Lead Induced Changes in the Growth and Antioxidant Metabolism of the Lead Accumulating and Non-accumulating Ecotypes of Sedum alfredii
Supported by the National Natural Science Foundation of China (20477039), the Program for Changjiang Scholars and Innovative Research Team in University (IRT0536), and the State Key Basic Research and Development Plan of China from the Science and Technology Ministry of China (2002CB410804).
Abstract
The phytotoxicity and antioxidative adaptations of lead (Pb) accumulating ecotype (AE) and non-accumulating ecotype (NAE) of Sedum alfredii Hance were investigated under different Pb treatments involving 0, 0.02 mmol/L Pb, 0.1 mmol/L Pb and 0.1 mmol/L Pb/0.1 mmol/L ethylenediaminetetraacetic acid (EDTA) for 6 days. With the increasing Pb level, the Pb concentration in the shoots of AE plants enhanced accordingly, and EDTA supply helped 51% of Pb translocation to shoots of AE compared with those treated with 0.1 mmol/L Pb alone. Moreover, the presence of EDTA alleviated Pb phytotoxicity through changes in plant biomass, root morphology and chlorophyll contents. Lead toxicity induced hydrogen peroxide (H2O2) accumulation and lipid peroxidation in both ecotypes of S. alfredii. The activities of superoxide dismutase (SOD), guaiacol peroxidase (G-POD), ascorbate peroxidase, and dehydroascorbate reductase elevated in both leaves and roots of AE as well as in leaves of NAE with the increasing Pb levels, but SOD and G-POD declined in roots of NAE. Enhancement in glutathione reductase activity was only detected in roots of NAE while a depression in catalase activity was recorded in the leaves of NAE. A significant enhancement in glutathione and ascorbic acid (AsA) levels occurred in both ecotypes exposed to Pb and Pb/EDTA treatment compared with the control, however, the differences between these two treatments were insignificant. The dehydroascorbate (DHA) contents in roots of both ecotypes were 1.41 to 11.22-fold higher than those in leaves, whereas the ratios of AsA to DHA (1.38 to 6.84) in leaves altering more to the reduced AsA form were much higher than those in roots. These results suggested that antioxidative enzymes and antioxidants play an important role in counteracting Pb stress in S. alfredii.
Heavy metal pollution is a widespread global dilemma, and has been a major environmental concern over the past several decades. A wide range of heavy metals have been detected in different biota. Lead (Pb) is one of the most abundant, ubiquitous toxic elements posing a critical concern to human and environmental health in that it is a persistent contaminant, has low solubility, and is classified as carcinogenic and mutagenic (Diels et al. 2002).
Conventional cleanup technologies are generally too costly to be used to restore contaminated sites, and are often harmful to the normal properties of the soil (Holden 1989). Well established cost effective and environmentally friendly phytoremediation techniques have grabbed increasing attention recently (Salt et al. 1998; Garbisu and Alkorta 2001). No reliable reports on Pb hyper-accumulating species under natural conditions are available; moreover, the phyto-availability of Pb is restricted by the strong complexation of Pb within solid soil fractions. To overcome this problem and increase Pb availability to plants, chelators have been used to enhance Pb solubility artificially in soil solutions (Kos and Lestan 2004). The role of ethylenediaminetetraacetic acid (EDTA) in increasing plant metal uptake has been documented by a number of researchers (Liphadzi and Kirkham 2006). Huang et al. (1997) reported that among five chelating agents, EDTA was the most efficient in increasing shoot Pb concentration in both pea and corn.
Pb exerts adverse effects on morphology, growth and photosynthetic pathways of plants and inhibits enzyme activities, water balance, causes alterations in membrane permeability and disturbs mineral nutrition (Sharma et al. 2005). Biochemical responses of plants to Pb include enhancement in the activity of antioxidative enzymes and production of antioxidants scavenging system controlling free radicals that are produced upon exposure to them. Pb can induce oxidative stress with over-production of reactive oxygen species (ROS) including superoxide radicals (O2•−), hydroxyl radicals (•OH) and hydrogen peroxide (H2O2) (Verma and Dubey 2003; Thomas Ruley et al. 2004; Reddy et al. 2005). Free radicals and hydrogen peroxide are reported to cause membrane damage that is often related to lipid peroxidation. The dismutation of superoxide into H2O2 and O2 by superoxide dismutases (SOD, EC 1.15.1.1) has been widely recorded as a detoxification mechanism. H2O2 formed can be decomposed into H2O and O2 within the peroxisome by catalase (CAT) that does not require an additional substrate. The scavenging of H2O2 in other cell compartments depends on distinct peroxidases such as guaiacol peroxidases (G-POD) and ascorbate peroxidases (APX) that use a substrate for their activity (Cakmak and Marschner 1992; Noctor and Foyer 1998). Glutathione reductase (GR) together with dehydroascorbate reductase (DHAR) is involved in the breakdown of H2O2 via the ascorbate-glutathione cycle (Noctor and Foyer 1998). Glutathione, the basic unit of phytochelatins is very mobile and can be found at high concentrations in all cell compartments, phloem and in roots (Foyer and Noctor 2000). Glutathione is an important antioxidant and forms, with oxidized glutathione (GSSG), an important redox couple, providing the conditions to support mitochondrial oxidative phosphorylation, generation of adenosine triphosphate (ATP), and hence key anabolic activities (May et al. 1998), and glutathione also takes part in thioredoxin-related regulation of many enzymes of photosynthetic metabolism. Ascorbate is a major metabolite in plants, which occurs in the cytosol, chloroplasts, vacuoles, mitochondria and cell wall.
Sedum alfredii Hance is a newly discovered Zn/Cd hyper-accumulator growing in the old Pb/Zn mined areas of southeast China (118°56′E, 29°17′N), and has been reported to be a Pb accumulating species (He et al. 2002; Yang et al. 2002b; Yang et al. 2004). Earlier studies on S. alfredii mainly focused on the accumulation and transportation mechanism (Yang et al. 2002a; Zhou and Qiu 2005; Yang et al. 2006), and less attention was paid to the detoxification mechanisms. In this context, a study was conducted to investigate the antioxidant metabolism of two ecotypes of S. alfredii under the stress of Pb, and the effects of EDTA on the detoxification of Pb were also studied. Pb toxicity was assayed using parameters such as biomass, root morphology, photosynthetic pigments, antioxidant enzymes activity and membrane structure.
Results
Lead uptake
After being treated with 0.02 mmol/L Pb, there were no significant differences of Pb concentration in the shoot of accumulating ecotype (AE) plants compared with control (CK) (P > 0.05) (Table 1). The addition of 0.1 mmol/L Pb significantly accelerated the Pb concentration in the shoot of AE and non-accumulating ecotype (NAE) plants resulting in 45.4- and 25.8-fold increases, compared with CK, respectively. Combined Pb and EDTA treatment caused a significant increase in the shoot Pb concentration of AE (increased by 51%) compared with those treated with 0.1 mmol/L Pb alone (P < 0.05).
| Treatment (mmol/L) | Shoot (mg/kg) | Root (mg/kg) | Ratio (shoot/root) | |||
|---|---|---|---|---|---|---|
| AE | NAE | AE | NAE | AE | NAE | |
| CK | 20.92c | 18.48d | 25.31c | 18.88d | 0.827 | 0.979 |
| 0.02 Pb | 125.18c | 72.00c | 666.19c | 1327.19c | 0.188 | 0.054 |
| 0.1 Pb | 949.73b* | 476.03b | 4712.46a | 7224.24a | 0.202 | 0.066 |
| 0.1 Pb + 0.1 EDTA | 1431.31a* | 667.14a | 3261.69b* | 5768.55b | 0.439 | 0.116 |
- Values are means ±SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05). CK, control; EDTA, ethylenediaminetetraacetic acid.
The Pb concentration in the shoot of AE was always higher than that of NAE, but the trend was on the contrary for the root (Table 1). After treatment with 0.1 mmol/L Pb, the concentration in roots of AE and NAE plants increased 186.2- and 382.7-fold compared with CK, respectively. With the combined EDTA and Pb treatment, the concentration of Pb in roots decreased 30.8% and 20.2% compared with the treatment of 0.1 mmol/L Pb alone for the AE and NAE plants, respectively.
Plant growth parameters and photosynthetic pigments
In the hydroponics experiment, AE plants exhibited strong tolerance to Pb toxicity, with erect stem, thicker and dark green colored leaves compared with NAE plants. The dry weights of both ecotypes of S. alfredii are presented in Table 2 (P < 0.05). Shoot dry weights of AE were higher than those of the NAE in both CK and treated plants. After increasing the application rate of Pb from 0.02 mmol/L to 0.1 mmol/L, the shoot Pb in AE decreased by 15.9%; however, 0.1 mmol/L Pb combined with 0.1 mmol/L EDTA addition caused an increase in the shoot dry weight by 5.1% compared with those treated with 0.1 mmol/L Pb alone. As for root, the same trend could also be traced; EDTA addition stimulated root growth compared with Pb treatment alone.
| Treatment (mmol/L) | Shoot (g/plant) | Root (g/plant) | Ratio (shoot/root) | |||
|---|---|---|---|---|---|---|
| AE | NAE | AE | NAE | AE | NAE | |
| CK | 0.817a | 0.490a | 0.057a | 0.046a | 14.25 | 10.58 |
| 0.02 Pb | 0.723ab* | 0.476a | 0.047ab | 0.044ab | 15.49 | 10.91 |
| 0.1 Pb | 0.608b* | 0.306c | 0.037b | 0.029c | 16.28 | 10.43 |
| 0.1 Pb + 0.1 EDTA | 0.638ab* | 0.377b | 0.040b | 0.034bc | 15.81 | 11.21 |
- Values are means ±SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05). CK, control; EDTA, ethylenediaminetetraacetic acid.
In order to compare the root morphological characteristics under various treatments, root length, root surface area, root diameter and root volume were measured (Table 3). Under the stress of 0.1 mmol/L Pb and 0.1 mmol/L Pb/0.1 mmol/L EDTA, root length of the AE was significantly reduced by 43.4% and 21.3%, respectively, as compared with CK (P < 0.05). A similar trend was traced in the NAE plants, although the root length was always lower than that of AE plants in both CK treatments. Both root surface area and root volume decreased after treatment with 0.1 mmol/L Pb or 0.1 mmol/LPb/0.1 mmol/L EDTA in both ecotypes of S. alfredii (P < 0.05); the parameter for AE was always higher than that of NAE. As for root diameter, no significant differences were observed between CK and treatments (P > 0.05) for both ecotypes.
| Treatment (mmol/L) | Length (cm/plant) | Surf. area (cm2/plant) | Avg. diam (mm/plant) | Volume (cm3/plant) | ||||
|---|---|---|---|---|---|---|---|---|
| AE | NAE | AE | NAE | AE | NAE | AE | NAE | |
| CK | 279.26a | 252.14a | 61.38a* | 44.15a | 0.430a | 0.400a | 0.917a | 0.650a |
| 0.02 Pb | 275.34ab* | 237.88ab | 58.18ab* | 40.18ab | 0.390a | 0.357a | 0.873a* | 0.567a |
| 0.1 Pb | 208.90c | 140.38c | 52.88b* | 35.57b | 0.373a | 0.290a | 0.563b | 0.407b |
| 0.1 Pb + 0.1 EDTA | 229.29bc* | 163.23bc | 55.41ab* | 41.32ab | 0.393a | 0.333a | 0.653b* | 0.433b |
- Values are means ±SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05). CK, control; EDTA, ethylenediaminetetraacetic acid.
The chlorophyll a, b, total chlorophyll and carotene contents of the AE plants were significantly higher (P < 0.05) than that of the NAE plants in all treatments (Figure 1). After treatment with Pb or combined with EDTA, there were no significant changes in chlorophyll a and b for AE plants (P > 0.05), while those of NAE decreased slightly compared with CK (P < 0.05) (Figure 1A, B). After treatment with 0.1 mmol/L Pb, the total chlorophyll content of NAE plants decreased significantly by 8.2% compared with CK (P < 0.05), while for AE plants treated with 0.1 mmol/L Pb/0.1 mmol/L EDTA, the total chlorophyll increased 4% compared with those treated with Pb alone (P > 0.05) (Figure 1C). As for the carotene content, after treatment with 0.1 mmol/L Pb or 0.1 mmol/L Pb/0.1 mmol/L EDTA, carotene contents decreased significantly in both ecotypes. It could also be seen that the carotene contents of AE plants were always higher than that of NAE (P < 0.05) (Figure 1D).
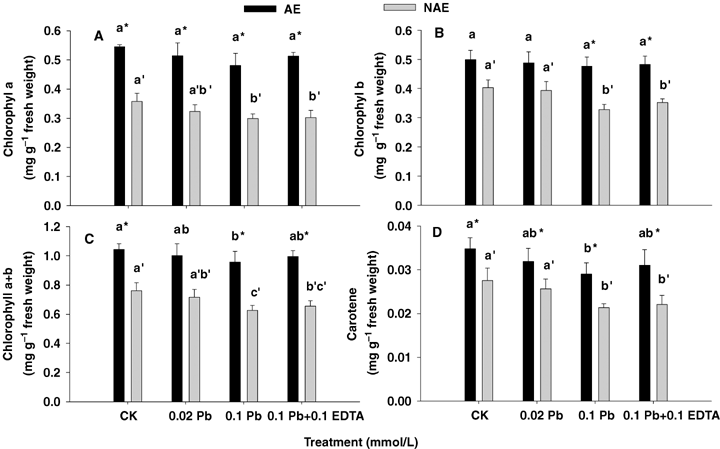
Effects of Pb and ethylenediaminetetraacetic acid (EDTA) on photosynthetic pigments.Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and carotene (D) of the two contrasting ecotypes of Sedum alfredii. Values are means ± SD (n = 3). Different letters among treatments indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
H2O2 concentrations and lipid peroxidation products
Treatments with Pb alone and combined with EDTA stimulated H2O2 accumulation (1.21 to 3.61-fold increases as compared with CK) in both leaves and roots tissues of AE and NAE, and the H2O2 concentrations in the roots were extremely higher than those in the leaves. Moreover, the H2O2 concentration was much higher in the roots of NAE than those of AE treated with Pb alone and its combination with EDTA, whereas it was opposite to those in leaves. Particularly, the H2O2 concentrations in the plants treated with 0.1 mmol/L Pb combined with 0.1 mmol/L EDTA were much lower than those in the plants treated with 0.1 mmol/L Pb alone (except for that in roots of AE) (P < 0.05) (Figure 2). Lead toxicity resulted in lipid peroxidation, where malondialdehyed (MDA) content increased significantly with increased Pb levels (Figure 3). Increased H2O2 concentration and lipid peroxidation with metal exposure revealed that Pb toxicity caused generation of reactive oxygen species and oxidative stress in S. alfredii.
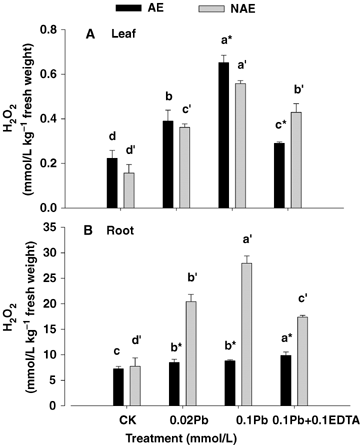
Concentrations of H2O2 in leaf (A) and root (B) of the two contrasting ecotypes of Sedum alfredii treated with Pb and ethylenediaminetetraacetic acid (EDTA).Values are means ±SD (n = 3). Different letters among treatments indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
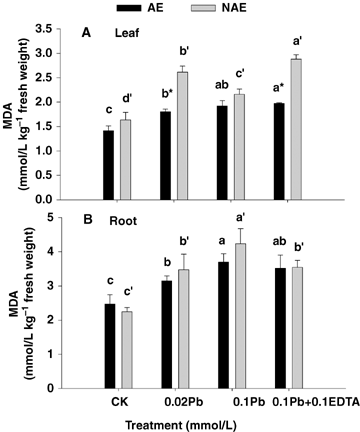
Effects of Pb and ethylenediaminetetraacetic acid (EDTA) on lipid peroxidation products (expressed in terms of malondialdehyed [MDA] concentration) in leaf (A) and root (B) tissues of the two contrasting ecotypes of Sedum alfredii.Values are means ±SD (n = 3). Different letters among treatments indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
Antioxidative enzymes activities
Superoxide dismutase is considered as the first defense against oxidative stress, which dismutates two superoxide radicals to H2O2 and oxygen and thus maintains superoxide radicals in steady state levels. SOD activity increased significantly in the leaves and roots of AE plants as well as in the leaves of NAE under Pb and its combinations with EDTA, whereas it was noticed that SOD activity decreased in roots of NAE (P < 0.05) (Figure 4A, B).
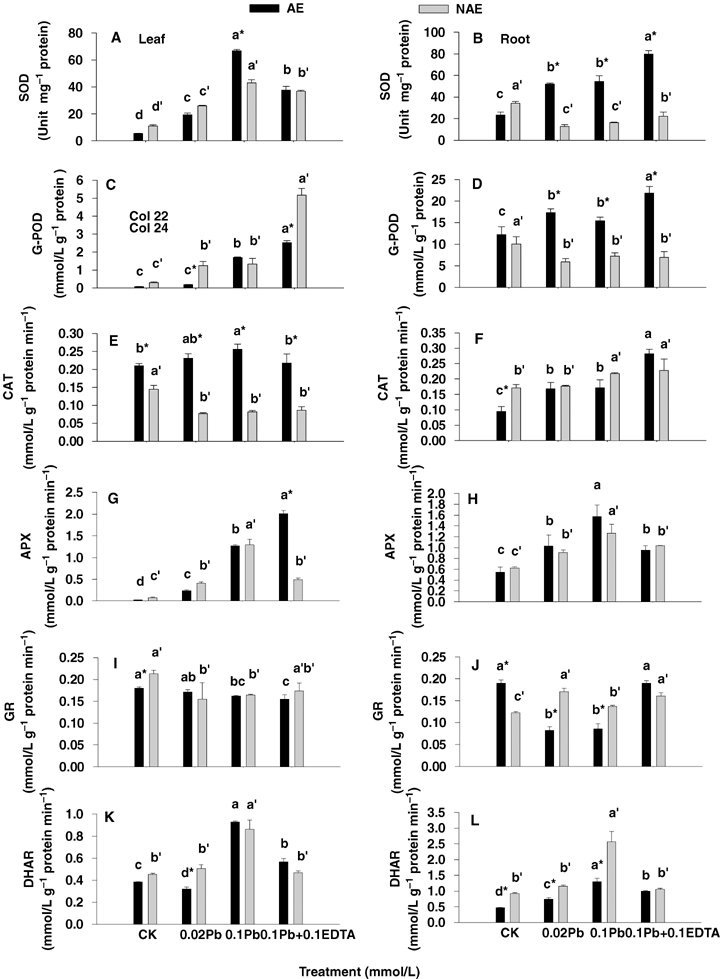
Changes in antioxidative enzymes activity in leaf (left panel) and root (right panel) of the two contrasting ecotypes of Sedum alfredii treated with Pb and ethylenediaminetetraacetic acid (EDTA).Superoxide dismutase (SOD) (A and B), guaiacol peroxidase (G-POD) (C and D), catalase (CAT) (E and F), ascorbate peroxidases (APX) (G and H), glutathione reductase (GR) (I and J) and dehydroascorbate reductase (DHAR) (K and L). Values are means ±SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
Catalase, APX, and G-POD contribute to the elimination of excessive H2O2 in plant cells. The activity of G-POD was dramatically increased by Pb treatments and Pb combined with EDTA in the leaves of both ecotypes, where the G-POD activity in leaves of AE and NAE grown under 0.1 mmol/L Pb +0.1 mmol/L EDTA were 1.48-fold and 3.88-fold higher, respectively, than those of the plants exposed to 0.1 mmol/L Pb alone (Figure 4C). Moreover, there was an elevated activity of G-POD in roots of AE other than NAE under Pb treatment (Figure 4D). However, CAT activity declined about 50% with increasing Pb toxicity in the leaves of NAE (Figure 4E), whereas an enhancement in CAT activity was observed in the roots of both AE and NAE as well as in the leaves of AE (Figure 4E, F). Nevertheless, the activities of APX in leaves and roots were significantly elevated in lead stressed plants of both ecotypes of S. alfredii, and the magnitude of elevation ranged from 1.67- to 77.8-fold over CK, whereas APX activity in the leaves of AE exposed to 0.1 mmol/L Pb/0.1 mmol/L EDTA was recorded 58.6% higher than that in presence of 0.1 mmol/L Pb alone (P < 0.05) (Figure 4G, H).
The two enzymes GR and DHAR, which use glutathione as reductant for converting DHA to ascorbic acid (AsA) are closely involved in glutathione (GSH) and AsA changes. Statistical analysis showed a significant increase in DHAR activity in both root and leaves of NAE and AE under Pb stress, and the highest DHAR activity in both leaves and roots were noted after exposing plants to 0.1 mmol/L Pb (Figure 4K, L). GR activity slightly decreased in leaves of both AE and NAE under lead stress as compared with their CK (Figure 4I), whereas GR activity was elevated in the roots of NAE and remarkably declined in the roots of AE exposed to 0.02 mmol/L and 0.1 mmol/L Pb, but recovered in the presence of 0.1 mmol/L Pb combined with 0.1 mmol/L EDTA (Figure 4J).
Antioxidants
Not only does the enzymatic antioxidative system comprise SOD, CAT, G-POD, APX and GR, but also the nonenzymatic antioxidants (e.g. glutathione and ascorbate) are involved in the scavenging system controlling ROS. A significant enhancement in GSH and AsA levels was observed in both the roots and leaves of NAE and AE in the presence of Pb alone and its combination with EDTA (P < 0.05) (Figures 5 and 6). Interestingly, the GSH contents were much higher in the leaves of AE than those of the NAE, but the situation was opposite in roots (Figure 5). Particularly, the AsA pools were much higher (ranging from 1.27- to 2.68-fold) in both NAE tissues than those in AE (P < 0.05) (Figure 6A, B). However, insignificant changes in GSH and AsA concentrations were noted in both ecotypes exposed to 0.1 mmol/L Pb +0.1 mmol/L EDTA compared with those treated at 0.1 mmol/L Pb alone. The DHA contents in the roots of both ecotypes displayed a 1.41−11.22-fold increase compared with leaves, whereby the ratios of AsA to DHA in leaves (reduced AsA/DHA ratio ranging from 1.38−6.84) were much higher than those in roots (reduced AsA/DHA ratio less than 1) in effect, altering more to the reduced AsA form (P < 0.05) (Figure 6).
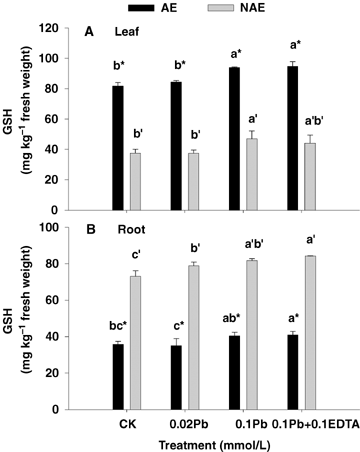
Concentrations of reduced glutathione (GSH) in leaf (A) and root (B) of the two contrasting ecotypes of Sedum alfredii treated with Pb and ethylenediaminetetraacetic acid (EDTA).Values are means ± SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
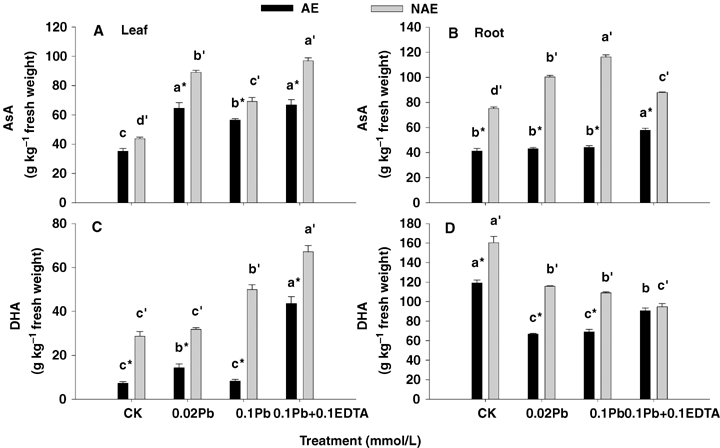
Concentrations of reduced AsA and dehydroascorbate (DHA) in leaf (left panel) and root (right panel) of the two contrasting ecotypes of Sedum alfredii treated with Pb and EDTA.Reduced ascorbic acid (AsA) (A and B) and DHA (C and D). Values are means ±SD (n = 3). Different letters among treatment indicate significant differences at P < 0.05. Asterisks (*) indicate significant difference between accumulating ecotype (AE) and non-accumulating ecotype (NAE) plants (P < 0.05).
Discussion
Lead belongs to an elemental group that is not involved in plant metabolism. It is quite important to understand the mechanisms behind unharmed survival of plants in an environment contaminated with Pb compounds (Piechalak et al. 2003). Earlier studies have shown that most of the lead remains in bound form either in cell walls or in vacuoles (Rebechini and Hanzely 1974; Verma and Dubey 2003). Mishra et al. (2006) reported that lead accumulation may be attributed to its binding to cell wall and other different ligands such as GSH, phytochelatins (PCs) etc.
Numerous reports on the use of synthetic chelators in enhancing uptake and transport of heavy metals by plants have been published in recent years (Lopez et al. 2005; Luo et al. 2005; Evangelou et al. 2006; Tandy et al. 2006). In our experiment, after 6 days of cultivation with 0.02 mmol/L lead nitrate, over 84.2% of Pb absorbed by plants was accumulated in the roots of AE, while 94.9% of Pb was accumulated in the roots of NAE (Table 1). Some earlier studies of legumes including Lupinus luteus (Tomaszewska et al. 1996), Pisum sativum, Vicia faba and Phaseolus vulgaris (Piechalak et al. 2002) have also shown that over 90% of Pb uptake was accumulated in the roots. With the increasing Pb treatment from 0.02 mmol/L to 0.1 mmol/L, Pb concentration in the shoot of AE plants increased by 6.7 times. EDTA addition at a concentration of 0.1 mmol/L made it possible to raise the Pb translocation to shoots from 16.8% to 30.5% (Table 1). This increase is not much if we consider the results of Huang et al. (1997) who found greater than a 100-fold increase in the amount of Pb accumulated in the shoots of pea under soil cultivation. Addition of EDTA to Pb-contaminated medium or soil results in the formation of Pb-EDTA complexes and in this form Pb enters the plant more easily (Vassil et al. 1998; Wu et al. 1999). The results obtained from the hydroponics studies may differ from the data concerning soil cultivation because in the soil, there are additional active factors that are absent in hydroponics. For instance in soil, soil chemistry and the presence of different types of organic compounds play the role of ligands for trace metals (Piechalak et al. 2003). Moreover, there are soil microorganisms that exert a significant effect on the activity of many metals including Pb, and are able to conduct methylation of chemical elements (Kabata-Pendias and Pendias 1999).
Compared with CK, the treatment of 0.02 mmol/L Pb had no effects on the growth of both ecotypes of S. alfredii; in contrast, in Pb treatment level up to 0.1 mmol/L, the shoot dry weights of both ecotypes decreased significantly (P < 0.05), and it could be found that the decrease for AE (25.5%) was less than that of NAE (37.5%) obviously showing that AE had more tolerance to the Pb toxicity (Table 2). At combination of 0.1 mmol/L EDTA with 0.1 mmol/L Pb, the shoot biomass of both ecotypes of S. alfredii increased slightly compared with those treated with Pb alone (P > 0.05), which may be due to the stimulatory effect of chelator. The toxic effects of heavy metal on plants are directly reflected by root morphology (Marschner 1995). Earlier studies concerning root morphology of S. alfredii were mainly focused on the root length (He et al. 2002), but other parameters of root morphology especially under the stress of Pb + EDTA have not been reported. It can be seen from Table 2 that Pb treatment inhibited the root growth in both ecotypes of S. alfredii, and the addition of EDTA alleviated Pb toxicity of root length to a certain extent. The same trend could be traced in the surface area and volume, while for root diameter; Pb treatments had no significant effect on both ecotypes of S. alfredii compared with CK (P > 0.05).
Besides affecting plant growth, Pb toxicity also adversely interrupted photosynthetic activity by causing distortion of chloroplast ultra-structure, inhibition of the synthesis of photosynthetic pigments and enzymes of Calvin cycle (Mishra et al. 2006). Reduction in chlorophyll content may be attributed to inhibition of δ-aminolevulinic acid dehydratase (ALAD) caused by Pb uptake (Prasad and Prasad 1987), impaired uptake of essential elements such as Mn and Fe or damage of photosynthetic apparatus or due to chlorophyll degradation by increased chlorophyllous activity (Sharma et al. 2005). It could be seen that both chlorophyll a and b did not decrease significantly for AE plants after treating with 0.1 mmol/L Pb or 0.1 mmol/L Pb +0.1 mmol/L EDTA (P < 0.05) (Figure 1A, B); while there was significant decrease for the NAE plants, displaying that AE plants have stronger tolerance to Pb toxicity than NAE. Total chlorophyll and carotene content of both the ecotypes of S. alfredii decreased significantly (P < 0.05) at 0.1 mmol/L Pb or 0.1 mmol/L Pb +0.1 mmol/L EDTA, showing Pb stress on the synthesis of photosynthetic pigments (Figure 1C, D).
Various studies have reported that heavy metal stress can cause an overproduction of hydrogen peroxides resulting in membrane damage (Verma and Dubey 2003; Thomas Ruley et al. 2004; Reddy et al. 2005; Mishra et al. 2006). In the present study, Pb toxicity also induced H2O2 accumulation and lipid peroxidation in S. alfredii (Figures 2 and 3). When lead entered the plants, significant changes in the activity of antioxidative enzymes were recorded. The activities of SOD, G-POD, CAT, APX and DHAR were all elevated in both leaves and roots of AE, but GR activity was depressed. Moreover, an enhancement was noticed in SOD, G-POD, APX and DHAR activities in leaves of NAE under Pb stresses, whereas SOD and G-POD were declined in the roots (Figure 4). The enhancement in activities of these enzymes may contribute to resist Pb-induced oxidative burst in the plants. An increase in CAT, which is a key enzyme involved in the removal of toxic peroxides, was observed in roots of both ecotypes as well as in the leaves of AE other than NAE (Figure 4E, F). The effect of Pb toxicity on CAT activity differed in plant species and tissues in previous reports. Verma and Dubey (2003) reported that Pb treatment resulted in a decline in catalase activity in roots, whereas in shoots catalase activity increased in rice seedlings grown at moderately toxic Pb levels (0.5 mmol/L), whereas a highly toxic Pb level (1 mmol/L) led to a marked inhibition in enzyme activity. Conversely in horsegram (Macrotyloma uniflorum (Lam.) Verdc.), bengalgram (Cicer arietinum L.) and lead accumulating plant Sesbania drummondii, CAT activity was elevated after Pb treatment (Thomas Ruley et al. 2004; Reddy et al. 2005).
The glutathione and ascorbate pools were remarkably increased under Pb stress (Figures 5 and 6) and these results are in agreement with previous studies by Sun et al. (2005) who also revealed that Pb treatments resulted in an increase of GSH for both ecotypes of S. alfredii. Interestingly, the induction of DHAR activity but depression in GR activity were observed in this plant (Figure 5), which suggested that Pb exposure might result in accelerated GSH synthesis in the S. alfredii to account for the high GSH levels in the tissues. However, compared with 0.1 mmol/L Pb alone, Pb supplement with EDTA showed an insignificant change in concentrations of GSH and AsA for both ecotypes. This was different from the results obtained by Piechalak et al. (2003) who demonstrated a significant effect of EDTA on the GSH content in Pisum sativum grown under Pb stress.
Materials and Methods
Plant materials and treatment condition
The AE of S. alfredii was collected from an old Pb/Zn mined area and the NAE of S. alfredii was obtained from a tea garden of Hangzhou in Zhejiang Province of China.
Healthy and equal-sized shoots of both the ecotypes were chosen and grown for 2 weeks in the modified nutrient solution, in which KH2PO4 concentration was adjusted to 0.01 mmol/L in order to prevent precipitation of lead. The composition of the nutrient solution used for Pb treatment was as follows (in mmol/L): 2 KNO3, 0.05 KCl, 0.5 Ca(NO3)2·4H2O, 0.2 MgSO4·7H2O, 0.1 NH4NO3, 0.01 KH2PO4, 0.012 H3BO3, 0.002 MnSO4·H2O, 5 x 10−4 ZnSO4·7H2O, 2 x 10−4 CuSO4·5H2O, 1 x 10−4 Na2MoO4, 1 x 10−4 NiSO4, 0.02 Fe-EDTA. After pre-culturing for 14 days, the plants were transferred to 2.5-L pots. After 4 days, different Pb treatments were given. Four treatments were used: (i) control (CK); (ii) 0.02 mmol/L Pb; (iii) 0.1 mmol/L Pb; and (iv) 0.1 mmol/L Pb/0.1 mmol/L EDTA. Pb was used in the form of Pb(NO3)2. The experiment was randomly arranged with three replicates for each treatment. Plants were grown under glasshouse conditions with natural light, day/night temperature of 25/20°C and humidity of 70–90%. Nutrient solution pH was adjusted daily to 5.8 with 0.1 mol/L NaOH or 0.1 mol/L HCl. The nutrient solution was continuously aerated and renewed with treatments after every 3 days. For better physiological response of enzyme activity under Pb toxicity, 6 days was selected as the treatment time.
Measurement of root morphological parameters
Root morphological parameters such as root length, surface area, diameter, and volume were measured after treatment for 6 days. Root length, surface area, diameter, and volume were determined with root automatism scan apparatus (MIN MAC, STD1600+, Tokyo, Japan), equipped with WinRHIZO software offered by Regent Instruments Company.
Element analysis
Different plant parts were separated and thoroughly washed with distilled and de-ionized water, oven-dried at 70°C for 72 h, weighed and ground with a stainless steel mill, then passed through 0.1 mm nylon sieve. About 0.1 g of the plant sample was digested using the HNO3/HClO4 digestion method. The digested solutions were washed into 50-mL flasks and made volume using de-ionized water. The plant Pb concentrations were determined using integrated couple plasma mass spectrophotometry (ICP-MS) (Agilent, Tokyo, Japan).
Chlorophyll contents
At the time of harvest, fresh leaves of both of the ecotypes were collected for the determination of chlorophyll contents. Leaves were cut into small pieces and 0.5 g of the sample was put into the glass tubes. Then 10 mL of 80% acetone was added to the tubes and kept overnight for complete extraction. Chlorophyll contents were determined spectrophotometrically using the visible wavelengths of 663 645 and 480 nm for chlorophyll a, b and carotene respectively.
Lipid peroxidation and H2O2 content assay
The measurement of lipid peroxidation and H2O2 content was carried out according to an earlier method (Velikova et al. 2000). Each sample (1.0 g) was homogenized with 4 mL 0.1% (w/v) trichloroacetic acid (TCA) under ice bath conditions. The homogenate was centrifuged at 12 000 g for 20 min and the supernatant was obtained for both lipid peroxidation and H2O2 analysis. The thiobarbituric acid (TBA) test, which determines malonaldehyde (MDA) as an end-product of lipid peroxidation, was used for the measurement of lipid peroxidation in samples. To 1 mL of the supernatant, 1 mL of 20% (w/v) TCA comprising 0.5% (w/v) TBA was added. The mixture was incubated in boiling water for 30 min and then the reaction was immediately stopped by placing the tubes in an ice bath. The tubes were centrifuged at 1500 g for 10 min, and the absorbance was read at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of MDA-TBA complex was calculated from the extinction coefficient 155 mM−1 cm−1. For assay of H2O2 concentration, 1 mL of the supernatant was added to 1 mL of 10 mmol/L potassium phosphate buffer (pH 7.0) and 2 mL of 1 mol/L KI. H2O2 concentration was estimated based on the absorbance at 390 nm.
Antioxidant enzyme activity assay
Both leaves and root samples of a known weight (1.0 g) were homogenized in 6 mL cold 50 mmol/L potassium phosphate buffer (pH 7.8) containing 0.2 mmol/L EDTA and 2% (w/v) polyvinylpyrrolidone (PVP) in an ice bath using a prechilled mortar and pestle. The homogenate was centrifuged for 20 min at 12 000 g at 4°C and the supernatant obtained was used for enzyme analysis. An aliquot of the extract was used to determine protein content following the methods described by Bradford (1976), using bovine serum albumin as standard.
Superoxide dismutase (EC 1.15.1.1)
Superoxide dismutase activity was determined by the photochemical method provided by Giannopolitis and Ries (1977). One unit of SOD activity was defined as the amount of enzyme required to cause a 50% inhibition of the rate of NBT reduction at 560 nm.
Guaiacol peroxidase (EC 1.11.1.7)
The activity of G-POD was assayed following the previous method of Cakmak et al. (1993). The reaction mixture contained 25 mmol/L potassium phosphate buffer (pH 7.0, containing 0.1 mmol/L EDTA), 0.05% guaiacol, 10 mmol/L H2O2, and the enzyme. Activity was measured by the increase in absorbance at 470 nm caused by guaiacol oxidation (E = 26.6 mM−1 cm−1).
Catalase (EC 1.11.1.6)
Catalase activity was assayed in a reaction mixture containing 25 mmol/L potassium phosphate buffer (pH 7.0, containing 0.1 mmol/L EDTA), 10 mmol/L H2O2, and the enzyme. The decomposition of H2O2 was followed at 240 nm (E = 39.4 mM−1 cm−1) (Cakmak and Marschner 1992).
Ascorbate peroxidase (EC 1.11.1.11)
Ascorbate peroxidase activity was measured using a previous procedure (Nakano and Asada 1981) through monitoring the rate of ascorbate oxidation at 290 nm (E = 2.8 mM−1 cm−1). The reaction mixture contained 25 mM potassium phosphate buffer (pH 7.0, containing 0.1 mmol/L EDTA), 100 mmol/L H2O2, and 0.25 mmol/LAsA, and the enzyme aliquot.
Glutathione reductase (EC 1.6.4.2)
Glutathione reductase activity was assayed followed the previous method (Foyer and Halliwell 1976) by monitoring the decrease in absorbance at 340 nm caused by nicotinamide-adenine dinucleotide phosphate (NADPH) oxidation (E = 6.2 mM−1 cm−1). The reaction mixture contained 25 mmol/L potassium phosphate buffer (pH 7.8, containing 0.2 mmol/L EDTA), 0.5 mmol/L glutathione disulphide (GSSG), 0.12 mmol/L NADPH, and the enzyme aliquot.
Dehydroascorbate reductase (EC 1.8.5.1)
Dehydroascorbate reductase activity was measured by monitoring the increase in absorbance at 265 nm (E = 14 mM−1 cm−1) due to ascorbate formation (Nakano and Asada 1981). The reaction mixture composed of 25 mmol/L potassium phosphate buffer (pH 7.0, containing 0.1 mmol/L EDTA), 3.5 mmol/LGSH, 0.4 mmol/L DHA and the enzyme aliquot.
Antioxidants assay
Glutathione concentration
The levels of GSH were estimated fluorimetrically following Hissin and Hilf (1976) with some modifications. Each sample was extracted on an ice bath with 3 mL of 100 mmol/L phosphate buffer (pH 8.0, containing 5 mmol/L EDTA) and 1 mL of 25% meta-phosphoric acid; and was centrifuged at 10 000 g for 30 min. The supernatant was further diluted five times with phosphate-EDTA buffer (pH 8.0). The final assay mixture (2.0 mL) contained 0.1 mL of the diluted tissue supernatant, 1.8 mL of phosphate-EDTA buffer, and 0.1 mL of the o-phthaladehyde (OPT) solution. After mixing thoroughly and incubating at room temperature for 15 min, fluorescence at 420 nm was determined with activation at 350 nm.
Ascorbic acid and dehydroascorbate content
Levels of AsA and DHA followed the procedure described by Singh et al. (2006) with a small modification. Briefly, a known weight (1.0 g) of sample was extracted with 3 mL of 5% (w/v) TCA and centrifuged at 18 000 g for 15 min. Total ascorbate (AsA+DHA) was determined in a reaction mixture consisting of 0.2 mL of supernatant, 0.5 mL of 150 mmol/L phosphate buffer (pH 7.4, containing 5 mmol/L EDTA) and 0.1 mL of 10 mmol/L dithiothreitol (DTT) to reduce DHA to AsA. After 10 min at room temperature, 0.1 mL of 0.5% (w/v) N-ethylmaleimide was added to remove excess DTT. AsA was assayed in a similar manner except that 200 mL of deionized water was substituted for DTT. Color was developed in both reaction mixtures with the addition of 0.4 mL of 10% (w/v) TCA, 0.4 mL of 44% (v/v) phosphoric acid, 0.4 mLof α,α′-dipyridyl in 70% (v/v) ethanol and 0.2 mL of 3%(w/v) FeCl3. The reaction mixtures were incubated at 40°C for 40 min and the absorbance was read at 532 nm. DHA was estimated from the difference between total ascorbate and AsA.
Data analysis
Statistical analysis was carried out using the SPSS statistical package (version 11.0). All values reported in this work are means of at least three independent replications. Data were tested at significant levels of P < 0.05 by two-way ANOVA.
(Handling editor: Jin-Zhong Cui)




