Enhanced resistance to foliar fungal pathogens in carrot by application of elicitors
Abstract
Treatment of greenhouse-grown carrot plants with salicylic acid (SA) (100 μm), chitosan (0.02%) and the nutrient-chelate product Alexin (1%) followed 10 h later by inoculation with the necrotrophic fungal pathogens Alternaria radicina and Botrytis cinerea significantly reduced disease development 10 days after inoculation (d.a.i.) compared with control plants sprayed with water. The most effective treatment was chitosan, followed by Alexin and SA. Additional sprays of elicitors resulted in significantly lower disease development 25 d.a.i. Treated plants had elevated transcript levels of pathogenesis-related protein 1 (PR1), chitinase, lipid transfer protein (LTP), chalcone synthase, nonexpressor of PR1 and pathogenesis-related protein 5 (PR5) genes compared with control plants when assayed 10–70 h after treatment. The activity of peroxidase, polyphenoloxidase, phenylalanine ammonia-lyase, chitinase, β-1,3-glucanase and lipoxygenase was significantly increased in elicitor-treated plants compared with control plants 12–72 h after treatment. Microscopic examination of treated leaves revealed reduced fungal growth and colonisation, 48 h after treatment, accompanied by fewer lesions at 120 h, compared with the control. Protein extracts from elicitor-treated plants reduced spore germination and germ tube elongation of the pathogens in vitro by 30–45%. Elicitor-treated plants accumulated higher amounts of total phenolics, 6-methoxymellin and H2O2 compared with the control. Both chitosan and Alexin induced responses similar to that of SA, suggesting that these elicitors may activate the salicylate pathway, leading to induction of defence genes, enzymes, phytoalexin and phenolics, which collectively reduced fungal colonisation.
Introduction
Carrot [Daucus carota ssp. sativus (Hoffm.) Acrang.] is an important root crop worldwide, which contains high levels of provitamin A and other nutrients (Ammirato, 1986). Fungal diseases pose a major constraint to carrot production in the field and during storage. Several foliar fungal diseases affect carrot, causing leaf spotting and blighting (Davis & Raid, 2002), and losses because of these diseases can be severe. Most often, control methods for foliar diseases involve fungicide applications because genetic resistance is frequently lacking (Peterson & Simon, 1986).
There have been few studies on the effectiveness of induced resistance for disease control on carrot. Enhanced resistance in plants to pathogens, both locally and systemically, can be achieved by treatment with different agents, such as virulent or avirulent pathogens, cell wall fragments, synthetic chemicals and plant extracts (Vidhyasekaran, 1997; Walters et al., 2005). This type of induced resistance is not absolute, but results in reduced incidence and severity of disease caused by fungal, bacterial and viral pathogens (Kuć, 2006). The corresponding plant defence responses following treatment include an oxidative burst leading to cell death, changes in cell wall composition, synthesis of antimicrobial compounds such as phytoalexins, activation of defence genes and priming of host cells (Kuć, 2006). Induced resistance in plants can be achieved with applications of salicylic acid (SA) as well as related compounds, such as benzo (1,2,3) thiadiazole-7-carbothioic acid S-methyl ester, 2,6-dichloro-isonicotinic acid and DL-3-amino-n-butyric acid (Walters et al., 2005). Chitosan, a deacetylated derivative of chitin, has also been reported to enhance disease resistance in plants (Benhamou et al., 1994) and induces defence responses similar to that of SA. The effects of these inducer chemicals have not been previously studied in carrot. An earlier report demonstrated the possibility of inducing systemic disease resistance in carrot leaves by inoculating Cercospora carotae (Pass.) Kazn. & Siemaszko as the agent for both induction and challenge. Infection of carrot leaves by C. carotae was reported to reduce the susceptibility of plants to subsequent infections by other foliar pathogens later in the growing season (Mercier & Kuć, 1996).
To determine if enhanced disease resistance could be achieved through application of chemical elicitors, we evaluated the effect of a commercial formulation of chitosan (963-Chitosan; Apollo-Canada Enterprises, Burnaby, BC, Canada) and a commercial nutrient-chelate compound (Alexin) and compared them with SA. For this investigation, we selected two foliar fungal pathogens of carrot, namely Alternaria radicina (Meier, Drechsler & Eddy) and Botrytis cinerea (De Bary) Whetzel, which are known to infect carrot and grow and sporulate well under laboratory conditions. Foliar symptoms of A. radicina (causing black rot) (Farrar et al., 2004) are characterised by dark brown to black necrotic lesions, which may be surrounded by a chlorotic halo. Conidia deposited on leaf surfaces will germinate and produce germ tubes, which penetrate the host cell directly to establish an infection site (Coles & Wicks, 2003; Farrar et al., 2004). B. cinerea infects senescent plant tissue and can also enter through wounds. The diseased tissue becomes light brown and water soaked, and as the lesions expand, they turn dark brown and the fungus sporulates profusely (Sharman & Heale, 1977). Carrot plants treated with the elicitors were assessed for enhanced resistance to A. radicina and B. cinerea foliar blights, and the potential mechanism of disease resistance was studied.
Materials and methods
Plant material and elicitor treatments
Seeds of the high carotene mass carrot (provided by Dr Phil Simon, University of Wisconsin, Madison, WI, USA) were surface sterilised in 10% Chlorox (0.525% NaOCl) for 10 min and rinsed in sterile water and planted in pots (12 cm diameter) containing Sunshine planting mix (Sun Gro Horticulture, Vancouver, British Columbia, Canada). The plants were grown in a greenhouse at 22–28°C, 70–85% relative humidity, 600–1000 μmol m−2 s−1 light intensity and 12-h photoperiod. Pure cultures of the fungal pathogens A. radicina (provided by Dr Barry M. Pryor, University of Arizona, Tucson, AZ, USA) and B. cinerea (isolated from naturally infected carrot leaves) were maintained on V8 and potato dextrose agar media, respectively. Ten-week-old carrot plants were sprayed with SA (100 μM; Sigma, St Louis, MO, USA), chitosan (0.02%) (963-Chitosan; Apollo-Canada Enterprises, Vancouver, BC, Canada) and an organic nutrient-chelate mixture, Alexin (1%) (containing 8% potassium, 2.4% calcium, 0.8% magnesium, 0.2% boron, oligosaccharides and salicylate derivatives) (Nutri-Ag Ltd, Toronto, Ontario, Canada; http://www.nutriag.com) using a microsprayer to run-off. Control plants received water.
Treated carrot plants were inoculated 10 h after treatment with a conidial suspension (1 × 106 mL−1) of A. radicina or B. cinerea using an atomiser and incubated in a humid chamber for 72 h. The inoculated plants were then transferred to a greenhouse and maintained under ambient conditions (22–28°C; 70–85% relative humidity; 600–800 μmol m−2 s−1; 12- to 13-h photoperiod). A second and third application of elicitors was made 10 and 20 days after inoculation to test the hypothesis that repeated applications might prolong the induced resistance phenomenon. Combinations of fungicide plus elicitor treatments were also made by applying chlorothalonil (Syngenta Canada, Guelph, Ontario, Canada) (500 μg mL−1) at 10 days instead of an elicitor treatment followed by an elicitor application at 20 days. The fungicide control was application of chlorothalonil at 10 days after inoculation (d.a.i.) without elicitors.
Plants were scored for disease severity 10 d.a.i. and again at 14 and 25 days using a 6-point disease rating scale based on the percentage of leaf area infected (1 = 0%; 2 = 1–10%; 3 = 11–25%; 4 = 26–40%; 5 = 41–55% and 6 =>56%) (Jayaraj & Punja, 2007). Disease index was calculated as the sum of disease ratings of individual leaves/(6 × number of leaves). Dry plant biomass was recorded at the end of the experiment. The experiment was conducted three times, with 30 plants per treatment in each experiment.
Microscopic observation of the infection process
To study the effect of elicitors on infection by the pathogens, 10-week-old carrot plants were treated with elicitors as described above. Ten hours after treatment, the plants were inoculated with a conidial suspension (1 × 106 mL−1) of A. radicina or B. cinerea using an atomizer and incubated in a humid chamber for 72 h. Leaf samples were collected at various time intervals (0, 12, 24, 48, 72, 96 and 120 h), cut into approximately 1 cm2 pieces and transferred to vials containing ethanol (95%) and glacial acetic acid (1:1) for clearing. After 2 days, the tissues were transferred to 80% lactic acid and incubated at room temperature for 5 days. Tissue segments were transferred to a microscope slide, the lactic acid was blotted away and the sample was stained with lactophenol blue solution (50%) (Fluka Chamie GmbH, Buchs, Switzerland) for 3 min. The tissues were destained in lactophenol (phenol crystals 20 g; lactic acid 20 mL; glycerine 40 mL and water 20 mL) and examined under phase contrast in a light microscope (Zeiss, Toronto, Canada). Stained spores of Botrytis and emerging hyphae of Botrytis and Alternaria appeared blue in colour. Cleared and unstained leaf segments were also examined for the number and size of lesions, which appeared as reddish-brown spots (Dhingra & Sinclair, 1995).
In vitro antifungal activity of protein extracts from elicitor-treated leaves
Total proteins from elicitor-treated carrot leaves were extracted in 0.1 M phosphate buffer (pH 6.5) from samples collected 24 and 48 h, after treatment as described by Jayaraj & Punja (2007). Five-hundred microgram equivalent of total protein was placed in a cavity slide and 50 μL spore suspensions (1 × 105 mL−1) from 10-day-old cultures of A. radicina and B. cinerea were added to the slides. The slides were incubated in a humid chamber at 23°C for 15 h. The spores were stained with lactophenol blue solution, and germination was determined by microscopic (×200 magnification) examination. A spore was considered to have germinated if the length of the germ tube was at least as long as the width of the spore. Four replicate slides were prepared for each sample and values of 10 microscope fields were averaged. The experiment was conducted twice.
Northern blotting
For protein and total RNA extraction, carrot leaf samples were collected in triplicate at 0, 12, 24, 48, 72 and 96 h after elicitor treatment, and immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from 300 mg of leaf tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s protocol. RNA (10 μg) was run on a 1.4% (w/v) agarose formaldehyde gel and transferred overnight onto membrane (Hybond N+; Amersham, Piscataway, NJ, USA). The blots were probed with [α-32P] dCTP-labelled probes of carrot pathogenesis-related protein-1 (PR1; AB127984.1), chitinase (U52848), lipid transfer protein (LTP; M64746), chalcone synthase-2 (AJ006779), Arabidopsis nonexpressor of PR1 (NPR-1) (NM_105102) and pathogenesis-related protein-1 (PR-5; NM_106161) cDNAs, as described by Jayaraj & Punja (2007).
Enzyme assays
For the extraction of total proteins, frozen leaf samples were homogenised with a pestle and mortar in liquid nitrogen. The frozen powder was suspended in 0.1 m phosphate buffer, pH 6.5, containing 0.5 mmM phenylmethyl sulphonyl fluoride and centrifuged at 10 000 g for 15 min at 4°C. Protein content of crude extracts was determined by the bicinchoninic acid microtitre plate assay kit (Pierce, Rockford, IL, USA). The activity of peroxidases (POs) was assayed spectrophotometrically using pyrogallol as a substrate. The PO activity was expressed as changes in absorbance s−1 g−1 fresh weight of tissue. Polyphenol oxidase (PPO) activity was assayed using catechol as a substrate and the enzyme activity was expressed as catechol equivalents. Phenylalanine ammonia-lyase (PAL) activity was assessed spectrophotometrically by assaying the rate of conversion of l-phenylalanine to trans-cinnamic acid at 290 nm, and the amount of trans-cinnamic acid synthesised was calculated using its absorption coefficient of 9630 μmol s−1 g−1 (Rahman & Punja, 2005). Chitinase activity was determined using n-acetyl glucosamine (NAG) as a substrate and activity was expressed as NAG units (Singh et al., 1999). Glucanase activity was assessed using laminarin as a substrate, and β-1,3-glucanase activity was expressed as micromole glucose equivalents (Wood & Bhat, 1988). Lipoxygenase activity was estimated employing linoleic acid as a substrate, and activity was expressed as linoleic acid equivalents (Alexrod et al., 1981).
Estimation of total phenolic content
Leaf tissues (1 g) were homogenised in 10 mL of 80% methanol and agitated for 15 min at 70°C. One millilitre of the methanolic extract was added to 5 mL of distilled water and 250 μL of Folin-Ciocaleau reagent (1 N) and the solution was kept at 25°C. After 3 min of incubation, 1 mL of saturated Na2CO3 solution and 1 mL of distilled water were added and the reaction mixture was further incubated for 1 h at 25°C. The absorption of the developed blue colour was measured using a spectrophotometer at 725 nm. The total phenolic content was calculated based on standards prepared with phenol and expressed as phenol equivalents g−1 fresh weight (Rahman & Punja, 2005).
Estimation of 6-methoxymellin
Leaf samples were extracted for 6-methoxymellin (6-ME) for 12 h at ambient temperature using spectrophotometric grade hexane (Lafuente et al., 1996). The solution was decanted and then re-extracted with an equal volume of 80% ethanol. Absorbance of the ethanol layer was measured at 267 nm, and 6-ME was calculated using a molar absorptivity of 14 800.
Staining for H2O2 production
Production of hydrogen peroxide from reactive oxygen species (ROS) following treatment with elicitors was visualised by staining of leaves collected at different time intervals with 3,3′-diaminobenzidine solution (DAB) (1 mg mL−1 and pH 3.8) for 3 h. Tissues were decolourised by boiling in 95% ethanol for 20 min and viewed in the light microscope (Thordal-Christensen et al., 1997).
Tissue nutrient analysis
Dried carrot leaf tissues were ground to a powder in a blender, and 2 g (dry weight) of the sample (in two replicates) was analysed for macronutrients (total nitrogen, phosphorus, potassium, calcium, magnesium, sodium and sulphur) and micronutrients (zinc, copper, iron, manganese, boron and molybdenum) using standard methods of analyses conducted by a commercial analytical laboratory (Norwest Labs, Calgary, Alberta, Canada).
Statistical analyses
All experiments were conducted at least twice with a minimum of three replicates per treatment and included appropriate controls. The greenhouse experiments were conducted three times with 30 replicate plants. All data were analysed by analysis of variance, and mean disease severity values were separated using Fisher’s protected least significant difference test (P = 0.05). All analyses were performed using Statistical Analysis System (SAS) computer program (SAS System Version 7, 1998; SAS Institute, Cary, NC, USA).
Results
Effect of elicitor treatment on disease incidence
Foliar application of three elicitor compounds to carrot plants significantly reduced disease development by A. radicina and B. cinerea (Table 1; Fig. 1[link]). Three consecutive greenhouse trials were conducted and a similar trend was observed in all experiments; results from one experiment are presented in Table 1. Chitosan application reduced Alternaria and Botrytis infection by up to 76% and 72%, respectively, followed by Alexin (57% and 43%) and SA (31% and 30%). Severe symptoms such as blighting or shrivelling of leaves did not develop on plants treated with chitosan or Alexin compared with control plants. The results (Table 1) also showed the positive effect of repeated applications (three times) of elicitors made at 10-day intervals. Integration of a chlorothalonil spray 10 d.a.i. with two sprays of Alexin provided better disease control than Alexin alone (Table 1). None of the elicitor treatments was phytotoxic on carrot plants at all stages of growth at the concentrations tested. Plants treated with Alexin or chitosan had significantly higher dry biomass 30 days after treatment. The mean values were 1.21, 1.10, 0.72 and 0.60 (g plant−1) for chitosan, Alexin, SA and control plants, respectively.

Effect of elicitor treatment on the incidence of Alternaria blight and growth of plants. Photographs were taken 25 days after inoculation. Treatments were made three times (0, 10 and 20 days). Fungal inoculation was made 10 h after the first elicitor treatment. Plants were placed in a humid chamber for 72 h and then maintained in a greenhouse. SA, salicylic acid.
| Treatments | Disease Severity Valuesb | |||
|---|---|---|---|---|
| Alternaria | Botrytis | |||
| 10 days | 25 days | 10 days | 25 days | |
| Control | 4.08 | 4.42 | 4.45 | 4.68 |
| SA | 3.30 | 3.05 | 3.25 | 3.32 |
| SA + F | 2.76 | 2.58 | 2.85 | 2.73 |
| Alexin | 2.16 | 1.94 | 2.41 | 2.67 |
| F | 2.06 | 1.70 | 2.25 | 2.05 |
| Alexin + F | 1.65 | 1.26 | 1.95 | 1.60 |
| Chi | 1.25 | 1.02 | 1.55 | 1.31 |
| Chi + F | 1.16 | 0.70 | 1.25 | 1.02 |
| SED (d.f.) | 0.080 (36) | 0.054 (36) | ||
| F value | 40.3 | 57.23 | ||
| LSD (P = 0.05) | 0.47 | 0.39 | ||
- Chi, chitosan; F, fungicide; SA, salicylic acid; SED, standard error of difference.
- a Elicitor application was made 10 h before inoculation and 10 and 20 days after inoculation (d.a.i), and disease observations were made 10 and 25 days after inoculation. For elicitor + F combinations, chlorothalonil was applied on the 10th day instead of an elicitor application, followed by an elicitor application at 20 days. SED, F, LSD values apply to both 10- and 25-day observations.
- b Disease severity values were calculated using a 6-point disease rating scale based on the percentage of leaf area infection.
A group of elicitor-treated plants was also analysed for nutrient composition (N, P, K, Mg, Na, S, Zn, Bo, Mn, Cu, Fe and Mo). No significant differences in nutrient levels and profiles were observed (data not shown) except for boron that was higher (3.3-fold) in Alexin-treated compared with control plants.
Microscopic observation of the infection process
Inoculated tissues following elicitor treatment were examined under the microscope to observe spore germination, germ tube formation, hyphal proliferation and tissue necrosis at different time intervals. No significant differences in the percentage of spores germinated were observed between the treatments and the control up to 24 h (data not shown). However, there were differences in germ tube length and hyphal growth of A. radicina and B. cinerea (Fig. 2, panel I and Fig. 2, panel II) in control tissues compared with the treatments. At 48 h, growth of hyphae from germinated spores in the control was extensive, while it was much less for both A. radicina and B. cinerea in leaves treated with SA, Alexin and chitosan (Fig. 2). Consequently, at 120 h, there were larger lesions on the control leaves compared with the smaller lesions seen on the elicitor-treated leaves (Fig. 2, panel III). When spore suspensions were mixed with protein extracts from control plants and those treated with elicitors in vitro, the percent spore germination was reduced by 30–45% in elicitor-treated plant extracts (Fig. 3).
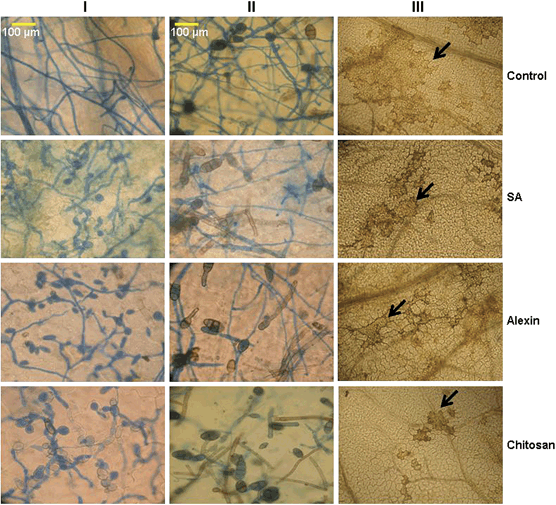
Effect of elicitor treatment on growth of Botrytis (panel I) and Alternaria (panel II) on carrot leaves. Leaves were cleared and stained with lactophenol blue 48 h after inoculation; Alternaria lesions (panel III) (black arrow) 120 h after inoculation. SA, salicylic acid.
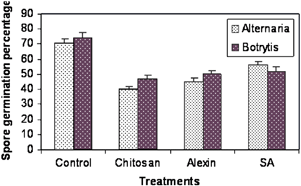
In vitro antifungal activity of protein extracts from elicitor-treated leaves. Bars represent percent spore germination of Alternaria and Botrytis in the presence of leaf extracts from elicitor-treated plants 48 h after treatment. Vertical bars indicate mean ± SE. SA, salicylic acid.
Defence gene transcript accumulation and defence enzyme activities in carrot leaves following elicitor treatments
Carrot plants treated with elicitors showed enhanced transcript levels of chitinase, ltp, chs-2 and PR-5 genes compared with the water control (Fig. 4). Enhanced transcripts appeared at 12 h up to 72 h and began to decline at 94 h. There was also a strong induction of NPR-1 and PR-1 genes following various elicitor treatments. The activity of PO, PPO, PAL, chitinase, β-1,3-glucanase and lipoxygenase were also significantly enhanced by elicitors compared with the water controls. The increase was pronounced at early time intervals (from 12 h onwards) and declined at later times (72 and 96 h after treatment). β-1,3-glucanase activity was highest in chitosan-treated plants followed by Alexin and SA (Fig. 5a). Highest chitinase activity was observed in Alexin-treated plants at 48 h followed by chitosan (48 h) and SA (24 h) (Fig. 5b). PO and PPO activities were higher in plants treated with Alexin (up to 96 h) and chitosan (48 h) compared with SA (Fig. 5c and Fig. 5d). Similarly, a higher activity of PAL was observed in chitosan and Alexin-treated plants compared with SA up to 96 h (Fig. 6a). There was also a significant increase in lipoxygenase activity in elicitor-treated plants compared with water controls, with the highest activity at 24 h after treatment (Fig. 6b).
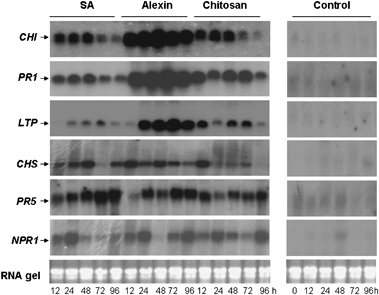
Northern blot showing accumulation of transcripts of chitinase (Chi), pathogenesis-related protein-1 (PR1), lipid transfer protein (LTP), chalcone synthase (Chs), pathogenesis-related protein 5 (PR5) and nonexpressor of PR1 (NPR-1) genes in carrot leaves following elicitor treatment. Ten micrograms of total RNA was used; bottom panel indicates RNA loading controls. SA, salicylic acid.
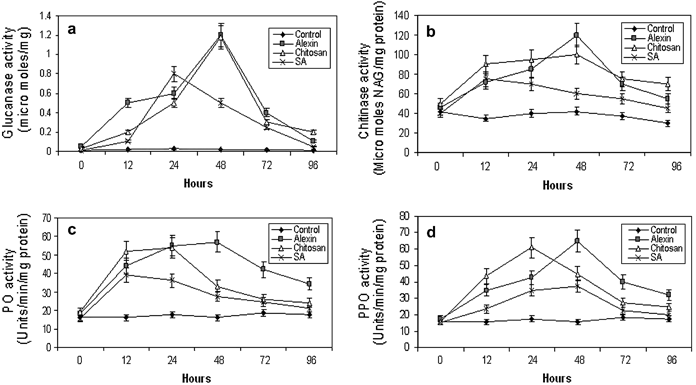
Enzyme activities in carrot leaves treated with three elicitor compounds compared with a water control. (a) Glucanase, (b) chitinase, (c) peroxidase (PO), (d) polyphenoloxidase (PPO). Vertical bars indicate mean ± SE. Data are means of three replicates. NAG, n-acetyl glucosamine; SA, salicylic acid.
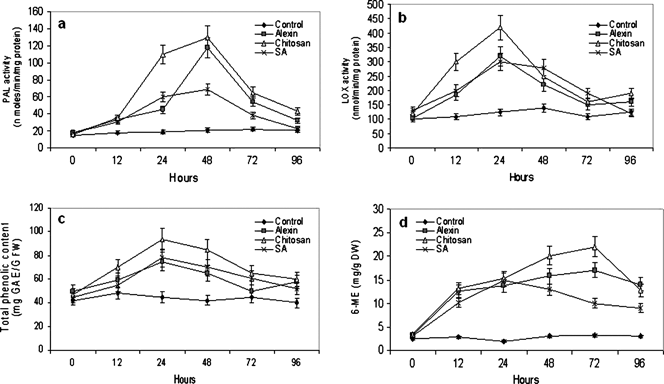
Enzyme activities, phenolic content and phytoalexin content of carrot leaves treated with three elicitor compounds compared with a water control. (a) Phenylalanine ammonia lyase (PAL), (b) lipoxygenase, (c) total phenolic content, (d) 6-methoxymellin (6-ME) content. Vertical bars indicate mean ± SE. Data are means of three replicates. SA, salicylic acid.
Phenolics, phytoalexin and H2O2 accumulation in carrot leaves following elicitor treatment
Total phenolic content of elicitor-treated leaves was higher compared with water controls (Fig. 6c). Maximum accumulation occurred in chitosan-treated leaves followed by Alexin and SA, which had similar levels. A carrot phytoalexin, 6-ME, was enhanced in elicitor-treated leaves compared with the water control (Fig. 6). The highest levels were detected in chitosan-treated leaves followed by Alexin and SA. Hydrogen peroxide accumulation in carrot leaf tissues was assessed by DAB staining. Leaves treated with elicitors showed enhanced accumulation of H2O2 as visualised by dark brown patches in the interveinal area (Fig. 7). Interveinal browning was seen as early as 6 h in all elicitor-treated leaves, while water-sprayed leaves had very few or no brown spots.

Production of hydrogen peroxide following treatment with elicitors. Carrot leaves were stained with 3′,3′-diaminobenzidine and decolourised in ethanol. Brown patches reflect H2O2 accumulation. Leaves were collected 12 h after treatment.
Discussion
There was a marked reduction in disease development because of A. radicina and B. cinerea on carrot plants under greenhouse conditions following elicitor treatments, and both chitosan and Alexin were very effective. To study the response of A. radicina and B. cinerea to elicitor treatments, microscopic observations of spore germination, germ tube growth and hyphal proliferation in elicitor-treated leaves were compared with the control. Hyphal proliferation and lesion number and size were reduced by the treatments at 48–120 h. The antifungal effects of protein extracts from elicitor-treated leaves were reflected by lower spore germination and germ tube growth in vitro, likely because of the activity of PR proteins that are well known for their antifungal properties (Jayaraj et al., 2004). For example, elevated levels of chitinase and glucanase and higher transcript levels of PR genes in northern blots indicate that PR protein expression was enhanced. However, a 30–45% reduction in pathogen growth suggests that PR proteins alone cannot account for the significant level of disease control observed. Therefore, it is likely that the concerted action of antifungal proteins and defence enzymes, together with phenolic, phytoalexin and H2O2 production, reduced development of A. radicina and B. cinerea in carrot leaves.
Following elicitor treatment, enhanced transcript levels of various genes were observed compared with the water control. Transcript levels were enhanced the most by Alexin followed by chitosan. For example, there was a strong induction of NPR-1, which is an essential modulator of the systemic acquired resistance (SAR) signalling pathway at downstream levels and interacts with members of the TGA family transcription factors (Cao et al., 1994). Some of these transcription factors bind to a promoter element within the PR-1 gene, suggesting a link between NPR-1 and transcriptional activation of PR-1 genes during the onset of SAR (Zhou et al., 2000). PR-1 transcript level was also strongly induced in this study. The transcript level of chalcone synthase was also elevated in elicitor-treated carrot plants. This is a key regulatory enzyme in flavonoid and isoflavonoid synthesis, whose expression is regulated by chemical and biological stimuli that include elicitor agents (Ichinose et al., 1992). A coordinated increase in transcript levels of PAL and Chs genes following microbial elicitor treatment was reported by Dixon (1986) in bean plants. In the present study, the transcript levels of chitinase, LTP and PR-5 were also enhanced by elicitor treatment in carrot.
The activity of PO, PPO, PAL, chitinase, β-1,3-glucanase and lipoxygenase enzymes was enhanced by elicitors compared with the water controls. Similar enzymes were induced in other plant species following elicitor treatments. In pepper plants, a rapid and transient induction of PAL, chitinase and glucanase activities was induced by treatment with acibenzolar-S-methyl (ASM) (Baysal et al., 2005), while SA treatment induced phenylpropanoid pathway enzymes in grape (Chen et al., 2006). Chitosan enhanced PO, chitinase, β-1,3-glucanase and lipoxygenase in potato and tomato tissues (Vasiukova et al., 2001). Chitinase action causes thinning of fungal cell walls leading to bursting of hyphae, while glucanases cause dissolution of cell wall glucans. A combination of chitinases and glucanases was demonstrated to be more effective than either enzyme alone against many fungi (Mauch et al., 1988). In addition to antifungal activities exerted by these enzymes, the degradation products resulting from enzyme action such as chito-oligosaccharides or glucans can act as powerful elicitors (Jayaraj et al., 2004).
Together with the enhanced transcript levels and activities of defence genes/enzymes as described above, total phenolic and 6-ME contents were higher in elicitor-treated plants compared with water controls. Phenolic accumulation following elicitor treatments has been commonly observed in other plant species. Infiltration of SA (150 μM) into harvested grape berries enhanced phenolic accumulation (Chen et al., 2006). Application of chitosan oligosaccharides to tomato induced several defence responses, including an accumulation of phenolics, which led to resistance to fungal infection (Benhamou et al., 1994). Carrot leaves in this study treated with Alexin, chitosan and SA accumulated more H2O2 as visualised by DAB staining, within 6 h after treatment. The intensity of browning is directly correlated with the level of ROS or H2O2 (Thordal-Christensen et al., 1997). ROS has been suggested to be the first defence line against pathogen invasion by directly killing the pathogen or slowing down its ingress. ROS also participates in other resistance mechanisms including cell wall lignification, phytoalexin production, hypersensitive response and defence signalling (Vidhyasekaran, 1997; Iriti & Faoro, 2003). A direct relationship between ASM-induced resistance in common bean to Uromyces appendiculatus (Pers.) Unger and H2O2 accumulation was observed in treated tissues (Iriti & Faoro, 2003). A similar phenomenon was observed by Małolepsza (2006) in tomato plants treated with ASM, which caused enhanced H2O2 accumulation leading to reduced infection by B. cinerea. A significant increase in lipoxigenase (LOX) activity was also observed in the present study. Lipoxygenase generates ROS and superoxide anion radicals and singlet oxygen (Vasiukova et al., 2001). The enhanced levels of ROS may participate in signal transduction leading to resistance responses (Sutherland, 1991). Oxidation of membrane lipids results in the production of several antifungal compounds, including phytoalexins, and initiation of cell wall lignification (Vidhyasekaran, 1997). Taken together, these findings indicate that elicitor treatment of carrot plants significantly increased defence gene expression as well as antifungal compounds.
Chitosan is a deacetylated derivative of chitin, which has been shown to induce various defence responses in plants, including PR protein and phytoalexin synthesis and activation of the phenylpropanoid pathway (Benhamou et al., 1994). It was proposed that chitosan can cause electrostatic disruptions of the plasma membrane, leading to signalling that involves rapid induction of a mitogen-activated protein kinase activity in tomato (Stratmann & Ryan, 1997) and can cause hydrogen peroxide production through the oxylipin pathway (Benhamou & Theriault, 1995). The activity of chitosan is dependent on the molecular weight and degree of acetylation (DA) of the molecule. Chitosan oligomers with a DA of over 10% stimulated chitinase activity (Roby et al., 1987) while 20% DA stimulated PAL activity in melon (Vander et al., 1998). The chitosan used in the present study has around 80% DA and demonstrated high elicitor activity and subsequent disease reduction. Chitosan application has reduced diseases in several other plant species, including grey mould on cucumber (Ben-Shalom et al., 2003) and tomato (Liu et al., 2007) caused by B. cinerea. Alexin also reduced disease incidence because of A. radicina and B. cinerea, likely through an induction of defence gene expression and antifungal compounds. Alexin is a product that contains chelated nutrients, oligosaccharides and salicylate derivatives. However, elemental analyses of Alexin-treated carrot plants revealed no significant changes in nutrient levels except for B detected in leaves under greenhouse conditions. There is only one previous report of the use of this compound for disease control. Alexin was as effective as fungicides in reducing the incidence of Septoria apiicola (Speg.) on celery (McDonald, 2006). The presence of salicylates and oligosaccharides in the product may be responsible for disease reduction through induced resistance.
Induction of disease resistance in crop plants is an attractive strategy because it can activate various defences in the plant, providing potential protection to multiple pathogens (Walters et al., 2005). With concerns regarding extensive use of chemical fungicides on horticultural crops, including carrot, alternative strategies that utilise non-fungicidal products need to be evaluated. In this context, the products chitosan and Alexin were found to be effective in reducing leaf necrosis caused by A. radicina and B. cinerea on carrot. However, additional studies under field conditions should be carried out to show efficacy and practical use of these compounds in controlling diseases on a broader scale. These products could be used in an integrated crop protection approach for carrots and other horticultural crops, perhaps in combination with fungicides.
Acknowledgements
This research was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. We thank Apollo-Canada Enterprises, Burnaby, British Columbia, Canada, for providing the chitosan sample, and Nutri-Ag Ltd, Toronto, Ontario, Canada, for providing Alexin.




