Terpenoid profiles of in vitro regenerated Artemisia petrosa subsp. eriantha (Apennines' genepì)*
We wish to dedicate this work to the students of L’Aquila University, victims of 6 April 2009 earthquake.
Abstract
Artemisia petrosa subsp. eriantha is a protected species whose essential oil is of great interest mainly for liqueur industry; it was micropropagated by in vitro culture technique followed by transfer to field. The content and the characteristics of the essential oils from regenerated plants collected after 1 and 2 years of field growing and from regenerated plants grown ‘in situ’ were assessed by gas chromatography/mass spectrometry (GC/MS) and compared with those of wild plants. Results indicate that in vitro propagated plants produce oils rich in sesquiterpenes. Forty seven compounds were identified, the main constituents being α- and β-thujone, whose content increased with the plant age. Quantitative, but not qualitative variations were observed both in wild and in micropropagated plants in relation with plant age and environmental factors. Thus, micropropagation provides plants suitable for the industrial exploitation of this species.
Introduction
Artemisia petrosa (Baumg.) Jan. ex D.C. ssp. eriantha (Ten.) (Giacomini & Pignatti) (alpine wormwood) of the Asteraceae family is a Central Apennines' subendemic species, which grows in rock crevices and on gravel slopes at altitudes between 2000 and 3100 m (Conti et al., 2005). It differs in morphological characters (length of basal leaves, ear density and length of glandular trichomes) from A. petrosa (Baumg.) Jan. ssp. eupetrosa Giac. & Pignatti, which typically grows in the Alps, Central Pyrenees, Carpathians and Balkans, but is absent in the Central Apennines (Tammaro, 1975). A. petrosa subsp. eriantha has been reported in different locations around the National Park of Gran Sasso and Monti della Laga, and also in the Majella, Sibillini mountains and Maritime Alps (Fig. 1) (Conti et al., 1992). It should be noted that the new combination Artemisia umbelliformis Lam. subsp. eriantha (Ten.) Vallès-Xirau & Brañas after Vallès-Xirau & Brañas (1991) has been proposed for this taxon.
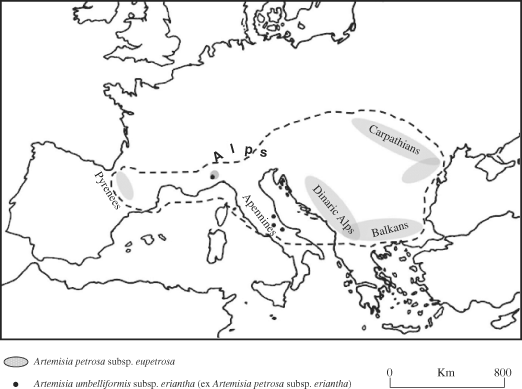
Distribution areas of A. petrosa subsp. eriantha (dots) and A. petrosa subsp. eupetrosa (ovals).
Alpine wormwood possesses a hard aromatic scent and its aerial parts are used as flavouring agent in the preparation of the strong-flavoured liqueur ‘genepì’ (Appendino et al., 1982; Rubiolo et al., 2009), which has a long tradition in North Italy. In folk medicine and in phytotherapy, this plant is known for its antispasmodic, tonic and antiinflammatory properties and as a remedy against cold (Simonnet et al., 2006). Previous research on this species evidenced essential oils rich in thujones with a great variability of concentrations ranging from 48% to 87% (probably owed to the different geographical areas of plant collection) (Rubiolo et al., 2009) and the absence, or very low concentration, of camphor (Bellomaria et al., 1981; Bicchi et al., 1982; Souleles, 1993; Mucciarelli et al., 1995; Rubiolo et al., 2009).
Thujones (4 methyl – 1 – (1 – methylethyl) bicyclo [3.1.0] – hexan – 3 – one), occurring in nature as a mixture of α and β isomers, are typical of some Artemisia species and known for their toxicity (Lachenmeier et al., 2008).
Several studies on the mechanism of the neurotoxicity of α-thujone indicate that it is a modulator of the γ-Aminobutyric acid (GABA) type A receptor (Meschler & Howlett, 1999; Höld et al., 2000), rapidly acting on the GABA-gated chloride channels. Suggestions that thujone activates the CB1 cannabinoid receptor, based on structural similarities of thujone enol to tetrahydrocannibinol, are still controversial (Meschler & Howlett, 1999).
For this reason, the use of thujone-containing plants and products is limited by food regulations: in fact, thujone, expressed as an isomeric mixture, is banned as a flavouring substance in the USA and in the EU is allowed with a limit of 10 mg L−1 in alcoholic beverages with more than 25% volume of alcohol and 35 mg L−1 in bitters (European Commission, 2002). Both α- and β-thujone were found in 24 food additives listed in the priority based assessment of food additives (PAFA) database (Food and Drug Administration, 1997).
Although protected by an Italian Regional Law (L.R. n.47, 11 September 1979) and reported as protected species in the European Habitat Directives 92/43/European Economic Community (EEC), annex V, A. petrosa subsp. eriantha is still an endangered species, and its survival is threatened by indiscriminate harvest by tourists.
To preserve alpine wormwood from extinction and, at the same time, to continue the exploitation of this plant in the liqueur industry, an in vitro propagation method was developed with the aim of obtaining cloned plants (Pace et al., 2004) to be transplanted and grown in field.
In this study we evaluated the content and characteristics of the essential oils from plants obtained by in vitro culture, transplanted to soil and harvested after 1 and 2 years of field growing. The results were compared with those of wild plants and regenerated plants grown in situ to gather information on the oil composition changes occurring during all the steps of this propagative technique; in vitro plantlets were also considered in this analysis.
Materials and methods
Wild plants
Wild plants were gathered in 2001 and 2006 at full flowering stage.
Plant micropropagation
Sterile plantlets of A. petrosa subsp. eriantha and tissue cultures thereof were obtained according to method reported in the study of Pace et al. (2004). Sterile seeds were obtained by 3 min immersion in 70% ethanol, followed by 30 min in 10% commercial bleach and several rinses with sterile water. They were germinated on Murashige and Skoog basal medium (pH 5.8) (Murashige & Skoog, 1962), containing 1% agar and supplemented with 3% sucrose and CaCO3 (500 mg L−1) in Phytatray II vessels (Sigma) at 24°C in the dark.
Callus induction was obtained on 6-benzylaminopurine (BAP) 0.5 mg L−1 and α-naphthaleneacetic acid (NAA) 0.25 mg L−1, whereas shoots were regenerated on BAP 0.4 mg L−1 and NAA 0.1 mg L−1. The presence of CaCO3 influences the rate of propagation which, under optimal conditions, reaches an average of 30–35 shoots/explant. Rooting of regenerated shoots was achieved using Murashige and Skoog basal medium supplemented with 500 mg L−1 CaCO3 and indole-3-butyric acid (IBA) 0.1 mg L−1.
Greenhouse-grown and field-grown plants
Rooted plantlets were transplanted to soil, and maintained under controlled conditions in a greenhouse as follows: roots were washed to remove agar and plantlets transferred to plastic sterile pots (Ø = 10 cm) containing 50% peat, 50% sand and Quoirin & Lepoivre medium (Quoirin & Lepoivre, 1977), and incubated in a growth chamber at 12–20°C with a 12-h light period for 2 months.
Plants were subsequently transferred to plastic pots (Ø = 16 cm) containing 75% peat and 25% sand and after 2 more weeks, transferred outdoors (Fig. 2). Plant samples were collected for the analysis at the beginning of flowering stage.

Regeneration of A. petrosa subsp. eriantha: (A) plant in nature (Gran Sasso); (B) Plantlets obtained by in vitro regeneration techniques; (C) Regenerated plantlets after transplant to soil.
In spring 2007, 10,000 plants derived from in vitro propagation were transferred in two experimental fields (Assergi and Barisciano) located at 1000 m above sea level within the Gran Sasso and Monti della Laga National Park. Samples of A. petrosa subsp. eriantha were collected the following year, during the flowering stage for analysis.
Oil isolation
Fresh material samples were water–steam distilled for 3 h in a Clevenger-type apparatus. This apparatus consists of a glass vessel filled with about 2 L of water and provided with a lifted perforated basket containing the fresh material to be distilled. In this way, there is no contact between water and herb sample and, as the water is heated, the steam passes through the plant material from bottom to top carrying the volatile compounds to a condenser tube.
The oils were decanted and stored in a dark glass bottle and kept at 4°C until analysis.
Oil analysis
A Carlo Erba High-Resolution Gas Chromatography (HRGC) 5160 mega gas chromatography (GC) equipped with a flame ionisation detector and a Hitachi D-2000 chromato-integrator was used for GC analysis employing a Supelco fused silica Supelco Phase Bonded 5 (SPB5) column (30 mm × 0.32 mm i.d., film thickness 0.25 µm) operating with a temperature gradient from 70 × 220°C at 5°C min−1, holding the initial temperature for 18 min. The carrier gas was helium at a flow rate 1 mL min−1. The detector temperature was 250°C and the injection system was on column. A Finnigan Mat ion trap detector (ITD), model 800 set at 70 eV was employed for mass spectral analyses under the above mentioned GC conditions.
The identification of compounds was established by comparing the peak retention times with those of pure substances, by enhancement of the peaks with standard compounds, by matching the obtained MS spectra with those reported in the ITD library and in literature (Adams, 1989) and confirmed by Kovats retention Index (KI).
The quantitative data were expressed as relative percentage of the oil constituents calculated from the GC peak areas without using correction factors and each chromatographic analysis was performed twice. Data analysis was performed with the software package Microsoft Excel, version 2002. Results are expressed as mean ± standard deviation (SD).
Results
The qualitative and quantitative composition of essential oils isolated from wild and in vitro regenerated plants of A. petrosa subsp. eriantha is reported in Table 1.
| Compound | Identification method | KI | Wild plants | In vitro plantlets | Soil-grown plants | In situ grown plants | |||
|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2006 | 2004 | First year (2005) | Second year (2006) | Assergi (2008) | Barisciano (2008) | |||
| α-Thujene | a,b,d | 930 | tr | tr | tr | tr | tr | tr | tr |
| α-Pinene | a,b,c,d | 940 | 0.14 | 0.35 | 0.03 | 0.16 | 0.27 | tr | tr |
| α-Fenchene | a,b,d | 952 | tr | tr | 0.07 | tr | tr | 1.17 | 0.42 |
| Sabinene | a,b,c,d | 975 | 1.46 | 2.77 | 0.12 | 0.40 | 1.64 | 5.86 | 3.16 |
| β-Pinene | a,b,c,d | 979 | 0.32 | 0.83 | 0.13 | 0.57 | 1.78 | 5.51 | 2.32 |
| Myrcene | a,b,c,d | 992 | 0.17 | 0.27 | 0.04 | 0.18 | tr | 0.53 | 0.31 |
| α-Terpinene | a,b,d | 1020 | 0.12 | 0.18 | tr | 0.10 | 0.17 | 0.15 | tr |
| p-Cimene | a,b,d | 1030 | 0.08 | 0.19 | 0.10 | 0.15 | 0.22 | tr | tr |
| Limonene | a,b,c,d | 1032 | 0.07 | 0.06 | 0.03 | 0.09 | tr | 0.50 | 0.20 |
| 1,8 Cineole | a,b,c,d | 1033 | 0.22 | 0.30 | 0.11 | 0.12 | 0.38 | 0.41 | 0.28 |
| γ-Terpinene | a,b,c,d | 1059 | 0.22 | 0.49 | 0.10 | 0.20 | 0.35 | 0.28 | 0.26 |
| (Z)-sabinene hydrate | a,b,d | 1066 | tr | tr | 0.10 | tr | tr | tr | tr |
| (Z)-linalool oxide | a,b,d | 1075 | tr | tr | tr | tr | tr | tr | tr |
| Fenchone | a,b,c,d | 1086 | tr | tr | tr | tr | tr | tr | tr |
| Terpinolene | a,b,c,d | 1088 | tr | 0.08 | tr | 0.07 | tr | tr | tr |
| (E)-sabinene hydrate | a,b,d | 1095 | tr | tr | tr | tr | tr | tr | tr |
| α -Thujone | a,b,c,d | 1100 | 78.33 | 52.22 | 29.99 | 33.85 | 42.82 | 40.18 | 40.59 |
| β -Thujone | a,b,c,d | 1112 | 8.92 | 19.72 | 13.33 | 13.82 | 16.82 | 20.24 | 20.19 |
| (E)-verbenol | a,b,d | 1114 | tr | tr | 0.07 | tr | tr | tr | tr |
| p-menta-1,3,8-triene | a,b,d | 1115 | tr | tr | 0.07 | tr | tr | tr | tr |
| (E)-pinocarveol | a,b,c,d | 1140 | tr | tr | 0.07 | tr | tr | tr | tr |
| (E)-sabinol | a,b,d | 1142 | tr | tr | 1.83 | 0.09 | tr | 0.64 | 0.97 |
| Camphor | a,b,c,d | 1145 | 0.35 | 0.24 | 0.07 | 1.17 | 1.53 | tr | tr |
| Pinocarvone | a,b,d | 1159 | tr | tr | 0.14 | tr | 0.01 | tr | tr |
| Terpinen-4-ol | a,b,c,d | 1180 | 0.29 | 1.02 | 0.32 | 0.45 | 0.65 | 0.51 | 0.59 |
| Thuj-3-en-10-al | a,b,d | 1183 | 0.05 | tr | 0.09 | 0.42 | 0.16 | tr | tr |
| α-Terpineol | a,b,c,d | 1190 | tr | tr | 0.11 | tr | 1.33 | tr | 0.19 |
| Myrtenal | a,b,d | 1192 | tr | tr | 0.24 | tr | tr | 0.28 | 0.30 |
| Methyl chavicol | a,b,d | 1197 | tr | tr | tr | tr | tr | tr | tr |
| Myrtenol | a,b,c,d | 1198 | tr | tr | 0.19 | tr | tr | tr | tr |
| Cuminaldheyde | a,b,c,d | 1240 | tr | tr | 0.04 | 0.09 | tr | tr | tr |
| (E)-sabinyl acetate | a,b,d | 1295 | tr | tr | 0.05 | tr | 0.14 | 0.25 | 0.30 |
| Neryl acetate | a,b,c,d | 1370 | tr | tr | 2.49 | tr | tr | tr | tr |
| α-Copaene | a,b,d | 1380 | tr | tr | 0.69 | tr | tr | 0.48 | 0.42 |
| β-Elemene | a,b,d | 1389 | tr | tr | 0.10 | 0.58 | tr | tr | tr |
| β-Caryophyllene | a,b,c,d | 1437 | tr | 1.92 | 5.50 | 7.94 | 5.20 | 3.92 | 4.94 |
| β-Farnesene | a,b,c,d | 1455 | tr | tr | 0.83 | 0.15 | tr | tr | tr |
| β-Chamigrene | a,b,d | 1472 | 0.08 | 0.10 | 0.06 | tr | tr | 0.10 | 0.12 |
| γ-Muurolene | a,b,d | 1475 | 0.09 | 0.18 | 1.43 | 2.17 | 1.15 | 0.46 | 0.29 |
| Bicyclogermacrene | a,b,d | 1496 | tr | tr | tr | tr | tr | tr | 0.14 |
| Unidentifiede | 1500 | 0.23 | 8.72 | 12.80 | 18.90 | 15.72 | 12.25 | 14.67 | |
| Unidentifiedf | 1510 | tr | 2.42 | 5.63 | 3.98 | 1.80 | 3.75 | 5.58 | |
| δ-Cadinene | a,b,d | 1522 | tr | tr | 0.12 | 0.14 | 0.08 | 0.11 | tr |
| Geranyl n-butyrate | a,b,d | 1566 | tr | tr | 0.61 | tr | tr | tr | tr |
| (E)-nerolidol | a,b,c,d | 1570 | tr | tr | 12.73 | 0.49 | 0.22 | tr | 0.29 |
| Epi-cubenol | a,b,d | 1628 | tr | tr | 1.12 | 0.25 | 0.15 | tr | tr |
| α-Bisabolol | a,b,c,d | 1684 | tr | tr | 0.10 | 0.16 | tr | tr | tr |
| Oil content % (v/w) | 0.33 | 0.52 | 0.02 | 0.12 | 0.21 | 0.30 | 0.27 | ||
- Compounds in bold are the content of thujones which are the most relevant. KL, Kovats retention Index.
- aRetention time.
- bKovats retention index.
- cPeak enrichment on co-injection with authentic standard.
- dMass spectrum.
- eMajor fragmentation ions (%) from mass spectral data: 41(100), 93 (55), 69 (53), 67 (48), 57 and 80 (38).
- fMajor fragmentation ions (%) from mass spectral data: 41(100), 93 (50), 69 (47), 67 (43), 80 (40), 81(36).
It is noteworthy that the lower altitude of growing of this alpine plant induced a higher productivity, but this has not been marked by an increase in fungal diseases and other pests, probably because of the content of antifungal, insecticide or repellent terpenoids (Abad et al. 2007; Chiasson et al. 2001; Soliman & Sallam, 2009). Oils were characterised by the presence of numerous compounds, 45 of which were identified. The majority of components (i.e. 33) were monoterpenes consisting of 12 hydrocarbon and 21 oxygenated derivatives, including thujones found as main oil constituents. The sesquiterpene fraction evidenced the presence of two unidentified compounds and β-cariophyllene as more representative components. All the oils were characterised by high content of total thujones with concentrations ranging from 43.32% to 87.25% and the predominance of α-thujone isomer. Significant differences in thujone content and α/β isomer ratios were evidenced in wild plants collected in different years; in particular, the oil from the wild plants showed the highest percentage of α-thujone (78.33%) and the highest ratio of α/β isomers (8.78), whereas in the other oils this ratio varied from 1.99 to 2.65 (Fig. 3). Variations in the biosynthetic rate of thujone, as well as in its relative distribution among the cis and trans-isomeric forms, can be influenced by the variation of environmental factors (Bellomaria et al., 1981; Jerkovic et al., 2003); accordingly, different climatic conditions were recorded during years 2001–2008 on the Gran Sasso mountain (Fig. 4).
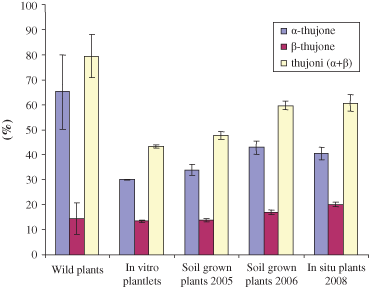
Mean values of α,β and total thujone contents of wild plants, in vitro plantlets, field-grown and in situ grown plants of A. petrosa subsp. eriantha with calculated SD for all collection years.
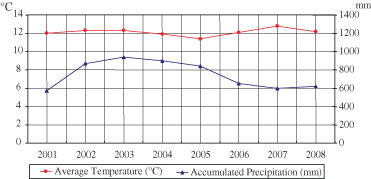
Metereological data relative to Gran Sasso mountain in the period 2001–2008 (Servizio Idrografico e Mareografico della Regione Abruzzo, 2008). Average Temperature (°C) and Accumulated Precipitation (mm) are expressed as annual mean values.
Oils from in vitro plantlets (2004) possess lower contents of α- and β-thujone (29.99% and 13.33%) in comparison with those of soil-grown plants harvested after 1 year (2005) (33.85% and 13.82%) or after 2 years (2006) (42.82% and 16.82%) (Table 1 and Fig. 3). In regenerated plants grown in situ, both in Assergi and Barisciano stations, the content of β-thujone increased further (20.24%, 20.19%), whereas that of α-thujone remained stable (40.18% and 40.59%) (Table 1 and Fig. 3).
An opposite trend was observed in the case of trans-nerolidol with a significant decrease from 12.73% found in the in vitro plantlets to less than 0.50% in soil-grown and in situ grown plants (Fig. 5).
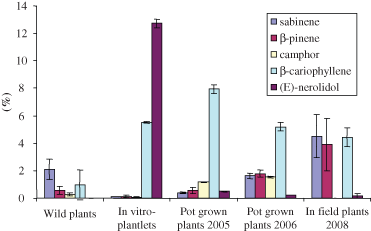
Mean values of sabinene, β-pinene, camphor, β-caryophyllene and E-nerolidol content of wild plants, in vitro plantlets, field-grown and in situ grown plants of A. petrosa subsp. eriantha with calculated SD for all collection years.
Soil-grown plants (2005 and 2006) showed scanty contents of camphor (1.17% and 1.53%); however these values, higher than those found in wild plants (0.24% and 0.35%), are reduced to ‘traces’ in plants grown in their natural environment (Assergi and Barisciano) (Fig. 5). The yield in essential oil ranged from 0.33% to 0.52% for wild plants, whereas that of regenerated plants grown in situ was lower, but more constant (0.27–0.30%).
Discussion
We have analysed the quality of essential oils derived from micropropagated A. petrosa subsp. eriantha plants, with the aim to evaluate the possible utilisation of in vitro-derived plants in the preparation of the strong-flavoured liqueur ‘genepì’. The practice of making hand-made liqueur, having a long tradition in Italy, is at present the main concern about the preservation of this species. Although protected by an Italian Regional Law (L.R. n.47, 11 September 1979) and reported as protected species in the European Habitat Directives 92/43/EEC, annex V, A. petrosa subsp. eriantha is still an endangered species, and its survival is threatened by indiscriminate harvest carried out by tourists.
The essential oils from wild and regenerated plants of Apennines' genepì showed a complex terpenoid profile characterised by high content of thujones (60–85%) with the predominance of the α isomer; the differentiation of the two isomeric forms occurs at the end of the biosynthetic pathway which diverge only at the last step, that is, the Nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction in (+)-sabinone, as suggested by Croteau (1987) (Croteau et al., 2000).
These findings extend those reported for Greek isolates of A. petrosa Frish by Souleles (1993) who demonstrated the presence of 41 compounds, the majority being 1,8-cineole, β-thujone and borneol. Interestingly, we did not detect this latter compound in our analysis, and found that 1,8 cineole concentration is rather low, ranging from 0.20–0.30% in wild plants to 0.28–0.41% in the regenerants. Our findings are in good agreement with that reported by Mucciarelli et al. (1995) who have proposed a partition of 15 different Artemisia species in two main groups, according to their oil composition; in particular, A. petrosa was grouped with Artemisia genepì and A. umbelliformis owing to the presence of α-thujone and the lack of camphor and 1,8 cineole.
Sabinyl-isovalerate and spathulenol, reported as the main constituents of the essential oil from A. umbelliformis, by Bicchi and collaborators (Bicchi et al., 1982), were not at all detected in our study; conversely, both sabinene and β-caryophyllene found in significant amount in our samples were not observed (Bicchi et al., 1982) or were present at low concentration (Rubiolo et al., 2009) in A. umbelliformis. These findings strongly support the conclusion that a different taxonomic state should be maintained for A. petrosa and A. umbelliformis that have instead recently been proposed as a unique taxonomic entity by Conti et al. (2005). However, the work by Rubiolo and collaborators (Rubiolo et al., 2009) has highlighted a remarkable and significant difference in both the volatile and non-volatile fractions of two geographic isolates of A. umbelliformis (from Italy and/or Switzerland). Thus, a precise and reliable conclusion cannot be drawn at present; a genomic approach, based on molecular marker characterisation, could complement and possibly simplify this analysis.
Differences in thujone contents and α/β isomer ratios, as well as in the relative amounts of other oil constituents such as camphor and nerolidol, were evidenced both in micropropagated and wild plants in relation to plant age and to environmental factors. These findings are consistent with results previously reported in the literature concerning secondary metabolism in general (Croteau et al., 2000; Sangwan et al., 2001) and terpene production in particular (Langenheim, 1994). Moreover, a close coordination between leaf ontogeny and oil accumulation, and biogenesis has been demonstrated in many aromatic plants (Sangwan et al., 2001; Marotti et al., 1994; McConkey et al., 2000) including Artemisia species (Chalchat et al., 1994; Simonnet et al., 2006).
In conclusion, in vitro regenerated alpine wormwood plants from Central Italy (Apennines' genepì) yielded amounts of essential oil comparable to those obtained from wild samples, with a similar qualitative composition. Micropropagation thus provides plants suitable for the industrial exploitation of this species that is rare in nature because it lives in a very selective environment (2200 m above sea level), and is at present threatened by the massive harvest carried out by tourists; moreover, the very short reproductive season does not guarantee its survival.
Considering the reported toxicity of thujones, and the limits imposed to their use by food regulations, the finding that these compounds are present in lower concentration in the regenerated plants, could represent a further advantage for their utilisation.
Eventually, the creation of experimental fields of alpine wormwood within the Gran Sasso-Monti della Laga National Park territory, might represent an opportunity for the mountain population's employment.
Acknowledgements
This research was in part supported by L. R. 9 April 1997, n. 835 ‘Tutela della biodiversita’ vegetale e la gestione dei giardini ed orti botanici’ Regione Abruzzo and Gran Sasso and Monti della Laga National Park. Thanks are due to Mrs Angela Vecchi for collaboration in the preparation of the manuscript.




