lipid peroxidation (Heath and Parker 1968). Five hundred milligrams of cells were collected and homogenized in 5 mL of 0.1% (w/v) trichloroacetic acid (TCA) solution. The homogenate was centrifuged at 10 000 g for 20 min and 0.5 mL of the supernatant was transferred to 1 mL of 0.5% (w/v) TBA in 20% TCA. The mixture was incubated in boiling water (100°C) for 30 min, and then put in an ice bath to stop the reaction. After centrifugation at 10 000 g for 5 min, the absorbency of the reaction mixtures was read at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of TBARS–TBA complex (red color) was calculated with the extinction coefficient of 155 mmol/cm.
Detection of pH value of cell culture medium
Cell suspensions were maintained in Murashige and Skoog medium adjusted to pH 5.5 to 5.6 with potassium hydroxide (KOH) (Haruta and Constabel 2003). For routine maintenance, 3 g of 1-week-old culture was transferred into 30 mL of medium in 50-mL flasks and maintained on an orbital shaker at 100 r.p.m. in the dark at room temperature. The medium pH values were measured every 5 min after treatment of 100 μmol/L Cu2+, using an Accumet pH meter (Fisher Scientific, Nepean, ON, Canada).
Detection of reduced AsA content
2,6-dichloroindophenol titration was used to detect the reduced AsA content. Two grams of suspension cells were homogenized in 20 mL 2% (v/v) oxalic acid in a mortar, and centrifuged to remove cell debris. The supernatant was diluted to 100 mL with 2% (v/v) oxalic acid, then 15 mL of diluted supernatant were titrated by 2,6-dichloroindophenol. The reduced AsA content was calculated as mg per 100 g suspension cells.
Isolation of plasma membranes and assay of NADPH oxidase activity
Plasma membranes (PMs) were isolated according to the method of Jiang and Zhang (2002). The PM pellets were resuspended in Tris-HCl (pH 6.8) and used for analysis immediately. All procedures were carried out at 4°C. Protein content of PMs was determined according to the method of Bradford (1976) with bovine serum albumin (BSA) as standard.
The NADPH oxidase activity was determined by the rate of superoxide dismutase-inhibitable ferricytochrome c reduction at 540 to 550 nm based on Xing's protocol (Xing et al. 2001). The membrane (10 mg protein/mL) was added in a reaction solution containing 50 mmol/L potassium phosphate buffer (pH 7.3), 50 mmol/L cytochrome c, 0.01% (v/v) TritonX-100, 2 mmol/L NaN3, 200 mmol/L NADPH, with or without 50 units of superoxide dismutase (SOD).
Expression of NADPH oxidase in transgenic BY-2 cells
According to the sequence published on the National Center for Biotechnology Information (NCBI), a tobacco DNA fragment encoding for NADPH oxidase D (NtrbohD, AJ309006) was cloned by RT-PCR and inserted into the pBI-121 vector with sequencing identification. Suspension cell transformation and maintenance were carried out as described by Simon-Plas et al. (2002).
For Western blot analysis to detect NADPH oxidase, membrane proteins of transgenic cell lines were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride membranes (Immun-Blot Membranes Bio-Rad, Hercules, CA, USA), and subjected to immunodetection with polyclonal antibodies against the C-terminal region of NADPH oxidase (Sagi and Fluhr 2001).
Semi-quantitative RT-PCR
Total RNA was extracted using an RNeasy plant mini kit (Qiagen, West Sussex, UK). Residual DNA was removed with DNase I treatment (Invitrogen, Strathclyde, UK). One microgram of total RNA was reverse-transcribed using 0.5 μg Oligo (dT) 12–18 (Invitrogen) and 200 units of Superscript II (Invitrogen) following the supplier's recommendation. cDNA samples were standardized by actin mRNA level. The gene-specific primers were designed as bellow for semi-quantitative RT-PCR:
GLDH (AB048530), 5′-TTTTAGGCTTTGACTGTGGTG-3′ and 5′-TCAGATGASAGAGCTTCTCASAG-3′; cAPX1 (X596 00), 5′-CTCAAGCTGTTGACAAATG-3′ and 5′-AGCTTCAGCA ACCAATTC-3′; sAPX (AB022274), 5′-TTGTTTCAGTTGGCC AGTGC-3′ and 5′-CGCTGCCTTGTGTAGG-3′; tAPX (AB022 273), 5′-TGTTTTCTACAGAATGGGC-3′ and 5′-GTTGAGTAT TTTG CTGCCAC-3′; AO (D43624), 5′-AACCAAAAACACCTC AAGGC-3′ and 5′- GGTGCTTGTTTTAGGACATC- 3′; DHAR (AY074787), 5′-TGCTGTGGGTGCCCCTAATGTCCTC-3′ and 5-AGCCACCTCA AGATGGTACA GTTTC-3; NtrbohD (AJ309 006), 5′-ASAGGGTAATAAATCAGGTTCAG-3′ and 5′-CAAGG CGTGTTGTCTTAGTTC-3′; NtPLDδ (Z84822), 5′-ATAAATGG GTTGAGATACTGGATAG-3′ and 5′ -CTTCTCTTTT CCGACT CTCC GCTGG-3′; Actin (X63603) 5′-CGCGAAAAGATGACT CAAATC-3′ and 5′-AGATCCTTTCTGATATCCACG-3′.
Semi-quantitative RT-PCR conditions included pre-denaturation at 94°C for 3 min and 30 cycles of denaturation at 94°C for 45 s, annealing at 52°C for 30 s, and extension at 72°C for 45s, followed by 10 min at 72°C, using 0.5 μL cDNA template of the RT reaction and 0.2 μmol/L of each primer in a total volume of 25 μL. PCR products were analyzed on 2% (w/v) agarose gel containing 0.5 μg/mL ethidium bromide. The images of PCR gels were analyzed for density comparison of DNA fragments.
(Handling editor: Jin-Zhong Cui)
Acknowledgements
We thank Dr Jun-Jun Liu (Pacific Forestry Center, Canada) for constructive comments to this manuscript.




