Grape powdery mildew as a food source for generalist predatory mites occurring in vineyards: effects on life-history traits
Abstract
In several perennial cropping systems, generalist or omnivorous species represent important biocontrol agents. They can persist on plants by feeding on alternative foods when prey is scarce and potentially limit pest outbreaks. Among beneficials characterised by a wide food range, those belonging to the acarine family Phytoseiidae represent important biocontrol agents. Generalist predatory mites can develop and reproduce using various food sources as alternatives to their tetranychid prey. The presence of alternative food sources can also induce switching feeding behaviour of generalist predators from prey to alternative foods. We evaluated in the laboratory the role of the grape powdery mildew (GPM) for the survival, development and reproduction of Amblyseius andersoni and Typhlodromus pyri, two important beneficial phytoseiid mites, in European and North-American vineyards. We also compared life-history parameters obtained when feeding on GPM with those obtained feeding on tetranychids mite prey or cattail pollen. Results indicated that GPM is an adequate food source for generalist mite survival and development. Results suggest that GPM can sustain mite populations in the absence of higher quality food sources. Based on optimal foraging theory, comparison of life-history parameters on GPM and mite prey suggests that the disruption of phytophagous mite control by these predatory mites in the presence of GPM appears unlikely. Implications for biological control in vineyards are discussed.
Introduction
The goals of conservation biological control tactics are to preserve or enhance beneficials in the agroecosystems. The study of the nature of interactions among beneficials and the other components of the agroecosystem can help in increasing the likelihood of the success of this pest control strategy (Barbosa, 1998). In several perennial cropping systems, generalist or omnivorous species represent important biocontrol agents, and by exploiting several food sources they are favoured, compared with less polyphagous species. Their populations can persist by feeding on alternative foods when prey are scarce and can potentially limit pest outbreaks, subsequently providing effective biocontrol (Symondson et al., 2002; Wäckers et al., 2007).
Among beneficials characterised by a wide food range, those belonging to the acarine family Phytoseiidae represent important biocontrol agents in several perennial cropping systems (Helle & Sabelis, 1985). Generalist predatory mites are able to develop and reproduce feeding on various food sources as alternatives to their tetranychid prey (McMurtry & Croft, 1997). Indeed, phytoseiids will engage in intraguild predation (Yao & Chant, 1989; Schausberger & Croft, 2000) and/or can exploit a wide range of other food resources (Overmeer, 1985). Generalist predatory mites are often present on plants in high numbers even when prey mites are scarce or absent for long periods (Eichhorn & Hoos, 1990; Bakker & Klein, 1992; Duso, 1992), and their persistence has been considered as a key factor in the biological control of phytophagous mites in perennial crops (Nyrop et al., 1998).
In several cropping systems, generalist phytoseiid abundance is positively correlated with pollen availability (Kennett et al., 1979; Duso et al., 1997, 2004; Addison et al., 2000; Roda et al., 2003). However, as pollen availability is temporally limited to spring and early summer in many areas (Eichhorn & Hoos, 1990; Addison et al., 2000; Duso et al., 2004), other food sources may be required to support phytoseiid populations before they enter into diapause in the fall. Plant fungal diseases have also been associated with presence or abundance of generalist phytoseiid mites. Field observations carried out in North-Italian vineyards indicated that infections by grape downy mildew Plasmopara viticola (Berk. & Curtis ex de Bary) Berlese & De Toni were associated with population increases of two phytoseiid species, Amblyseius andersoni (Chant) and Typhlodromus pyri Scheuten (Duso et al., 2003) that are important beneficials for European and North-American viticulture (Schruft, 1985; Duso & de Lillo, 1996; Marshall & Lester, 2001; Prischmann et al., 2006). Downy mildew was recently shown to be an alternative food source that allows population maintenance and increase for both phytoseiid species (Pozzebon & Duso, 2008). Concerning the other widely distributed grape plant pathogenic fungus, Uncinula necator (Schw.) Burr., the causal agent of grape powdery mildew (GPM), it has been suggested by Eichhorn & Hoos (1990) that T. pyri feeds on some stages. However, its role for the development and reproduction of predatory mites has yet to be examined, and its importance for generalist predatory mite persistence is currently not known.
The present paper aims to assess if GPM can be considered an alternative food for two generalist phytoseiids, A. andersoni and T. pyri, and if it is an adequate nutritional resource for the survival, development and reproduction of both species. Because the availability of alternative food sources can induce switching feeding behaviour of generalist predators (Murdoch, 1969; van Baalen et al., 2001), the consequences for predator–prey interactions are not obvious and depend on the relative quality/suitability of the alternate food resources (van Baalen et al., 2001). Hence, we also compared life-history parameters of A. andersoni and T. pyri feeding on GPM and feeding on tetranychid mite prey or pollen.
Materials and methods
Stock cultures
Typhlodromus pyri and A. andersoni individuals used in experiments were obtained from laboratory colonies reared in a walk-in growth chamber held at 25°C and 70 ± 10% of relative humidity (RH). Colonies of A. andersoni and T. pyri originated from individuals collected from an experimental vineyard and apple orchard, respectively, at New York State Agricultural Experiment Station, Cornell University, Geneva, NY, USA. The T. pyri colony was initiated about 2 months before experiments while A. andersoni was in colony for 1.5 years. Phytoseiids were reared on detached grape leaves of Baco Noir variety, a Vitis vinifera L. interspecific hybrid grapevine, or on excised bean leaves infested with the two-spotted spider mite (TSSM) Tetranychus urticae Koch. TSSM were reared on bean plants (Phaseolus vulgaris L.) grown under glasshouse conditions. A GPM colony, to be used for laboratory experiments, was maintained on potted ‘Baco Noir’ grapevines growing in a walk-in growth chamber [20–30°C and 70–80% relative humidity (RH)].
Laboratory experiments
In laboratory experiments we determined whether GPM, at levels comparable with other food sources, supports the development and oviposition of T. pyri and A. andersoni. Developmental times of T. pyri and A. andersoni feeding on GPM were compared with developmental times feeding on TSSM T. urticae and on cattail pollen Typha spp. Cattail pollen was chosen as a reference alternative food because it has been shown to be suitable for the development and reproduction of these generalist predatory mites in preliminary experiments. As the control for this experiment, mites of the two species were isolated on leaf rearing discs without supplemental food. Two leaf discs were used as part of an experimental unit to provide different food: ‘base leaf disc’ and ‘food leaf disc’. ‘Base leaf discs’ (1 cm in diameter) were obtained from the interveinal areas of fully expanded leaves collected from Baco Noir grapevine grown under greenhouse conditions. Base leaf discs were floated ventral side up on water saturated sponges to prevent desiccation and mite escape.
Foods were offered to phytoseiids through the food leaf disc (0.5 cm in diameter). Pollen (0.5 mg) or 15–20 motile forms (females and immatures) of T. urticae were placed on the food leaf disc using a fine brush. Additional food leaf discs were obtained from leaves of Baco Noir variety infected by U. necator to provide fresh GPM to phytoseiids. Food leaf discs were set bottom side down on base leaf discs so that phytoseiid mites could easily move between the smaller food disc and the larger base disc. Food sources were provided at levels that exceed the amount that could be eaten by predatory mites in the recording period as observed in preliminary assays. Cattail pollen used in the experiments had been previously collected and stored at −20°C. To obtain a cohort of even-aged larvae, females of T. pyri and A. andersoni were collected from laboratory colonies, allowed to lay eggs on a leaf disc for 24 h and then removed. At egg hatching, a single larva was transferred to each experimental unit and monitored from larva through the adult stage or death. We made three observations per day (every 8 h) to evaluate the duration of each developmental stage. Food sources were replenished every 2 days. Protonymphs could be distinguished from larvae by the number of legs. Deutonymphs were distinguished by the presence of an eight-legged exuvium. Once the adult stage was reached after an additional moulting, a male, reared using the same food source, was added to each disc. Daily observations continued until the first egg was laid. The developmental times of at least 20 females and 15 males per species were measured per treatment. Hence, the experiments comprised four groups of 10–20 eggs per species, per treatment, for a total amount of about 50–60 eggs/species/treatment. We evaluated the oviposition rates and survival of T. pyri and A. andersoni feeding on the same food sources. Experimental units were constructed using the above-mentioned protocol. A single, recently moulted adult female, developed using the different food sources, was placed on each disc with a male reared in the same treatment. The experiment involved a total of 15 females and 15 males per species and per treatment. All experiments were carried out in climate chambers held at 23°C, 80–85% RH with a 16:8 h light:dark regime.
Data analysis
We assess the effects of different food sources on phytoseiids development and oviposition with a restricted maximum likelihood one-way analysis of variance (ANOVA) using the PROC MIXED of SAS (SAS Institute Inc., 1999). Only individuals that reached the adult stage were included in the analysis. Differences among food treatments were evaluated with the least significant difference method to the least squares means (α = 0.05), and standard error of the differences (SED) are presented (Littell et al., 2002). We considered time (hours) to reach each development stage, pre-oviposition times and mean fecundity (total number of eggs per female) as dependent variables. Data on the development times of females and males were analysed separately. Data were checked for ANOVA assumptions prior to the analysis. Juvenile survival (number of females + males/number of initial eggs) and sex ratio (number of females/number of females + males) were analysed using a logit model and performing a likelihood ratio χ2-test (α = 0.05) with the GENMOD procedure of SAS and applying a Wald χ2-test (α = 0.05) to examine differences in least squares means (SAS Institute Inc., 1999). Mites lost during experiment were excluded from the analyses. The effect of treatments on the survival curves and on median longevity of females and males were evaluated with the Kaplan–Meier method and were compared by a pairwise Wilcoxon χ2-test (α = 0.05) with the LIFETEST procedure of SAS (SAS Institute Inc., 1999).
Results
Amblyseius andersoni
All food sources were suitable for the development of A. andersoni, while no development was observed in the control, where protonymphs never moulted to deutonymphs. Survival to adult of A. andersoni was higher when provided TSSM or pollen than GPM (likelihood ratio χ2 = 28.56; d.f. = 2; P < 0.001; Table 1). Survival to adult on GPM was lower than on TSSM (Wald χ2 = 14.29; d.f. = 1; P < 0.01) and pollen (Wald χ2 = 13.05; d.f. = 1; P < 0.01). Survival to adult on TSSM and pollen was similar (Wald χ2 = 0.20; d.f. = 1; P = 0.65). The sex ratio was not affected by diet (likelihood ratio χ2 = 0.30; d.f. = 2; P = 0.86; Table 1).
| TSSM | Pollen | GPM | |
|---|---|---|---|
| A. andersoni | |||
| Survival to adulta | 0.94 ± 0.03 (n = 49) | 0.93 ± 0.03 (n = 46) | 0.59 ± 0.08 (n = 35) |
| Sex ratiob | 0.61 ± 0.01 (f = 30; m = 19) | 0.63 ± 0.04 (f = 29; m = 17) | 0.57 ± 0.07 (f = 20; m = 15) |
| T. pyri | |||
| Survival to adulta | 0.89 ± 0.06 (n = 43) | 0.92 ± 0.02 (n = 46) | 0.68 ± 0.07 (n = 40) |
| Sex ratiob | 0.65 ± 0.05 (f = 28; m = 15) | 0.63 ± 0.03 (f = 29; m = 17) | 0.62 ± 0.05 (f = 25; m = 15) |
- a Number of females + males/number of initial eggs.
- b Number of females/number of female + males.
The developmental times of predatory mites from larvae to protonymphs were similar across the different food sources (females: F2,73 = 2.47; P = 0.09; males: F2,46 = 2.89; P = 0.06) (Table 2). No effects of diet were observed on the development from protonymphs to deutonymphs (females: F2,73 = 1.65; P = 0.19; males: F2,46 = 1.46; P = 0.24) (Table 2). An effect of food source was observed on development from deutonymph to adult female (F2,73 = 88.35; P < 0.001) (Table 2), with development longer for predators reared on GPM compared with pollen (SED = 4.27; t73 = 9.67; P < 0.001) or TSSM (SED = 4.24; t73 = 13.10; P < 0.001) (Table 2). Moreover, development from deutonymph to adult female was longer on pollen compared with TSSM (SED = 3.80; t73 = 3.77; P < 0.001; Table 2). No effects of different food sources were found for male development (F2,46 = 0.30; P = 0.73; Table 2). Diet influenced the developmental time from egg to adult female (F2,73 = 36.75; P < 0.001); this was longer for A. andersoni reared on GPM than on TSSM (SED = 6.75; t73 = 8.26; P < 0.001) or pollen (SED = 6.78; t73 = 6.79; P < 0.01) (Table 2). No differences were observed for development from egg to adult female between TSSM and pollen (SED = 6.05; t73 = 1.60; P = 0.11; Table 2). No differences were found in development time from egg to adult males (F2,46 = 0.54; P = 0.58; Table 2). A. andersoni oviposition was observed for females reared on pollen and TSSM but not for those reared on GPM. Pre-oviposition times were similar among treatments (F1,25 = 0.47; P = 0.49; Table 2). TSSM supported greater oviposition than pollen (F1,25 = 15.36; P < 0.001; Table 2). Effects of food source were observed on survival of A. andersoni adult females (Wilcoxon χ2 = 29.22; d.f. = 2; P < 0.001; Table 3 and Fig. 1[link]). A. andersoni females that fed on GPM survived for a shorter time period than females fed on TSSM (Wilcoxon χ2 = 25.96; d.f. = 1; P < 0.0001) and pollen (Wilcoxon χ2 = 11.96; d.f. = 1; P < 0.001; Table 3), while survival was similar between TSSM and pollen (Wilcoxon χ2 = 0.24; d.f. = 1; P = 0.61; Table 3). No effect of food source was observed for male survivorship (Wilcoxon χ2 = 0.12; d.f. = 2; P = 0.94).
| Control | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| TSSM | Pollen | GPM | TSSM | Pollen | GPM | ||
| A. andersoni | |||||||
| Egg | 29.2 ± 1.6 | 28.8 ± 1.6 | 29.4 ± 1.7 | 27.6 ± 2.0 | 27.2 ± 1.2 | 24.0 ± 1.0 | 24.0 ± 1.3 |
| Larvae | 23.9 ± 1.3 | 24.5 ± 1.0 | 25.2 ± 0.7 | 27.8 ± 1.7 | 22.7 ± 0.9 | 24.7 ± 0.5 | 24.0 ± 1.0 |
| Protonymph | 128.5 ± 5.8 | 35.8 ± 3.2 | 29.7 ± 1.1 | 32.6 ± 3.0 | 26.2 ± 1.0 | 28.5 ± 1.8 | 30.5 ± 2.4 |
| Deutonymph | — | 71.7 ± 2.4 | 86.1 ± 2.9 | 127.8 ± 3.6 | 57.4 ± 3.1 | 61.2 ± 3.3 | 62.8 ± 3.3 |
| Eggs–adult | — | 160.9 ± 4.8 | 170.4 ± 4.1 | 215.8 ± 4.3 | 133.5 ± 3.8 | 138.4 ± 4.7 | 141.3 ± 3.4 |
| Pre-oviposition | — | 67.7 ± 3.2 | 70.7 ± 2.9 | — | |||
| Fecundity | — | 22.0 ± 1.5 | 13.7 ± 1.1 | — | |||
| T. pyri | |||||||
| Egg | 47.6 ± 1.1 | 48.9 ± 0.9 | 47.7 ± 1.0 | 47.7 ± 1.2 | 48.4 ± 1.2 | 47.5 ± 1.3 | 48.0 ± 1.4 |
| Larvae | 26.0 ± 1.8 | 24.3 ± 1.3 | 25.1 ± 1.1 | 25.9 ± 1.0 | 25.6 ± 0.9 | 25.9 ± 1.1 | 25.6 ± 0.9 |
| Protonymph | 148.9 ± 6.5 | 51.4 ± 2.1 | 46.4 ± 2.6 | 57.3 ± 4.5 | 48.3 ± 1.1 | 42.7 ± 1.4 | 50.1 ± 3.2 |
| Deutonymph | — | 71.1 ± 1.7 | 73.6 ± 1.5 | 120.2 ± 2.2 | 50.7 ± 2.3 | 53.2 ± 3.6 | 50.1 ± 2.5 |
| Eggs–adult | — | 195.8 ± 2.8 | 192.8 ± 3.3 | 251.0 ± 4.7 | 172.9 ± 3.2 | 175.3 ± 4.1 | 177.9 ± 4.7 |
| Pre-oviposition | — | 79.3 ± 3.5 | 75.6 ± 4.9 | — | |||
| Fecundity | — | 13.6 ± 0.9 | 14.7 ± 1.3 | — | |||
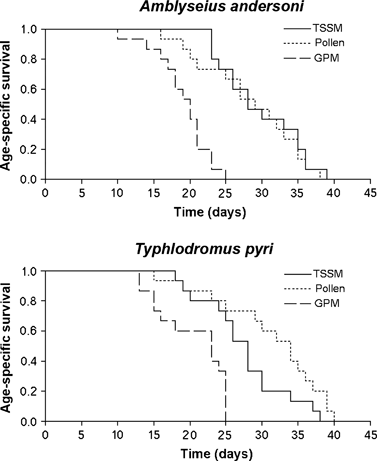
Age-specific survival of Amblyseius andersoni and Typhlodromus pyri females reared on three diets [two-spotted spider mite (TSSM), cattail pollen and grape powdery mildew (GPM)]. Data on age-specific survival curves were analysed using the Kaplan–Meier method.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Median longevity | Confidence interval (95%) | Median longevity | Confidence interval (95%) | |||
| Lower | Upper | Lower | Upper | |||
| A. andersoni | ||||||
| TSSM | 28 | 26 | 35 | 10 | 9 | 11 |
| Pollen | 29 | 25 | 33 | 10 | 9 | 12 |
| GPM | 20 | 18 | 21 | 9 | 9 | 11 |
| T. pyri | ||||||
| TSSM | 28 | 25 | 30 | 12 | 10 | 12 |
| Pollen | 34 | 29 | 36 | 12 | 11 | 13 |
| GPM | 23 | 16 | 25 | 10 | 10 | 12 |
- GPM, grape powdery mildew; TSSM, two-spotted spider mite.
Typhlodromus pyri
Typhlodromus pyri reached the adult stage in all treatments except the control. Survival to adult was higher for T. pyri feeding on TSSM and pollen than feeding on GPM (likelihood ratio χ2 = 11.07; d.f. = 2; P = 0.003; Table 1). Survival to adult was lower on GPM than on TSSM (Wald χ2 = 5.07; d.f. = 1; P = 0.02) and on pollen (Wald χ2 = 7.68; d.f. = 1; P < 0.01), while similar survival was observed between the latter and the TSSM (Wald χ2 = 0.49; d.f. = 1; P = 0.48). No effect of diet was observed on sex ratio (likelihood ratio χ2 = 0.08; d.f. = 2; P = 0.95; Table 1).
The duration of the larval stage was not affected by food source (females: F2,77 = 0.49; P = 0.61 males: F2,41 = 0.02; P = 0.97; Table 2). In the control, larvae became protonymphs but died soon thereafter. The duration of the protonymphal stage was not influenced by food sources (females: F2,77 = 2.81; P = 0.06; males: F2,41 = 2.47; P = 0.09; Table 2). An effect of diet was observed on the development from deutonymph to adult female (F2,77 = 217.15; P < 0.001; Table 2). Deutonymphs provided with GPM required longer to reach the adult female stage compared with deutonymphs provided with TSSM (SED = 2.64; t77 = 18.60; P < 0.01) or pollen (SED = 2.59; t77 = 17.95; P < 0.01) (Table 2). No differences were observed between TSSM and pollen (SED = 2.51; t77 = 1.01; P = 0.31; Table 2). However, there were no differences in time to develop from the deutonymph to adult stage for males (F2,41 = 0.15; P = 0.86; Table 2). The developmental time from egg to adult females of T. pyri was influenced by different food supplies (F2,77 = 73.31; P < 0.001; Table 2). Predatory mite females reared on GPM developed more slowly than females reared on pollen (SED = 5.31; t77 = 11.11; P < 0.01) and TSSM (SED = 5.22; t77 = 10.38; P < 0.01) (Table 2). No differences were observed between pollen and TSSM (SED = 5.06; t77 = 0.56; P = 0.57; Table 2). For males, diet did not influence development time (F2, 41 = 0.34; P = 0.71; Table 2). No eggs were laid by females reared on GPM. Pre-oviposition periods were similar among the different food sources other than GPM (F1,25 = 0.98; P = 0.33; Table 2). Moreover, ovipositions on pollen and on TSSM were not different (F1,25 = 0.57; P = 0.45; Table 2). Effects of food source were observed on survival of T. pyri females (Wilcoxon χ2 = 20.01; d.f. = 2; P < 0.001; Table 3 and Fig. 1[link]). Survival of females was shorter on GPM than on TSSM (Wilcoxon χ2 = 11.01; d.f. = 1; P < 0.001) and on pollen (Wilcoxon χ2 = 12.55; d.f. = 1; P < 0.001) (Table 3 and Fig. 1[link]). Similar levels of survival were observed on TSSM and on pollen (Wilcoxon χ2 = 2.66; d.f. = 1; P = 0.10; Table 3 and Fig. 1[link]). No effect of food source was observed on survival of males (Wilcoxon χ2 = 4.97; d.f. = 2; P = 0.08; Table 3).
Discussion
Results from the laboratory experiment indicated that leaves infected by GPM could be considered as suitable substrate for development and survival of T. pyri and A. andersoni. Mites reared on leaf discs infected by GPM reached the adult stage and survived for several days, while phytoseiids without food were unable to develop to deutonymphs and survived for only a few days at the protonymphal stage. Because the presence of leaf substrate alone did not allow for the development of phytoseiids (control treatment), we can assume that the presence of powdery mildew represented an adequate nutritional supply for mite development. This result reflects previous studies on feeding and development of phytoseiids on some other powdery mildew species. T. pyri can feed on Oidium fragariae Harz. from strawberry leaves, feed and develop on apple leaves infected by Podosphaera leucotricha (Ell. & Everh.) and on Erysiphe orontii Cast. from tobacco leaves, although very low level of reproduction was reported (Chant, 1959; Zemek & Prenerova, 1997). No data on powdery mildews as food are available for A. andersoni. With respect to other predatory mite species, apple leaves infected by the apple powdery mildew P. leucotricha were an appropriate substrate for the development of Kampimodromus aberrans (Oudemans) and Amblyseius umbraticus Chant and for the development and reproduction of Euseius finlandicus (Oud.) (Kropczynska-Linkiewicz, 1971; Daftari, 1979). The cassava mildew, Oidium manihotis Henn., appeared to be an adequate food source for Typhlodromalus limonicus (German & McGregor) and Typhlodromalus aripo DeLeon (Bakker & Klein, 1992; Bakker, 1993).
Our comparative experiments highlighted the effect of diet on life-history parameters of the two generalist phytoseiids. Feeding on GPM resulted in reduced juvenile survival compared with the other food sources. Nevertheless, GPM is a suitable food source for the development of both phytoseiid species, even if development is delayed compared with other food sources. The effect of diet on development first appeared during the deutonymphal stage, and this was reflected in the overall developmental time. Food sources did not affect the development from larvae to protonymphs and from protonymphs to deutonymphs for either phytoseiid species. T. pyri have nonfeeding larvae, while those of A. andersoni are considered as facultative feeding, that is, they feed rarely. Larvae of both predatory mite species can survive and develop to protonymphs without food (Zhang & Croft, 1994; Schausberger & Croft, 1999). Food sources considered in this study equally meet the nutritional requirements for the development from protonymphs to deutonymphs of both predatory mites. A diet based solely on GPM was inadequate for the oviposition by A. andersoni and T. pyri. Because it has been suggested that reproduction has a higher nutritional requirement than development (Sabelis, 1985b), we can assume that GPM has a lower nutritional value compared with the other food sources involved in this study.
In fact, the well-known alternative food cattail pollen not only supported development but also oviposition for T. pyri and A. andersoni. The prey TSSM supported complete development for both phytoseiids and, in particular, development of A. andersoni from deutonymph to adult was faster on this food compared with other food sources. The high nutritional value of TSSM was consistent with oviposition level: the highest egg production was recorded for A. andersoni females that fed on spider mites, while for T. pyri, the effects of T. urticae and cattail pollen were comparable. Diet effect was also observed on survival of females, which was lower on GPM than on pollen or T. urticae. The results of diet on oviposition indicate that A. andersoni and T. pyri respond differently to spider mite prey.
Our results showing lack of reproduction, but successful development and survival when fed GPM suggests that it should be considered a supplementary food based on definitions provided by Overmeer (1985). However, Overmeer (1985) emphasises that supplementary food can be important for survival of predatory mites. Indeed, in open-field conditions high phytoseiid abundance was associated with the presence of GPM mycelium (A. Pozzebon, unpublished data). Recently, the role as alternative food for generalist predatory mites has been documented for another grape pathogenic fungus, P. viticola (Pozzebon & Duso, 2008). In this study, a diet based on P. viticola met the nutritional requirement for increase and maintenance of predatory mite populations. Based on results presented here, the increase and maintenance of phytoseiid populations does not appear possible feeding on GPM exclusively. However, the effect of GPM on development and survival highlight that it is an additional food source that can be exploited to overcome the lack of other more suitable food sources over a short-term period. In fact, generalist predatory mites can exploit many different food sources and combinations, including those that promote only a low rate of increase (McMurtry, 1992). In general, they are able to prey upon small insects (e.g. thrips) and mites other than tetranychids (Camporese & Duso, 1995; Schausberger, 2003; Duso et al., 2005; Negloh et al., 2008), and they can also exploit food sources such as honeydew and plant-based substances (Duso & Camporese, 1991; Engel & Ohnesorge, 1994; van Rijn & Tanigoshi, 1999a, b; Papadopoulos & Papadoulis, 2008).
For phytoseiids, the generalist feeding habit is a favourable attribute for persistence when pests are rare or absent. Persistence in the system, even at low number, likely increases chances for successful biological control of herbivorous mites when they colonise the crop (McMurtry, 1992; Walde et al., 1992; Nyrop et al., 1998). Alternative food sources are often temporally limited, thus the ability to forage on a wide range of foods, including GPM or other fungi, increases the chance of persistence. Field studies carried out by Duso et al. (2003) revealed that densities of A. andersoni and T. pyri increased in response to downy mildew symptoms, which developed late in the season when other food (i.e. pollen) was limited. Additional studies on this aspect are required for the GPM.
This study also has implications in the context of trophic supplements to the predator–prey interactions. Life-history parameters can be considered as estimation of the relative suitability of different food sources for predatory mites (Tanigoshi, 1982; Sabelis, 1985a; Dicke et al., 1990). The results of the present study indicate a lower suitability of GPM for both predatory mite species compared with spider mite prey. In previous studies, the presence of alternative foods (e.g. apple powdery mildew, Typha latifolia pollen) appeared to reduce the short-term predation pressure on the prey (Wei & Walde, 1997; Zemek, 2005). Optimal foraging theory would predict that a predator should exploit a food source with high energy content (related to handling time) and switch to a less profitable food when the first is rare, increasing the probability of predator persistence (Murdoch, 1969; van Baalen et al., 2001). Considering the life-history parameters as estimation of the nutritional value of food sources, the present results suggests that a switch to GPM for these predatory mites possibly occurs at very low prey density and high level of fungus infection. In this scenario, the disruption of phytophagous mite control by these predatory mites appears unlikely.
The generalist predatory mites involved here are among the most effective biocontrol agents of phytophagous mites on grapevine worldwide (Duso, 1989; Kreiter et al., 2000; Marshall & Lester, 2001; Prischmann et al., 2006). To attain the successful control of phytophagous mites in viticulture, biocontrol strategies based on inoculative releases of generalist predatory mites were widely applied. Released phytoseiids can persist on vines, reducing herbivorous mite densities for a long time (Duso & Vettorazzo, 1999; Marshall & Lester, 2001). Established predatory mite populations can also persist on vines when prey is scarce or absent preventing pest outbreaks. They can exploit GPM, U. necator which is an obligate grape pathogen on cultivated and wild grapevines (Weltzien, 1981) and could be considered a suitable food resource that favour the persistence in the absence of pest mite prey. The role of GPM for phytoseiid persistence on plants may be particularly important in natural perennial systems such as wild grapes where GPM can naturally spread. In commercial vineyards, GPM is likely maintained under control with fungicide applications. Nevertheless, pathogen infections may occur at non injurious levels or spread late in the season when fungicides are applied less frequently. In these cases, they can contribute to predatory mite persistence on cultivated grapes as well.
Acknowledgements
We are grateful to Steve Hesler, Jason Nyrop, Sara Villani, Regina Lynd, Charles Moser and Adam Bordenaro for their helpful assistance. We thank Jan Nyrop and Karen Wentworth for suggestions and for providing T. pyri colonies. We also thank Jim McNicol for statistical advice. This research was funded by ‘Ing. Aldo Gini’ foundation fellowship to A. P., National Research Initiative of the USDA Co-operative State Research, Education and Extension Service (grant number 2001-35316-11039) to GML and the University of Padua (grants ex- 60%) to CD.




