Cryotherapy of shoot tips: a technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation
Abstract
Cryotherapy of shoot tips is a new method for pathogen eradication based on cryopreservation techniques. Cryopreservation refers to the storage of biological samples at ultra-low temperature, usually that of liquid nitrogen (−196°C), and is considered as an ideal means for long-term storage of plant germplasm. In cryotherapy, plant pathogens such as viruses, phytoplasmas and bacteria are eradicated from shoot tips by exposing them briefly to liquid nitrogen. Uneven distribution of viruses and obligate vasculature-limited microbes in shoot tips allows elimination of the infected cells by injuring them with the cryo-treatment and regeneration of healthy shoots from the surviving pathogen-free meristematic cells. Thermotherapy followed by cryotherapy of shoot tips can be used to enhance virus eradication. Cryotherapy of shoot tips is easy to implement. It allows treatment of large numbers of samples and results in a high frequency of pathogen-free regenerants. Difficulties related to excision and regeneration of small meristems are largely circumvented. To date, severe pathogens in banana (Musa spp.), Citrus spp., grapevine (Vitis vinifera), Prunus spp., raspberry (Rubus idaeus), potato (Solanum tuberosum) and sweet potato (Ipomoea batatas) have been eradicated using cryotherapy. These pathogens include nine viruses (banana streak virus, cucumber mosaic virus, grapevine virus A, plum pox virus, potato leaf roll virus, potato virus Y, raspberry bushy dwarf virus, sweet potato feathery mottle virus and sweet potato chlorotic stunt virus), sweet potato little leaf phytoplasma and Huanglongbing bacterium causing ‘citrus greening’. Cryopreservation protocols have been developed for a wide variety of plant species, including agricultural and horticultural crops and ornamental plants, and can be used as such or adjusted for the purpose of cryotherapy.
Conservation of plant genetic resources for food security
The world population today is 6.6 billion and is predicted to increase by 50% before 2050 (UNFPA, 2007). Providing food security for such a rapidly growing population will require a dramatic increase in crop production. Obviously, the challenge for further development of crop quality and quantity is much greater than ever (Hobbs, 2007). Availability of plant genetic resources of cultivated crops and their wild relatives is a prerequisite for breeding of elite cultivars via classical breeding and biotechnological techniques. Sustainable agricultural production that ensures both crop quality and quantity largely depends on efficient conservation and proper utilisation of plant genetic resources (Esquinas-Alcázar, 2005). It is estimated that up to 100 000 plants, that is more than one third of all plant species of the world, are threatened or face extinction in the wild (GSPC, 2002). Industrialisation, urbanisation and especially global warming will worsen this situation (Esquinas-Alcázar, 2005; Thuiller et al., 2005; Lynch et al., 2007). The International Treaty for Plant Genetic Resources for Food and Agriculture was made within the framework of the Food and Agriculture Organization of the United Nations and entered into force in 2004. The objectives are the conservation and sustainable utilisation of plant genetic resources for food and agriculture and the fair and equitable sharing of benefits arising out of their usage for sustainable agriculture and food security.
Cryopreservation of plant germplasm
Cryopreservation, that is the storage of living cells, tissues and organs at ultra-low temperature, usually in liquid nitrogen (−196°C), is considered an ideal means for long-term storage of plant germplasm. Cellular divisions and metabolic processes cease at the temperature of liquid nitrogen and, theoretically, plant materials can be preserved without any change for an indefinite period of time (Engelmann, 1997; Benson, 2008). Moreover, the storage requires little space, largely avoids contamination and demands only limited maintenance. For the long-term storage of vegetatively propagated plant germplasm, organised tissues such as shoot tips are preferred over cell and callus cultures because they are genetically more stable (Bajaj, 1991). Introduction of the plants to tissue culture helps to equalise the excised shoot tips in terms of physiology and growth stage (Engelmann, 2004). In addition, in vitro shoot tips are easy to handle, available any time of the year and amenable to plant regeneration (Reed, 2002).
Shoot tips used in cryopreservation or cryotherapy are anatomically defined as structures that consist of the apical or lateral shoot meristem (1–1.5 mm in size) with three to four leaf primordia (Fig. 1) (Takada & Tasaka, 2002; Benson, 2007). Cells in the meristems are tightly packed without intercellular cavities, are small and isodiametric in shape and possess a large nucleocytoplasmic volume ratio (Helliot et al., 2003; Wang et al., 2008). Vacuoles are small and scattered in the cell and the protoplasm contains a large number of cell organelles such as proplastids, mitochondria, Golgi apparatus and ribosomes associated with the endoplasmic reticulum (Fahn, 1985). Sizes of cells and vacuoles increase and nucleocytoplasmic ratio decreases with increasing distance from the apical dome (AD) (Helliot et al., 2002, 2003; Wang et al., 2008). Meristematic cells are able to self-renew and produce daughter cells that differentiate, which results in production of organs of the plant (Dello Ioio et al., 2008). Thus, cell division and differentiation are two basic physiological processes required for shoot regeneration from meristems.
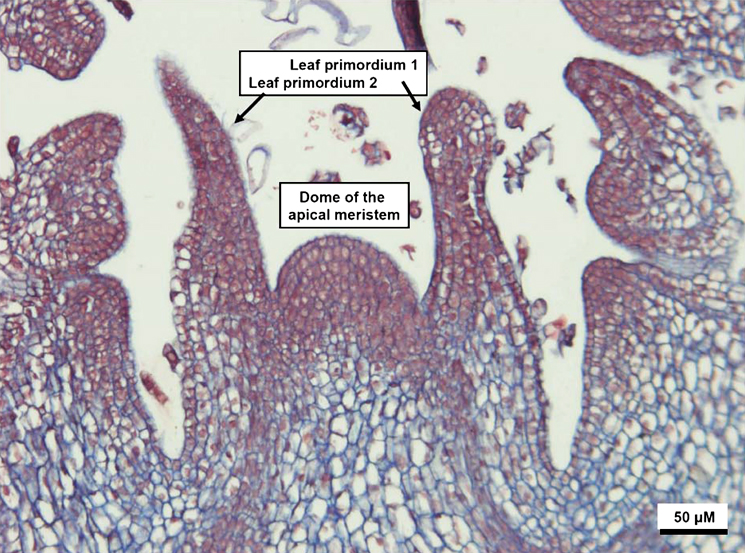
A typical shoot tip of potato (Solanum tuberosum) used for cryotherapy.
Phytohormones play a central role in balancing meristem maintenance and organ production (Shani et al., 2006; Kyozuka, 2007; Dello Ioio et al., 2008). Cytokinins positively regulate cell division. A high cytokinin activity sustains cell division, while high gibberellin and auxin activities are beneficial to initiation and differentiation of lateral organs (Shani et al., 2006; Kyozuka, 2007). In general, a combination of specific concentrations of cytokinin and auxin should be supplied to the meristem culture medium to optimise shoot regeneration (Faccioli & Marani, 1998).
To date, cryogenic techniques have been successfully applied to a large number of plant species including agricultural and horticultural crops from temperate and tropical regions (Reed, 2002; Lambardi & De Carlo, 2003; Engelmann, 2004; Panis & Lambardi, 2006; Wang & Perl, 2006; Gonzalez-Arnao et al., 2008). Cryopreservation of shoot tips has been applied in some genebanks for long-term storage of genetic resources of vegetatively propagated species such as potato in Germany (Mix-Wagner, 2003; Keller et al., 2008) and at the International Potato Center (CIP) in Peru (Golmirzaie & Panta, 2000), pear in the USA (Reed et al., 2000) and banana in Belgium (Panis & Lambardi, 2006). Cryopreservation protocols are also being tested on a large scale for their routine usage for long-term storage of various vegetatively propagated crops, such as mint, cassava, sweet potato, taro and strawberry (Reed, 2002, 2008; Engelmann, 2004; Gonzalez-Arnao et al., 2008).
Cryobiology and cryopreservation of photosynthesising organisms and the operational, risk and safety issues that must be considered in movement of cryobiological research into practice have been comprehensively reviewed by Benson (2007, 2008). Those issues are relevant also to cryotherapy of plants. We refer the reader to the aforementioned papers of Benson in these aspects. A glossary of terms related to cryotherapy is provided in Table 1.
| Cryopreservation: storage of living cells, tissues and organs at ultra-low temperatures, often that of liquid nitrogen (−196°C) |
| Cryotherapy: a technique that uses extreme cold (e.g. that of liquid nitrogen) to eradicate microorganisms from diseased tissue |
| Meristem: a group of undifferentiated cells capable of continuous cellular division giving rise to a shoot or root |
| Meristem culture (or shoot tip culture): culturing of meristems (or shoot tips) on artificial medium under aseptic conditions |
| Pathogen: a microbe or virus that causes disease |
| Pathogen eradication: removal of pathogens from living plants using techniques that allow the resultant pathogen-free plants be propagated and grown |
| Plant genetic resources: all genetic material of plants |
| Plant regeneration: formation of an entire plant from a single cell or group of cells |
| Systemic infection: a process in which the pathogen multiplies and moves from the initial site of infection to other parts of the plant |
| Thermotherapy: the use of heat to slow down multiplication of a pathogen. Used in combination with meristem/shoot tip culture to eradicate viruses and phytoplasmas |
| Vegetative propagation: a process by which new plant individuals are produced by somatic regeneration of organs (shoots or roots), without sexual reproduction. The new plants are identical in genotype to the mother plant |
| Vitrification: a physical transition process of an aqueous solution to an amorphous or glassy solid, which avoids the formation of ice crystals in cells |
Needs and traditional methods for pathogen eradication from crop plants
The availability of pathogen-free planting materials is crucial for high yields and quality of all crops. Plant diseases threaten the productivity and sustainability of agricultural production (Waterworth & Hadidi, 1998). Crop species such as potato, sweet potato, cooking bananas and cassava that ensure food security in many parts of the world are vegetatively propagated and therefore particularly prone to losses caused by viruses that are transmitted from generation to generation in the planting materials (Hadidi et al., 1998; Loebenstein et al., 2001; Loebenstein & Thottapilly, 2003). Similar problems also occur in many economically important horticultural crops such as citrus, pome and stone fruit trees, berry crops and in ornamental plants (Németh, 1984; Hadidi et al., 1998).
Phytoplasmas are obligate parasites of plants that were not discovered until the 1960s (Doi et al., 1967), but since then, their economical impact on agricultural production has been well documented (Hogenhout et al., 2008). For example, epidemics of the phytoplasma-related disease, papaya dieback, resulted in up to 100% crop losses in south-east Queensland and Western Australia in 2002 (Streten & Gibb, 2006). Occurrence of coconut lethal yellowing caused by a phytoplasma destroyed entire plantations in the Caribbean, Central and South America, Mexico and areas of Africa (Mpunami et al., 1999). The Huanglongbing (HLB) disease or ‘citrus greening’ caused by a bacterium is spread in infected planting materials and has already devastated the citrus industry in many Asian countries, killed hundreds of thousands of trees in Brazil and swept through 26 Florida counties since it was first reported there in 2005 (Callaway, 2008). It is considered the most destructive citrus disease (Bové, 2006; Callaway, 2008).
It is known for a long time that viruses are unevenly distributed in plants (Holmes, 1948; Kassanis, 1950). Phytoplasmas and the obligate bacterial parasites inhabit the plant vasculature, invade only tissues that support their movement and are unevenly distributed (Li et al., 2006; Hogenhout et al., 2008). Therefore, in infected plants, the youngest part (dome) of the meristematic tissue is generally either pathogen-free or contains a very low concentration of viruses and phytoplasmas, while the contrary is found with increasing distance from the meristem (Fig. 2). In meristem culture for pathogen eradication, it is therefore important to excise meristem tips as small as possible for culture and regeneration in vitro to ensure their freedom of viruses and phytoplasmas.

Combination of thermotherapy and cryotherapy for enhanced elimination of viruses that are able to invade the meristematic cells efficiently. I: Most of the more developed, infected cells are lethally injured whereas the youngest cells in the meristem survive the cryo-treatment. If the virus was not able to enter the meristem, the treatment would result in virus-free plants. However, in cases like here where the virus penetrates the meristem, shoots regenerated after cryo-treatment will remain infected. II: Additional suppression of virus and increased propensity of infected cells to be injured by cryo-treatment can be achieved by subjecting shoots to thermotherapy before excising shoot tips for treatment in liquid nitrogen. Thermotherapy causes stress and reduces survival of the more developed cells, and also accelerates degradation of viral RNA (Wang et al. 2008). An encapsulated raspberry shoot tip (1.5 mm) is illustrated in the middle (courtesy Jenni Kesulahti). AD, apical dome (top layer of cells of the apical meristem); HC, healthy cells; KC, killed cells; LP1, leaf primordium 1 (the youngest leaf primordium); LP2, leaf primordium 2; SC, surviving cells; SVIC, surviving, virus-infected cells; VIC, virus-infected cells.
The meristem consists of actively dividing cells and is 0.1 mm in diameter and 0.25 mm long, on average, depending on the plant species (Quak, 1987). However, the excision of such small meristems is difficult and time-consuming and their regeneration is often problematic. Therefore, larger ‘meristem tips’ of approximately 1.0 mm are usually excised but the chances of obtaining virus-free plants are then reduced. Conventional methods of virus eradication involve heat treatment (thermotherapy) (Kassanis, 1950) which can be applied on shoots introduced to tissue culture before they are subjected to ‘meristem-tip culture’, which suppresses pathogen titres and enhances virus eradication (Walkey & Cooper, 1975).
Cryopreservation techniques applied for cryotherapy of shoot tips
In essence, cryopreservation of shoot tips is a process in which shoot tips are exposed to ultra-low temperature, stored and regenerated for multiplication. A critical step in the process is dehydration of cells before immersing the tissues into liquid nitrogen (Engelmann, 2004). Otherwise, crystallisation of intracellular water inside cells damages them lethally because the crystals penetrate membrane structures. Thus, for successful cryopreservation, lethal intracellular injury must be avoided. Classical cryotechniques (slow freezing) are based on freeze-induced dehydration (Kartha, 1985). ‘One-step freezing’ cryotechniques (i.e. techniques allowing the direct immersion of explants in liquid nitrogen) have been established since 1990 (Engelmann, 1997). The modern dehydration techniques are based on vitrification, that is, the physical transition of water directly from the liquid phase into an amorphous phase or glass (Fahy et al., 1984). Encapsulation–dehydration, encapsulation–vitrification, vitrification, droplet-freezing and droplet-vitrification are among the most popular new ‘one-step freezing’ cryotechniques (Engelmann, 1997, 2004; Wang & Perl, 2006; Wang & Valkonen, 2007; Gonzalez-Arnao et al., 2008; Reed, 2008). In these vitrification-based procedures, before direct immersion of shoot tips into liquid nitrogen, most of the freezable intracellular water is removed by exposure of naked or encapsulated shoot tips to a highly concentrated vitrification solution such as PVS2 (plant vitrification solution no. 2; Sakai et al., 1990) or by air drying (Fabre & Dereuddre, 1990).
In cryotherapy, lethal injury of cells during cryo-treatment is utilised to kill infected tissues. Cells that are located more distant from the AD and are more likely to be infected by viruses or phytoplasmas are more sensitive to injury than the cells that are located in the meristematic zone of AD. Hence, the cells in the AD and in the first leaf primordia are those likely to survive and regenerate into pathogen-free shoots. Consequently, a larger proportion of plants regenerated following cryotherapy is likely to be pathogen-free than what is achieved through the traditional meristem culture (Table 2).
| Plant | Pathogen | Plant regeneration from shoot tips (%) | Pathogen-free regenerants (%) | References | ||
|---|---|---|---|---|---|---|
| Meristem | Cryo | Meristem | Cryo | |||
| Banana | CMV | 100 | 76 | 4 | 34 | Helliot et al. (2002) |
| Banana | BSV | 100 | 76 | 76 | 90 | Helliot et al. (2002) |
| Grapevine | GVA | 75 | 60 | 12 | 96 | Wang et al. (2003) |
| Potato | PLRV | 55 | 87 | 56 | 85 | Wang et al. (2006) |
| Potato | PVY | 55 | 87 | 62 | 93 | Wang et al. (2006) |
| Prunus hybrid | PPV | 85 | 50 | 19 | 50 | Brison et al. (1997) |
| Raspberrya | RBDV | 60 | 30 | 0 | 35 | Wang et al. (2008) |
| Sweet orangeb | HLB | 69 | 85 | 25 | 98 | Ding et al. (2008) |
| Sweet potatoc | SPCSV | 100 | 87 | 100 | 100 | Wang & Valkonen (2008b) |
| Sweet potatoc | SPFMV | 100 | 87 | 10 | 100 | Wang & Valkonen (2008b) |
| Sweet potatoc | SPCSV + SPFMV | 100 | 87 | 7 | 100 | Wang & Valkonen (2008b) |
| Sweet potatod | SPLL | 100 | 85 | 10 | 100 | Wang & Valkonen (2008a) |
- BSV, banana streak virus; CMV, cucumber mosaic virus; GVA, grapevine virus A; HLB, huanglongbing bacterium; PLRV, potato leaf roll virus; PVY, potato virus Y; RBDV, raspberry bushy dwarf virus; SPCSV, sweet potato chlorotic stunt virus; SPFMV, sweet potato feathery mottle virus; SPLL, sweet potato little leaf phytoplasma.
- a Shoots were subjected to thermotherapy followed by cryotherapy of the excised shoot tips.
- b Applied also to another accession of sweet orange, Beijing lemon, mandarin and pummelo, which resulted in HBL-free regenerants at 93%, 91%, 93% and 94% frequency, respectively.
- c Shoot tips size 1.5 mm.
- d Shoot tip size 1.0 mm.
To achieve a high efficiency of pathogen eradication, it may be necessary to adjust the established cryopreservation protocol to increase mortality of cells. Furthermore, thermotherapy of shoots before excision of shoot tips may be helpful for further enhancement of pathogen eradication by cryotherapy (Fig. 2) (Wang et al., 2008).
Case studies on pathogen eradication by cryotherapy
Brison et al. (1997) were the first to successfully apply cryotherapy to eliminate plant pathogens. Plum pox virus (PPV) was eradicated from an interspecific Prunus rootstock using a vitrification protocol. Since then, this technique has been successfully applied to eradicate viruses, phytoplasma or bacteria from cultivated plants of six families (Table 2). The main steps of the cryotherapy procedure are summarised in Fig. 3.
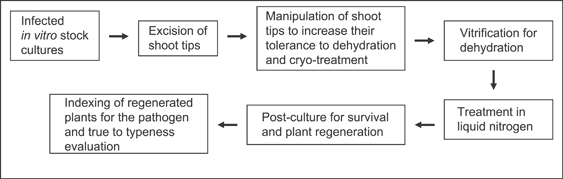
Main steps involved in cryotherapy of shoot tips. The cryotherapy-specific steps of an encapsulation–vitrification protocol will add only 4 days to the total time of 2–3 months needed to complete virus eradication from potato or sweet potato using conventional meristem culture (Wang et al. 2006; Wang & Valkonen 2008b). Manipulation of shoot tips to increase their tolerance to dehydration and cryo-treatment takes 3 days. Vitrification and treatment in liquid nitrogen take 2 and 1 h, respectively. Postculture for survival and plant regeneration takes 2.5 months, which does not differ from the time needed for shoot regeneration following meristem tip culture.
Virus eradication from staple crops
Sweet potato feathery mottle virus (SPFMV; genus Potyvirus) is transmitted in a non-persistent manner by aphids (Loebenstein & Thottapilly, 2003), whereas sweet potato chlorotic stunt virus (SPCSV; genus Crinivirus) is transmitted by whiteflies in a semipersistent, non-circulative manner (Sim et al., 2000). SPCSV is restricted to phloem, whereas SPFMV infects all types of tissues in sweet potato plants (Karyeija et al. 2000; Wang & Valkonen, 2008b). SPFMV and SPCSV are among the most common viruses infecting sweet potatoes [Ipomoea batatas (L.) Lam.; Convolvulaceae] (Tairo et al., 2005; Njeru et al., 2008, and references therein). They interact synergistically and co-infection results in development of the sweet potato virus disease (SPVD), the most damaging disease of sweet potato crops that causes yield reductions up to 90% (Ngeve & Bouwkamp, 1991; Milgram et al., 1996; Gibson et al., 1998; Karyeija et al., 1998; Gutiérrez et al., 2003; Mukasa et al., 2006). Recent studies have shown that regardless of the size of shoot tips (0.5–1.5 mm) and infection status (single or co-infection) of the plants, SPFMV and SPCSV can be eradicated at 100% efficiency from sweet potato plants using cryotherapy. In contrast, meristem tip culture produced only 7–10% SPFMV-free regenerants from the co-infected plants (Table 2) (Wang & Valkonen, 2008b).
Potato leaf roll virus (PLRV), the type member of genus Polerovirus, is limited to the phloem of infected plants. PLRV can be persistently transmitted by many aphid species (Kennedy et al., 1962). Potato virus Y (PVY) is the type species of genus Potyvirus transmitted by more than 40 aphid species (Kennedy et al., 1962; Sigvald, 1984). PLRV and PVY occur in almost all potato-growing areas of the world and are the most important viruses limiting profitable and sustainable potato production (Loebenstein et al., 2001; Valkonen, 2007). Wang et al. (2006) applied three cryogenic procedures (encapsulation–dehydration, encapsulation–vitrification and droplet-vitrification) to potato plants (Solanum tuberosum L., Solanaceae) naturally double infected with PLRV and PVY (Table 2). Both viruses could be efficiently eliminated by the three procedures resulting in 83–86% and 91–95% plants free of PLRV and PVY, respectively. No differences were found in the efficiency of virus eradication among the three cryogenic procedures.
Cucumber mosaic virus (CMV; genus Cucumovirus) causes a mosaic disease or infectious chlorosis in banana (Musa spp; Musaceae) (Ayo-John et al., 2008). CMV is transmitted by more than 60 aphid species in a non-persistent manner in a wide variety of plant species, and also in seeds (Wardlaw, 1972). Banana streak virus (BSV), the causal agent of banana streak disease (Lockhart, 1986), belongs to genus Badnavirus and is transmitted by mealybugs. CMV and BSV cause severe problems in banana production (Dahal et al., 1999). They reduce fruit yield (Lockhart, 2000) and restrict plant breeding and germplasm dissemination (Harper et al., 2005). Cryotherapy by vitrification has been tested for eradication of CMV and BSV from in vitro cultures of meristematic clumps of dessert banana cv. Williams (AAA, Cavendish subgroup). Up to 30% and 90% of the plants regenerated following cryotherapy were free from CMV and BSV, respectively (Helliot et al., 2002).
Virus eradication from economically important fruit and berry plants
Plum pox virus (genus Potyvirus) is the agent causing the most destructive viral disease, sharka, in stone fruits of Prunus spp. including peach (Prunus persica L.), plum (Prunus domestica L.), apricot (Prunus armeniaca L.), nectarine (P. persica variety nectarine), almonds (Prunus amygdalus Batch), sweet cherry (Prunus avium L.) and sour cherry (Prunus cerasus L.) (Levy et al., 2000; Damsteegt et al., 2007). It occurs in most of the Prunus-growing regions of the world (Németh, 1984). PPV is transmitted by aphids in a non-persistent manner and by grafting (Damsteegt et al., 2007). Although much effort has been invested to obtain PPV-resistant cultivars using breeding and genetic engineering methods (López-Moya et al., 2000), the use of virus-free plants for establishing orchards is still the main measure for an effective control of this virus. Brison et al. (1997) obtained virus-free plants for the first time using cryotherapy. They applied a vitrification protocol for cryotherapy and used in vitro grown shoot tips from experimentally inoculated, PPV-infected stock cultures of an interspecific Prunus rootstock ‘Fereley-Jaspi’. Virus-free plants were obtained with high frequencies (45–60%) irrespective of the size of shoot tips used (Table 2). Larger shoot tips (0.5–2 mm) showed better regrowth (56–70%) compared with the smallest ones (0.3–0.5 mm; 11%) (Brison et al., 1997).
Grapevine virus A (GVA; genus Vitivirus) is associated with Kober stem grooving, a component of the grapevine rugose wood disease complex that occurs in most grapevine-growing regions and causes severe damage to production of grapevine (Vitis vinifera; Vitaceae) (Garau et al., 1994). GVA is transmitted by many pseudococcid mealybug species (Rosciglione et al., 1983; Garau et al., 1994). Grapevine ‘Bruti’ infected with GVA was used for virus eradication by cryotherapy of shoot tips (Wang et al., 2003). About 96% of the plants regenerated from cryotreated shoot tips were virus-free, whereas only few virus-free plants (12%) were obtained using meristem culture (Table 2). No GVA-free plantlet was obtained when all other steps of the cryotherapy procedure were carried out but the freezing step was omitted. Therefore, virus eradication was dependent on treatment of shoot tips in liquid nitrogen. Damage to cryotreated shoot tips varies depending on the cryogenic protocol employed (Matsumoto, 2001; Wang et al., 2005) and may affect virus eradication efficiency. The authors therefore tested two cryogenic procedures (encapsulation–dehydration and vitrification) on GVA eradication but found no differences in the eradication efficiency (Wang et al., 2003).
Raspberry bushy dwarf virus (RBDV) is the only member of genus Idaeovirus. It infects many soft fruit crops of the genus Rubus (family Rosaceae), including raspberry (Rubus idaeus L.), members of the group ‘Loganobaccus’ (loganberry and boysenberry) and black raspberry (Rubus occidentalis L.). RBDV is a pollen-born virus, transmitted to progeny seedlings through the ovule and among flowering plants through infected pollen (Cadman, 1965; Murant et al., 1974). RBDV is widely distributed in most of the Rubus-growing regions of the world and drastically reduces yield and quality of fruits (Strik & Martin, 2003). Control of this virus is dependent on growing healthy plants in isolation from sources of possible infection, or growing RBDV-resistant cultivars. RBDV has proven difficult to eliminate from infected plants through traditional methods such as meristem culture, thermotherapy and thermotherapy followed by meristem culture (Theiler-Hedtrich & Baumann, 1989; Lankes, 1995; Karesova et al., 2002). Recent attempts to eliminate RBDV from diseased plants of raspberry by meristem culture (shoot tip size 0.1–0.3 mm), thermotherapy followed by meristem culture (1 mm) or cryotherapy of shoot tips (1 mm) did not result in RBDV-free plants (Wang et al., 2008). Therefore, a new procedure was established in which RBDV-infected shoot cultures were first subjected to thermotherapy in vitro with a regime of daily 16-h light and 8-h dark period at 38°C and 26°C, respectively, for 28–35 days (Wang et al., 2008). Subsequently, tips (1 mm) excised from the heat-treated shoots were subjected to cryotherapy. Using this method, about 36% survival and 30% regrowth of cryotreated shoot tips were obtained when stock shoots were heat-treated for 28 days. Among the plantlets regenerated from the treatment combining thermotherapy and cryotherapy, 33–35% were RBDV-free (Table 2; Wang et al., 2008).
Eradication of phytoplasmas and bacteria
Phytoplasmas are intracellular cell wall-less bacteria belonging to the class Mollicutes that live as obligate parasites in plants and their insect vectors (Lee et al., 2000; Hogenhout et al., 2008). Phytoplasmas colonise only the phloem tissues, mainly sieve elements, and are transmitted by phloem-feeding leafhoppers (Cicadellidae), planthoppers (Fulgoridae) and psyllids (Psyllidae) (Lee et al., 2000). The sweet potato little leaf (SPLL) phytoplasma (Candidatus Phytoplasma aurantifolia) occurs in some sweet potato-growing areas and causes heavy yield losses that can reach up to 50% (Pearson et al., 1984; Gibb et al., 1995; Davis et al., 2003; Tairo et al., 2006). Wang & Valkonen (2008a) tested eradication of SPLL phytoplasma from a sweet potato line using cryotherapy of shoot tips. Three sizes of shoot tips were tested for survival and regrowth following cryotherapy. High survival (83–87%) was obtained in shoot tips ranging from 0.5 to 1.5 mm in size, but regrowth of 0.5-mm-long shoot tips was poor (15%) compared with the 1.0- and 1.5-mm-long shoot tips (regrowth 86% and 80%, respectively). When 1.0-mm-long shoot tips were used for cryotherapy, all regenerated shoots were phytoplasma free (Table 2).
Citrus HLB disease, also known as ‘citrus greening’, is caused by phloem-limited, non-culturable, Gram-negative bacteria (Jagoueix et al., 1994; Bové, 2006). HLB is widely distributed in more than 40 countries in Asia and Africa (Garnier et al., 1984; Bové, 2006). In the Americas, HLB disease occurs in two of the largest citrus-growing regions in the world, namely in the State of São Paulo in Brazil (Coletta-Filho et al., 2004) and in Florida in the USA (Zhou et al., 2007). Three bacterial species have been identified in the diseased plants: Candidatus Liberibacter asiaticus in Asia (Jagoueix et al., 1994), Candidatus Liberibacter africanus in Africa (Jagoueix et al., 1994) and Candidatus Liberibacter americanus in the American continent (Teixeira et al., 2005). The disease attacks Citrus, Fortunella and Poncirus species (Rutaceae) and is transmitted by two psyllid insects, Diaphorina citri in Asia and America, and Trioza erytreae in Africa (Teixeira et al., 2005; Bové, 2006). Ding et al. (2008) eliminated HLB from infected in vitro plantlets of sweet orange cv. ‘Hongjiang’ [Citrus sinensis (L.) Osbeck] by cryotherapy of shoot tips and obtained HLB-free plants at a frequency of 98% (Table 2). Similarly, cryotherapy of shoot tips was applied to the sweet orange accession ‘Luogang’ (C. sinensis), mandarin ‘Ponkan’ (Citrus reticulata Blanco), Shatianyou pummelo [Citrus grandis (L.) Osbeck] and Beijing lemon [Citrus limon (L.) Burm.f.] and almost all regenerated plants were HLB-free (93%, 93%, 94% and 91%, respectively) (Ding et al., 2008).
Efficiency of cryotherapy
The efficiency of pathogen eradication through cryotherapy compared with conventional meristem culture depends on the efficiency of plant regeneration and the frequency by which pathogen-free regenerants are obtained. A comparison of these techniques is provided in Table 2.
As expected, higher survival and regrowth frequencies are observed following meristem culture compared with cryotherapy of shoot tips (Brison et al., 1997; Wang et al., 2003; Panis et al., 2005; Wang & Valkonen, 2007, 2008a,b). However, it should be emphasised that much higher frequencies of pathogen-free plants are produced with cryotherapy of shoot tips than with shoot tip culture (Brison et al., 1997, Helliot et al., 2002; Panis et al., 2005; Wang et al., 2003, 2006, 2008; Ding et al., 2008; Wang & Valkonen, 2008a,b) or thermotherapy followed by meristem culture (Wang et al., 2006, 2008).
Brison et al. (1997) found that shoot regrowth rates of meristem cultures and cryotreated shoot tips increased up to 97% and 70%, respectively, with an increasing shoot tip size from 0.3 to 2.0 mm. However, the frequency of virus eradication by meristem culture decreased from 31% to 12% when the size of shoot tips increased from 0.3 to 1.5 mm. With cryotherapy, high proportions (45–60%) of virus-free plants were obtained irrespective of the shoot tip size. Similar results have been obtained with other pathogens and hosts (Wang et al., 2003, 2006; Ding et al., 2008; Wang & Valkonen, 2008b). Indeed, efficiency of pathogen eradication by meristem tip culture can be increased by using smaller shoot tips which, however, compromises survival and regrowth (Faccioli & Marani, 1998). Furthermore, small shoot tips (meristems) less than 0.5 mm are tedious to excise. Therefore, one benefit of cryotherapy is that it allows high-efficiency pathogen eradication and good rates of regeneration independent of the size of shoot tips used.
In pathogen–host combinations where the use of meristem culture alone eradicates the pathogen reasonably well, incorporation of cryotherapy to the procedure may further improve eradication efficiency (Table 2) and adds only marginally to the time needed to execute the eradication protocol (Fig. 3). Cryotherapy does not require tedious excision of small meristems for secure, high-frequency elimination of the pathogen (Wang & Valkonen, 2008b) and hence allows treating large numbers of samples. In addition, cryotherapy can be easily incorporated to the procedures used in genebanks for plant cryopreservation (Brison et al., 1997; Panis et al., 2005) and hence the long-term storage of pathogen-free materials and conservation of the germplasm may be enhanced.
Mechanism of pathogen eradication by cryotherapy of shoot tips
Since the first report on efficient virus eradication by cryotherapy of shoot tips was published (Brison et al., 1997), efforts have been directed to unravel the mechanism behind cryotherapy. Wang & Valkonen (2008b) obtained only virus-free plants when 1.0-mm-long shoot tips containing three leaf primordia were excised from sweet potato plants infected with SPCSV, SPFMV or both viruses. Virus localisation in the shoot tips demonstrated that SPCSV reached only the fifth leaf primordium but not the younger leaf primordia, whereas SPFMV reached the fourth leaf primordium. Localisation of these viruses was similar irrespective of whether they infected plants alone or in the combination that results in the synergistic, severe SPVD (Wang & Valkonen, 2008b). The SPLL phytoplasmas were detected in shoot tips of the same sweet potato genotype only in sieve elements below the second leaf primordium (Wang & Valkonen, 2008a,b). Following cryotherapy, only cells in the AD and a few cells in the first two leaf primordia survived, whereas other cells of the shoot tip showed signs of lethal injury (Wang & Valkonen, 2008a). Hence, localisation of the viruses and phytoplasma in shoot tips before cryotherapy and the pattern of surviving cells in shoot tips after cryotherapy provided an explanation as to why cryotherapy of shoot tips efficiently eliminated SPCSV, SPFMV and the SPLL phytoplasma. Similar reasons may also explain the high efficiency of virus eradication observed with many other viruses (Brison et al., 1997; Helliot et al., 2002; Wang et al., 2003, 2006), phytoplasma and vasculature-limited obligate bacteria that are not known to efficiently infect meristematic cells of shoot tips (Ding et al., 2008; Hogenhout et al., 2008).
Eradication of pathogens that infect meristematic cells efficiently is more difficult. Helliot et al. (2002, 2003) compared the ultrastructure of untreated meristematic cells of banana with those that had undergone cryotherapy. Cells in the AD were small and contained small vacuoles. Nucleolus was readily detected and cells had a high nucleocytoplasmic ratio in contrast to the more differentiated cells outside the meristematic zone. Following cryotherapy, only cells in a small area located in the AD and at the base of the youngest leaf primordia survived, while other cells were lethally injured. Similar results on higher tolerance of the cells in meristematic zone towards the cryo-treatment have been reported in other studies (Engelmann, 2004; Wang et al., 2005, 2008; Volk & Caspersen, 2007). The surviving cells were able to divide, differentiate and regenerate to plantlets. As discussed above, cryotherapy of proliferating meristems of banana produced approximately 30% and 90% CMV- and BSV-free plants, respectively, whereas controls resulted in only few CMV-free plants and 76% BSV-free plants (Helliot et al., 2002). The authors hypothesised that unlike BSV, CMV may invade the cells in the AD and the youngest leaf primordia in banana and hence be difficult to eradicate by cryotherapy, which was supported by detection of CMV in the AD and young leaf primordia in a more recent study (Helliot et al., 2007).
In raspberry plants infected with RBDV, cryotherapy of shoot tips did not produce any virus-free plant (Wang et al., 2008). Localisation of RBDV by immunostaining revealed strong signals for the virus in almost all tissues including the first two leaf primordia and the basal part of the meristem. Only a small group of cells in the AD contained no visible virus signals, indicating that these cells were virus-free or contained virus amounts below the detection limit. Following cryotherapy, surviving cells were observed in the AD and in the first two leaf primordia of shoot tips. RBDV was also detected in some of these tissues, which explained why no virus-free plants could be obtained (Wang et al., 2008).
Heat treatment of plants reduces virus titres and enhances virus eradication (Kassanis, 1950). Thermotherapy of shoot tip cultures in vitro can also be used to enhance pathogen eradication (Grondeau & Samson, 1994; Mink et al., 1998). Therefore, combination of thermotherapy and cryotherapy was tested with the RBDV-infected shoot cultures. This approach resulted in virus-free plants from 33% to 35% of the regenerated shoot tips. Virus localisation, histological and subcellular changes, and viral RNA degradation in shoot tips were studied. While distribution of virus signals was not significantly different between the non-treated and heat-treated shoot tips, signal intensity was greatly reduced in heat-treated shoot tips. Furthermore, northern blot analysis revealed that the amounts of viral RNA decreased rapidly and only degradation products were detectable after 5 days of thermotherapy. Thermotherapy also induced structural changes in tissues outside the AD. Tissue structure became looser, densely stained nucleoli were not observed, the cells were significantly enlarged (about twofold) and contained markedly enlarged vacuoles, and some cells were affected by plasmolysis. Hence, the heat-treated tissues showed signs of stress and damage, which probably increased their propensity to lethal injury at ultra-low temperature, during events preceding vitrification or during subsequent warming, or in all of these steps of the cryo-treatment. Following cryotherapy, only a few surviving cells were observed in the AD that maintained its histological integrity during thermotherapy (Wang et al., 2008). This part of the meristem contained no virus signals. These results suggest a model in which thermotherapy enhances degradation of RBDV in shoot tips, reduces the number of virus-infected cells that survive cryotherapy and hence increases the proportion of regenerants that are virus-free (Fig. 2).
Conclusion and prospects for future research
Conventional methods such as meristem culture and thermotherapy followed by meristem culture have been well established and are now widely applied to produce pathogen-free plants. However, to meet the demands for increased food production and improved productivity of crops, more efficient methods for production of pathogen-free plants are needed. To date, cryotherapy of shoot tips has been successfully applied to eradicate nine viruses, a phytoplasma and an obligate bacterial pathogen from crop plants belonging to six families. The current reasonable understanding of mechanisms behind pathogen eradication by cryotherapy of shoot tips is useful for designing pathogen eradication schemes for new pathogen–host combinations but cellular and molecular details of the mechanism deserve to be addressed also in future studies.
Cryotherapy-based procedures are easy to implement and do not require special equipment in addition to those typically available in a plant tissue culture laboratory. Cryotherapy facilitates treatment of large numbers of samples. It yields pathogen-free plants at a high frequency avoiding also the difficulties associated with excision of very small meristems. Functional cryopreservation protocols are available for many economically important monocot, herbaceous and woody plant species in addition to those mentioned above (Reed, 2008). There is hence a lot of scope to use cryotherapy more widely than reported to date. The main limitation lies in genotype-specificity of many cryopreservation protocols, which may preclude wider application of the available method to all genotypes or cultivars of the species. Similar limitations are also experienced with tissue culture techniques used for conventional meristem culture for pathogen eradication. These limitations will be alleviated and overcome by further development and adjustment of cryoprotocols for plants.
Cryotherapy provides already now an alternative, efficient strategy for eradication of plant pathogens in many species. Cryopreserved shoot tips may also be considered to be safer for exchange of germplasm between countries and regions because the cryogenic procedure reduces the amount of viable infected tissue (Panis et al., 2005). However, the phytosanitary status of regenerated plants still needs to be tested. Tissue culture may induce genetic instability in plants and therefore plants regenerated following cryotherapy should be checked for the true-to-typeness, which is performed also in the conventional pathogen eradication schemes based on meristem culture. However, as no callus formation occurs using the optimised meristem regeneration protocols (Engelmann, 2004), the risk for somaclonal variation is considered minimal.
Acknowledgements
The authors’ studies on cryotherapy have been supported by The Ministry of Agriculture and Forestry and University of Helsinki (Finland), East African Regional Network for Biotechnology, Biosafety and Biotechnology Policy Development programme (BIO-EARN) funded by Sida/SAREC (Q. W. and J. P. T. V.). B. P. would like to acknowledge the financial support of DGDC (Directorate-General for Development Cooperation), Belgium through a grant INIBAP (International Network for the Improvement of Banana and Plantain, currently Bioversity International).




