Possible Indirect Effects of Mammal Hunting on Dung Beetle Assemblages in Panama
Received 8 September 2005; revision accepted 20 March 2006.
ABSTRACT
enHunting often impacts rain forest mammal communities but little is known about its indirect effects on other taxa. We examined dung beetle assemblages using pitfall and flight-intercept traps at six rain forest sites in Panama that ranged in hunting intensity. Heavily hunted sites showed altered community composition, significantly fewer species (based on rarefaction), and lower abundances of dung beetles than did sites with little hunting. Our results suggest that intensive hunting affects nontarget forest taxa and is potentially altering ecosystem functioning.
RESUMEN
esLa cacería generalmente tiene impactos sobre las comunidades de mamíferos en bosques tropicales, sin embargo poco se sabe sobre los efectos indirectos sobre otros taxa. Examinamos los ensamblajes de escarabajos coprófagos usando trampas de caída y de intercepción de vuelo en seis sitios en Panamá que varían en su intensidad de cacería. Sitios con mucha cacería mostraron alteración en la composición de especies, significativamente menos especies (basado en rarefacción), y menores abundancias de escarabajos coprófagos, que sitios con poca cacería. Nuestros resultados sugieren que la cacería intensa de mamíferos está afectando a otros taxa indirectamente, con potenciales consecuencias para el funcionamiento del ecosistema.
Numerous studies have assessed the direct effects of anthropogenic disturbances on ecosystem components. Often these effects are straightforward and easy to predict. For example, selective logging and hunting will cause a decrease in the populations of most organisms targeted for harvest. Besides direct effects, disturbances may also have indirect effects, often referred to in the literature as higher-order effects, or cascading effects, when several steps of consequential effects are involved (e.g.,Redford 1992, Wright 2003, Letorneau et al. 2004, Terborgh et al. 2006). Although it is common practice to infer the likelihood of indirect effects based on measured direct effects, recent studies have shown that indirect effects on biotic components of the ecosystems are site-, time-, and species-specific. Consequently, it is difficult and could be misleading to predict such indirect effects, and they ought to be determined empirically (Didham et al. 1996, Wright 2003, Hamer et al. 2005).
An important biotic component of most terrestrial ecosystems is the community of beetles belonging to the subfamily Scarabaeinae, commonly known as dung beetles (Hanski & Cambefort 1991). Through their dung-burying behavior, dung beetles contribute to several ecosystem functions such as soil fertilization, soil aeration, nutrient cycling, pest control, and secondary seed dispersal (Bergstrom et al. 1976, Nealis 1977, Mittal 1993, Miranda et al. 1998, Andresen 2002, Andresen & Levey 2004). Several studies have documented direct and indirect effects on dung beetle communities caused by disturbances such as deforestation, forest fragmentation, and selective logging, using these insects as bioindicators (Halffter & Favila 1993, Davis 2000, Davis et al. 2001, Estrada & Coates-Estrada 2002, Halffter & Arellano 2002, Vulinec 2002, Andresen 2003, Larsen et al. 2005, Pineda et al. 2005, Quintero & Roslin 2005). However, the indirect effects of hunting of mammals on dung beetles have not, to our knowledge, been assessed previously.
Hunting is considered the second greatest threat, after habitat destruction, for many tropical mammal species (Redford 1992, Entwistle & Dunstone 2000, Milner-Gulland & Bennett 2003), and it is recognized that the indirect effects of hunting are diverse (Wright & Duber 2001, Wright 2003). Because of the dependence of dung beetles on mammal dung, reductions in the abundance and/or richness of these insects in areas where mammals have been decimated by hunting would be expected. Studies on the effects of forest fragmentation generally attribute the decrease in the dung beetle fauna, at least partially, to impoverished mammal communities (Klein 1989, Estrada et al. 1998, Vulinec 2000, Estrada & Coates-Estrada 2002, Andresen 2003, Feer & Hingrat 2005). Two studies have even found a positive correlation between the abundance of nonflying mammals and the abundance and richness of the dung beetles in forest fragments (Estrada et al. 1998, 1999; Feer & Hingrat 2005).
In fragmentation studies it is difficult to attribute dung beetle declines to a decrease in mammal abundances because both animal groups could be responding to a common cause such as habitat deterioration. Moreover, hunting does not affect all mammal species equally, and an increase in the populations of some species could compensate in terms of dung production (Wright 2003). Also, it is known that most tropical rain forest dung beetles are generalists in their feeding preferences (Hanski & Cambefort 1991), and thus they may switch to or increase their use of other resources when dung is scarce (Peck & Forsyth 1982). Finally, species-rich and abundant dung beetle assemblages have been reported for sites where mammal hunting is intense (Howden & Nealis 1975, Peck & Forsyth 1982). Here we present preliminary data to resolve the question: Does mammal hunting have a negative indirect effect on dung beetles via its direct negative effect on mammals?
We censused dung beetles at six of the eight sites used by Wright et al. (2000) in a study of hunting impacts in central Panama: Barro Colorado Island (BCI), Gigante Peninsula (GIG), Limite (LIM), Plantation Road (PLA), Sendero Las Cruces (SLC), and Carretera 25 (C25; Table 1). The first two sites are within the Barro Colorado Nature Monument, while the others are within Soberanía and Camino de Cruces National Parks. We excluded two of the sites used by Wright et al. because of differences in bedrock and tree-species composition (Rio Macho) and security issues (Rio Mandinga). All sites are within the Panama Canal Zone, and have similar lowland secondary rain forest with 20–30 m-tall canopies (Wright et al. 2000). The maximum linear distance among sites was ca 18 km (for a map see Wright et al. 2000). Long-term monthly averages for temperature at BCI range from 23–32°C with mean annual rainfall from 2188–2612 mm (Windsor 1990). A pronounced dry season occurs between January and April, with < 100 mm of monthly precipitation.
| Sitea | Hunting intensityb | Mammal abundancec | Number of beetle individuals | Number of beetle species | Rarefied number of speciesd | Sampling datee | Dry-season daysf |
|---|---|---|---|---|---|---|---|
| BCI | 1 | 5.31 | 849 | 28 | 20.22 | Feb 11 | 60 |
| (17–23) | |||||||
| GIG | 2 | 3.50 | 296 | 18 | 13.12 | Mar 16 | 93 |
| (11–16) | |||||||
| LIM | 3 | 2.23 | 284 | 24 | 16.38 | Feb 17 | 66 |
| (13–20) | |||||||
| PLA | 3 | 1.49 | 172 | 18 | 14.86 | Feb 21 | 70 |
| (12–17) | |||||||
| SLC | 4 | 1.01 | 78 | 12 | 12.00 | Mar 10 | 87 |
| (12–12) | |||||||
| C25 | 4 | 0.95 | 258 | 14 | 11.38 | Feb 24 | 73 |
| (9–13) | |||||||
- a BCI = Barro Colorado Island; GIG = Gigante Peninsula; LIM = Límite; PLA = Plantation Road; SLC = Sendero Las Cruces; C25 = Carretera C-25.
- b From Wright et al. (2000); ranks are from lowest (1) to highest (4) hunting intensity.
- c Expressed per linear km of transect. Calculated from data in Table 2 from Wright et al. (2000), by adding the number of troops for primates (three species), and the number of individuals for the other mammals (seven species) and dividing this number by the total number of km in transects.
- d Mean values and 95% confidence intervals (in parentheses) calculated with an individual-based rarefaction using 78 individuals as the baseline abundance level.
- e The dates given correspond to day two of each sampling period; traps were set out on day one, and dung beetles collected on day three.
- f Number of days elapsed since the beginning of the dry season, on 14 December 2004.
In 1997, Wright et al. (2000) collected transect data on mammal abundance for ten species (diurnal species: Geoffroy's tamarin, howler monkey, white-faced monkey, coati, collared peccary, red-tailed squirrel, agouti; diurnal/nocturnal species: anteater, brocket deer, white-tailed deer). They also ranked hunting intensity at each site by combining information provided by forest guards and physical evidence of poaching (see Wright et al. for details). In January 2006 we re-interviewed forest guards and reviewed poacher-arrest records, which led us to conclude that hunting intensity has not changed significantly since 1997; hence we use the same ranking system as did Wright et al. (2000) to describe hunting intensity at our study sites (Table 1). To estimate overall mammal abundance, we used the data in Wright et al. (2000) and pooled abundances of all ten species by adding the number of troops (for the three primate species) and the number of individuals (for all other species) encountered per kilometer of transect (Table 1). A very strong and significant negative correlation was found between hunting intensity and pooled mammal abundance (R2=−0.977, P < 0.001).
Dung beetles were sampled over a 5-week period (February 10–March 17) during the dry season of 2005, using ten pitfall and six flight-intercept traps per site. At each site, traps were set out along two perpendicular transects: a pitfall-trap transect (with traps spaced 10 m apart) and a flight-intercept-trap transect (with traps 20 m apart). Pitfall traps consisted of plastic containers (12 cm high × 12 cm wide) buried with the rim at ground level. Flight-intercept traps were squares of black nylon mosquito-net (1.5 × 1.5 m) stretched perpendicularly to the forest floor between trees above a set of shallow aluminum-foil pans. The lower edge of the trap was approximately 20 cm above the soil surface and extended upwards 1.5 m. Containers and pans were partly filled with water containing salt and detergent, and were protected from rain by either a plastic plate (20 cm above each pitfall) or a plastic tarpaulin. All traps were baited with 40 g of a homogenized mixture of fresh tapir, deer, primate, and peccary dung collected from the nearby Summit Zoo. Traps were set out in the morning and kept open for 48 h. All captured beetles were collected, counted, and identified to species.
Rainfall seasonality is known to affect dung beetle assemblages (Janzen 1983, Hanski & Cambefort 1991, Andresen 2005). At BCI, for example, biomass values for dung beetle communities peak in the early dry season (Howden & Young 1981), after which populations commonly decline dramatically as the dry season progresses (H. Stockwell, pers. comm.). The dry season of 2004–2005 had a length of 143 d (14 December 2004 to 6 May 2005; Panama Canal Authority, pers. comm.). We calculated the number of days since the beginning of the dry season until the sampling date for each study site (Table 1), and included this variable (number of dry-season days) in our analyses. The smallest number of individuals captured in a site (78 individuals, see Table 1) was used as the baseline abundance level to obtain a rarefied, comparable, mean number of species, with their 95% confidence interval, for each of the sites (Table 1). Individual-based rarefaction was performed with 1000 iterations using the program Ecosim 7.0 (Gotelli & Entsminger 2006). Beetle abundance and rarefied richness values were examined separately using best-subsets regression with two potential predictors: mammal abundance and number of dry-season days. The best predictor(s) were then included in multiple regression analyses. Because mammal abundance and hunting intensity were so strongly correlated, we used only mammal abundance in our analyses and assumed it to be a surrogate of hunting intensity. We calculated the proportions of diurnal and nocturnal species and individuals for each of the sites and, using arcsine-square root-transformed data, tested whether these were related to mammal abundance. Finally, we compared dung beetle communities among sites using nonmetric multidimensional scaling (NMS) with Sørenson's similarity index on the PC-ORD package (McCune & Mefford 1999). The significance of ordination results was tested using a Monte-Carlo permutation procedure (100 runs). Individual beetle species were correlated with ordination axes using Bonferroni-corrected alpha values to reduce the experiment-wise error rate. Finally, we used linear regression to determine relationships among ordination axes, mammal abundance, and number of dry-season days.
We captured a total of 1937 dung beetles belonging to 30 species of Scarabaeinae (Table S1). Beetle abundance and rarefied richness were highest at BCI, the site with the lowest hunting intensity and highest pooled mammal abundance. SLC, one of the two sites with lowest mammal abundances, had the lowest beetle abundance; on the other hand, C25, the other site with lowest mammal abundance, had the lowest rarefied species richness (Table 1). Most (73%) beetle species collected were classified as diurnally active.
The relationship between the log abundances of dung beetles and mammals was positive and significant (Fig. 1a; F1,5= 7.73, P < 0.05), with mammal abundance explaining 66 percent of the variation of beetle abundance among the study sites. Number of dry-season days at the time of sampling did not appear to affect dung beetle abundance (P > 0.05). In terms of the proportional abundance of nocturnal and diurnal beetles we found that the proportion of the former declined significantly with increasing mammal abundance (Fig. 1b; F1,5= 12.89, P < 0.023), while the latter, as a logical consequence, increased.
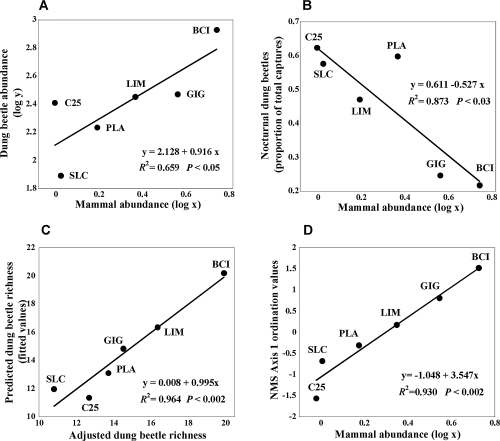
The relationship between (a) total dung beetle abundance and mammal abundance, (b) relative abundance of nocturnal dung beetles (proportion of total captures) and mammal abundance, (c) the observed and fitted values of dung beetle rarefied species richness, based on a multiple-regression model with two significant predictor variables, mammal abundance and time since onset of the dry season, and (d) the ordination (NMS) axis of the dung beetle community and mammal abundance at six rain forest sites in Panama.
The rarefied species richness of dung beetles was also significantly affected by mammal abundance (P < 0.05) and showed a significant effect of the number of dry-season days (P < 0.05), such that a multiple regression model with these two explanatory variables was significant (F2,5= 16.44, P < 0.03). The fitted values of the multiple regression explained 96 percent of the rarefied species richness across our sites (Fig. 1c). Unlike with the proportional dung beetle abundances, the proportion of diurnal and nocturnal species was not associated with mammal abundance (F1,5= 2.34, P= 0.20).
The community composition of dung beetles differed among sites, with BCI supporting the most diverse community, with 28 of the 30 recorded species, and the other sites each having a smaller subset of this total (Table 1). BCI had the most abundant species (Canthon lamprimus Bates, N= 382), whereas two other common species were found at the hunted sites LIM (Canthon aequinoctialis Harold, N= 294) and C25 (Uroxys micros Bates, N= 273). Rare species (< 5 individuals) were also more frequently captured at BCI, with two of the three rarest species captured there (Table S1). An ordination analysis of the dung beetle community identified a single axis (Axis 1) as significantly capturing 80 percent of the variation among sites (stress value = 11.04; Monte-Carlo test, P < 0.05). A linear regression of Axis 1 and mammal abundance strongly suggests that the dung beetle community at our sites responds to a significant gradient in mammal abundance (Fig. 1d; F1,5= 67.05, P < 0.005) that is most likely related to hunting intensity. Of the 30 beetle species, 20 were positively and significantly correlated with Axis 1, and ten of these species showed highly significant correlations (P < 0.0001; Table S1). There was no relationship between Axis 1 and number of dry-season days.
We consider this study preliminary, as our sampling effort was limited both spatially and temporally. Yet our results clearly suggest that the hunting of mammals in a Panamanian rain forest is having a number of significant impacts on the dry-season dung beetle assemblages. First, total abundance of dung beetles was found to decline with decreasing mammal abundances. Second, rarefied species richness of dung beetles was also found to decline. Third, a conservative estimate of individual species responses found that 20 out of the 30 collected species declined in response to decreasing mammal abundances. Finally, we observed that the proportional abundance of nocturnal beetles showed a significant increase with decreasing mammal abundances.
It is not possible from our study to determine whether the overall availability of dung is responsible for the observed effects, or whether the availability of the dung of a particular taxon is causing beetle declines. For the Neotropics several studies have suggested that howler monkeys (Alouatta spp.) are very important in maintaining diverse and abundant dung beetle communities (Howden & Young 1981, Anzures-Dadda 1998, Estrada et al. 1999, Andresen 2003) and that the dung of these primates is a preferred food-resource for dung beetles (Ponce-Santizo et al. 2006). Howler monkeys were present at all of our study sites (E. Andresen, pers. obs.), but their abundance showed a significant negative correlation with hunting intensity (Wright et al. 2000).
The decline in the proportion of diurnal beetle abundance could be a consequence of dung availability decreasing during the day, but not at night in the heavily hunted sites. This could indicate that common diurnal mammals (such as agouti and peccary) are being more heavily hunted than common nocturnal mammals (such as brocket deer, white-tailed deer, and paca). Alternatively, it could indicate that mammals are changing their activity periods in response to hunting (Griffiths & van Shaik 1993), becoming more active at night, such as was found for white-tailed deer elsewhere (Kilgo et al. 1998). Also, nocturnal beetles are often more tolerant than diurnal beetles to the disturbance of forest structure (Vulinec 2002). Although hunting per se does not affect forest structure, and indeed all our sites had comparable forest structure and composition (Wright et al. 2000), nocturnal beetles may be more tolerant to other kinds of disturbances, such as hunting and the associated reduced mammal and dung abundances. The exact mechanism of such tolerance would need to be elucidated, but it could include characteristics such as higher dietary plasticity in nocturnal than diurnal beetles, or higher competitive ability of the former in depleting a scarce resource.
Regarding rainfall seasonality, we did detect a significant effect of the number of dry-season days at the time of sampling on species richness but not on abundance of dung beetles. This research took place during the middle of the dry season, that is, the harshest period for most dung beetle species. During this period, at our study sites, many species survive only as immatures buried in the soil, and are thus not captured in traps. It would be interesting to see if hunting intensity and mammal abundance continue having similar effects on the dung beetle community during the rainy season. It is possible that the effects would be more pronounced due to increased competition for dung as many adult beetles start emerging with the beginning of the rains. But the contrary scenario could also occur if, for example, during the rainy season alternative food, like carrion (Hanski & Cambefort 1991), became more abundant. Short-term assessments of the effects of anthropogenic disturbances, like the present study, that do not consider seasonal variation could potentially produce misleading results (Hamer et al. 2005). Thus, until complementary information is available, caution should be taken when generalizing the results of this study beyond the dry season.
Dung availability is certainly not the only important factor affecting dung beetle communities. Factors such as forest structure, soil type, and rainfall seasonality, among others, can also have important effects on dung beetle community structure and composition (Hanski & Cambefort 1991). Forest structure and soil type were, in general, very similar among our study sites (Wright et al. 2000). Moreover, differences in dung beetle assemblages caused by such characteristics would not fall along a gradient, like that we observed for mammal abundance, they would merely increase variability.
Dung beetles perform several very important ecological roles, derived from their dung-burying behavior (Andresen & Feer 2005 and references therein). It is tempting to infer that these ecological functions will also be affected negatively in areas with high hunting intensity. However, such higher-order indirect effects ought to be assessed directly because the outcome of complex species-interactions can be highly unpredictable (Didham et al. 1996, Andresen 2003, Wright 2003).
Hunting is a pervasive activity, taking place in any site readily accessed by humans, including supposedly protected areas (Redford 1992, Peres & Lake 2003). Thus, it is becoming increasingly important to quantify not only the direct effects, but also the many possible indirect effects of mammal hunting (Wright 2003) in order to fully understand the implications of hunting on ecosystems and to assess the real conservation status of protected areas.
ACKNOWLEDGMENTS
We sincerely thank J. Wright for sponsoring this project; H. Stockwell for beetle identifications; A. Aiello for help with beetle labelling; N. Fabbroni and R. Ewers for field and lab help; Alberto Valencia García for technical assistance with the Figure; and R. Ewers, R. Didham, W. Laurance, and two anonymous reviewers for comments on the manuscript. The Summit Zoo provided fresh mammal dung, and the Autoridad Nacional del Ambiente of Panama provided collecting permits. Finally, we thank the Smithsonian Tropical Research Institute and Universidad Nacional Autónoma de México for financial and logistical support.




