Construction of a simple sequence repeat marker-based genetic linkage map in the autotetraploid forage grass Dactylis glomerata L.
Abstract
A molecular linkage map was developed for the tetraploid orchardgrass (Dactylis glomerata L.) from an F1 pseudo-testcross population of 76 individuals derived from the cross between two individual plants of Akimidori, a Japanese cultivar and Loke, a Swedish cultivar. Two simple sequence repeat (SSR)-based parental linkage maps were constructed from 314 polymorphic homologous SSR markers derived from the same species. The Akimidori map consisted of 24 linkage groups (LGs) and had a total length of 562 cM, with 168 loci and an average map density of 3.3 cM. Five homologous LGs were detected on this map. The Loke map consisted of 26 LGs and had a total length of 745 cM, with 227 loci and an average map density of 3.3 cM. Seven homologous LGs were detected on this map. A consensus map with seven homologous LGs was developed from these using TetraploidMap software based on the double-simplex (segregated as 3:1) and duplex (segregated as 5:1) markers. This is the first linkage map of the tetraploid Dactlysis and these maps will be useful for quantitative trait locus (QTL) analysis, marker-assisted selection and breeding for important traits in orchardgrass in the future.
Introduction
Orchardgrass or cocksfoot (Dactylis glomerata L.) is naturally distributed through Eurasia and North Africa and widely used as forage grass in major temperate regions with annual seed usage 4th in importance behind the Festuca, Lolium and Phleum genera (Bondesen 2007). Three levels of ploidy; diploid, tetraploid and hexaploid occur within Dactylis, with tetraploids (2n = 4x = 28) the most widely distributed in both nature and cultivation (Borrill 1991; Stewart and Ellison 2011).
Although Dactylis glomerata has been widely studied (Lumaret 1988), its limited importance combined with its auto-tetraploid nature and moderately large genome (4312 Mb; Creber et al. 1994) has delayed the development of molecular markers. Until now, almost all of the molecular markers used in Dactylis have been non-species-specific markers such as random amplified polymorphic DNA (RAPD) (Kölliker et al. 1999), amplified fragment length polymorphism (AFLP) (Peng et al. 2006, 2008) and simple sequence repeat (SSR) markers (Xie et al. 2008). Recently, SSR markers derived from SSR-enriched genomic libraries have been developed (Hirata et al. 2010).
Simple sequence repeat markers occur at high frequencies in eukaryotic genomes (Li et al. 2002). They have a number of advantages over other types of markers such as RAPD, RFLP (restriction fragment length polymorphism) and AFLP: they are polymerase chain reaction (PCR)-based, multi-allelic, co-dominant and highly polymorphic. For these reasons they have been widely used for plant genome analysis. Many SSR-based genetic linkage maps of forage grasses have been constructed, including perennial ryegrass (Jones et al. 2002), meadow fescue (Alm et al. 2003), tall fescue (Saha et al. 2005), Italian ryegrass (Hirata et al. 2006) and zoysiagrass (Li et al. 2009, 2010), and more recently Xie et al. (2011) have constructed an SSR and (sequence-related amplified polymorphism [SRAP]) markers-based linkage map of diploid orchardgrass. In the auto-polyploid species, linkage maps have been reported for sugarcane (Al-Janabi et al. 1993), alfalfa (Julier et al. 2003; Sledge et al. 2005) and potato (Meyer et al. 1998), but not for forage grasses.
Although there have been several reports of the use of molecular markers in diversity studies of Dactylis spp, no molecular linkage map of tetraploid Dactylis has yet been published. Here, we report the construction of an SSR marker-based linkage map of tetraploid cultivated Dactylis glomerata and the detection of homologous linkage groups (LGs).
Materials and methods
Plant materials
Two tetraploid D. glomerata cultivars, Akimidori and Loke, were used as parents of the mapping population. Akimidori originated in Japan, whereas Loke originated in Sweden. A pseudo-testcross F1 mapping population consisting of 76 individuals was derived from the cross between two individuals, Akimidori 4 (as female parent) and Loke 1 (as male parent), and their true F1 status was confirmed using two SSR markers with different heterozygous alleles in both parents.
DNA extraction and DNA marker
DNA was extracted from fresh leaf tissue by using the CTAB (cetyl trimethyl ammonium bromide) procedure (Murray and Thompson 1980). The quality of the DNA was checked in 0.8% agarose gels, and DNA concentrations were estimated by using a SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA).
A total of 969 primer pairs for D. glomerata SSRs developed from SSR-enriched genomic libraries by Hirata et al. (2010) were used to survey polymorphism between the two parents, and 314 SSR markers showing polymorphism were used in the map construction.
Polymerase chain reaction and PAGE
An M13-tagged forward primer (Rampling et al. 2001) method was used in the PCR reaction. We used 5 pmol of labeled M13 (–29) primers (IRD700- or IRD800- CACGACGTTGTAAAACGAC; LI-COR, Lincoln, NE, USA), 5 pmol of reverse primer and 1 pmol of 5′-tagged forward primer for each particular SSR, with the M13 sequence added to the primer’s 5′ end. The following thermocycling conditions were used: an initial denaturation at 94°C for 5 min; two cycles of 94°C for 1 min, 65°C for 1 min and 72°C for 1.5 min; 10 cycles of 94°C for 1 min, 65–55°C for 1 min (decreasing by 1°C per cycle) and 72°C for 1.5 min; 30 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1.5 min; and a final extension at 72°C for 7 min, followed by 4°C for keeping. SSR fragments were analyzed on 6% denatured acrylamide gel with a LI-COR (Lincoln, NE, USA) sequencer (Global Edition IR2 DNA Analyzer).
Data scoring and map construction
First, the segregating bands (SSR alleles) were scored as present or absent for each parent because the SSR primers amplified 1–4 bands in our tetraploid mapping population, which we could not distinguish the homozygous or heterozygous. Then, the segregation ratios of the SSR alleles were analyzed by using TetraploidMap software (Hackett and Luo 2003; http://www.bioss.ac.uk/knowledge/tetraploidmap); only SSR alleles that segregated as 1:1 were used in map construction using Joinmap 3.0 (Stam 1993).
Marker data were scored as cross-pollination data according to the definition of JoinMap 3.0. Linkage maps for the two parents were constructed at a logarithm of odds (LOD) score of 8.0 by using the Kosambi map function (Kosambi 1944), and the linkage maps were drawn with MapChart 2.0 (Voorrips 2002).
Consensus maps for both parents were constructed using TetraploidMap. First we used simple markers to construct the overall consensus maps for each parent. Then the double-simplex (segregated as 3:1) and duplex (segregated as 5:1) markers were mapped on each parental map and used to detect seven homologous LGs between the two parents.
Results
Polymorphism survey and marker distortion
Using the 314 polymorphic primers, two types of marker were detected in the F1 population. Type I was a band present in only one parent and showing a segregation ratio of present to absent of 1:1 or 5:1. Type II was a band present in both parents and showing a segregation ratio of present to absent of 3:1, 11:1, 35:1 or 1:0.
A total of 269 type I markers generated from 191 primer pairs were present in the Akimidori parent; 241 (89.6%) had a 1:1 segregation ratio and 28 (10.4%) had a 5:1 segregation ratio. There were 326 type I markers generated from 215 primer pairs in the Loke parent; 308 (94.5%) had a 1:1 segregation ratio and 18 (5.5%) had a 5:1 segregation ratio. Among the type I markers with 1:1 segregating ratios, 17 showed segregation distortion in Akimodori and 29 in Loke.
There were a total of 93 markers of type II. Thirty-four loci showed no segregation. Fifty-nine loci generated from 56 primer pairs segregated, and of these 31, 21 and seven segregated at 3:1, 11:1 and 35:1, respectively, at a P > 0.05 significance level (Table S1).
Construction of parental linkage maps
Two genetic linkage maps were constructed on the basis of only those type I markers showing 1:1 segregation ratios; we used 76 F1 individuals from the population Akimidori 4 × Loke 1. In total, 168 out of 241 loci were mapped on the Akimidori parental map and 227 out of 308 loci on the Loke parental map; the other loci could not be mapped. Of the mapped loci, 70 generated from 60 primer pairs were mapped only on Akimidori and 119 generated from 82 primer pairs were mapped only on Loke. The markers generated from 69 primer pairs were mapped on both parental maps, comprising 98 loci in Akimidori and 108 loci in Loke.
The Akimidori parental map consisted of 24 LGs that spanned a total of 562 cM, with an average of 23.4 cM per LG, ranging from 7 cM in LG Akimidori 18–44 cM in LG Akimidori 21 (Table 1). It had an average of 7.0 markers per LG; the LGs Akimidori 5 and Akimidori 13 both contained 13 markers (the largest number). The LGs Akimidori 18, Akimidori 23 and Akimidori 24 each contained only three markers (the smallest number). The density of the Akimidori parental map ranged from 1.3 to 8.8 cM between flanking markers, with an average of 3.3 cM.
| Linkage group (LG) | Map length (cM) | No. loci | Map density (cM) | Inter-locus gap distance (cM) | Homologous linkage group |
|---|---|---|---|---|---|
| Akimidori 1 | 25 | 9 | 2.8 | 3.1 | 1 |
| Akimidori 2 | 23 | 5 | 4.6 | 5.8 | 1 |
| Akimidori 3 | 40 | 7 | 5.7 | 6.7 | 1 |
| Akimidori 4 | 29 | 4 | 7.3 | 9.7 | 1 |
| Akimidori 5 | 35 | 13 | 2.7 | 2.9 | 2 |
| Akimidori 6 | 29 | 9 | 3.2 | 3.6 | 2 |
| Akimidori 7 | 15 | 10 | 1.5 | 1.7 | 2 |
| Akimidori 8 | 19 | 8 | 2.4 | 2.7 | 2 |
| Akimidori 9 | 24 | 9 | 2.7 | 3.0 | 3 |
| Akimidori 10 | 24 | 6 | 4.0 | 4.8 | 3 |
| Akimidori 11 | 28 | 4 | 7.0 | 9.3 | 3 |
| Akimidori 12 | 20 | 4 | 5.0 | 6.7 | 5 |
| Akimidori 13 | 38 | 13 | 2.9 | 3.2 | 4 |
| Akimidori 14 | 34 | 10 | 3.4 | 3.8 | 4 |
| Akimidori 15 | 24 | 10 | 2.4 | 2.7 | 7 |
| Akimidori 16 | 19 | 10 | 1.9 | 2.1 | 5 |
| Akimidori 17 | 16 | 6 | 2.7 | 3.2 | 5 |
| Akimidori 18 | 7 | 3 | 2.3 | 3.5 | 7 or 4 |
| Akimidori 19 | 23 | 5 | 4.6 | 5.8 | 6 |
| Akimidori 20 | 10 | 8 | 1.3 | 1.4 | 3 |
| Akimidori 21 | 44 | 5 | 8.8 | 11.0 | 3 |
| Akimidori 22 | 10 | 4 | 2.5 | 3.3 | 4 |
| Akimidori 23 | 17 | 3 | 5.7 | 8.5 | 5 |
| Akimidori 24 | 9 | 3 | 3.0 | 4.5 | – |
| Total | 562 | 168 | – | – | – |
| Mean | 23.4 | 7.0 | 3.3 | 3.9 | – |
The Loke parental map consisted of 26 LGs that spanned a total of 745 cM, with an average of 28.7 cM per LG, ranging from 13 cM in LG Loke 18–65 cM in LG Loke 13 (Table 2). It had an average of 8.7 markers per LG; LG Loke 13 contained 19 markers (the largest number). LG Loke 21 contained only three markers (the smallest number). The density of the Loke parental map ranged from 1.5 to 6.7 cM between flanking markers, with an average of 3.3 cM.
| Linkage group (LG) | Map length (cM) | No. loci | Map density (cM) | Inter-locus gap distance (cM) | Homologous linkage group |
|---|---|---|---|---|---|
| Loke 1 | 54 | 12 | 4.5 | 4.9 | 4 |
| Loke 2 | 17 | 11 | 1.5 | 1.7 | 4 |
| Loke 3 | 34 | 10 | 3.4 | 3.8 | 4 |
| Loke 4 | 34 | 7 | 4.9 | 5.7 | 4 |
| Loke 5 | 24 | 11 | 2.2 | 2.4 | 7 |
| Loke 6 | 32 | 9 | 3.6 | 4.0 | 7 |
| Loke 7 | 19 | 9 | 2.1 | 2.4 | 7 |
| Loke 8 | 26 | 8 | 3.3 | 3.7 | 7 |
| Loke 9 | 17 | 7 | 2.4 | 2.8 | 1 |
| Loke 10 | 24 | 4 | 6.0 | 8.0 | 1 |
| Loke 11 | 33 | 8 | 4.1 | 4.7 | 1 |
| Loke 12 | 27 | 8 | 3.4 | 3.9 | 1 |
| Loke 13 | 65 | 19 | 3.4 | 3.6 | 5 |
| Loke 14 | 34 | 10 | 3.4 | 3.8 | 5 |
| Loke 15 | 32 | 7 | 4.6 | 5.3 | 5 |
| Loke 16 | 31 | 10 | 3.1 | 3.4 | 2 |
| Loke 17 | 14 | 8 | 1.8 | 2.0 | 2 |
| Loke 18 | 13 | 8 | 1.6 | 1.9 | 2 |
| Loke 19 | 31 | 6 | 5.2 | 6.2 | 2 |
| Loke 20 | 30 | 11 | 2.7 | 3.0 | 6 |
| Loke 21 | 20 | 3 | 6.7 | 10.0 | 3 |
| Loke 22 | 18 | 10 | 1.8 | 2.0 | 6 |
| Loke 23 | 39 | 8 | 4.9 | 5.6 | 6 |
| Loke 24 | 17 | 7 | 2.4 | 2.8 | 3 |
| Loke 25 | 39 | 11 | 3.5 | 3.9 | 3 |
| Loke 26 | 21 | 5 | 4.2 | 5.3 | 3 |
| Total | 745 | 227 | – | – | – |
| Mean | 28.7 | 8.7 | 3.3 | 3.7 | – |
Homologous linkage groups detected within the parental maps
Homologous linkage groups (HLGs) were detected by comparing products from the same SSR primers. On the Akimidori parental map, all 168 loci were mapped to 24 LGs; among which five HLGs were detected: Akimidori 1 and 3; Akimidori 5, 6, 7 and 8; Akimidori 9, 10, 11, 20 and 21; Akimidori 13, 14 and 22; and Akimidori 16, 17 and 23 (Figure 1). The remaining nine LGs did not belong to any HLGs.
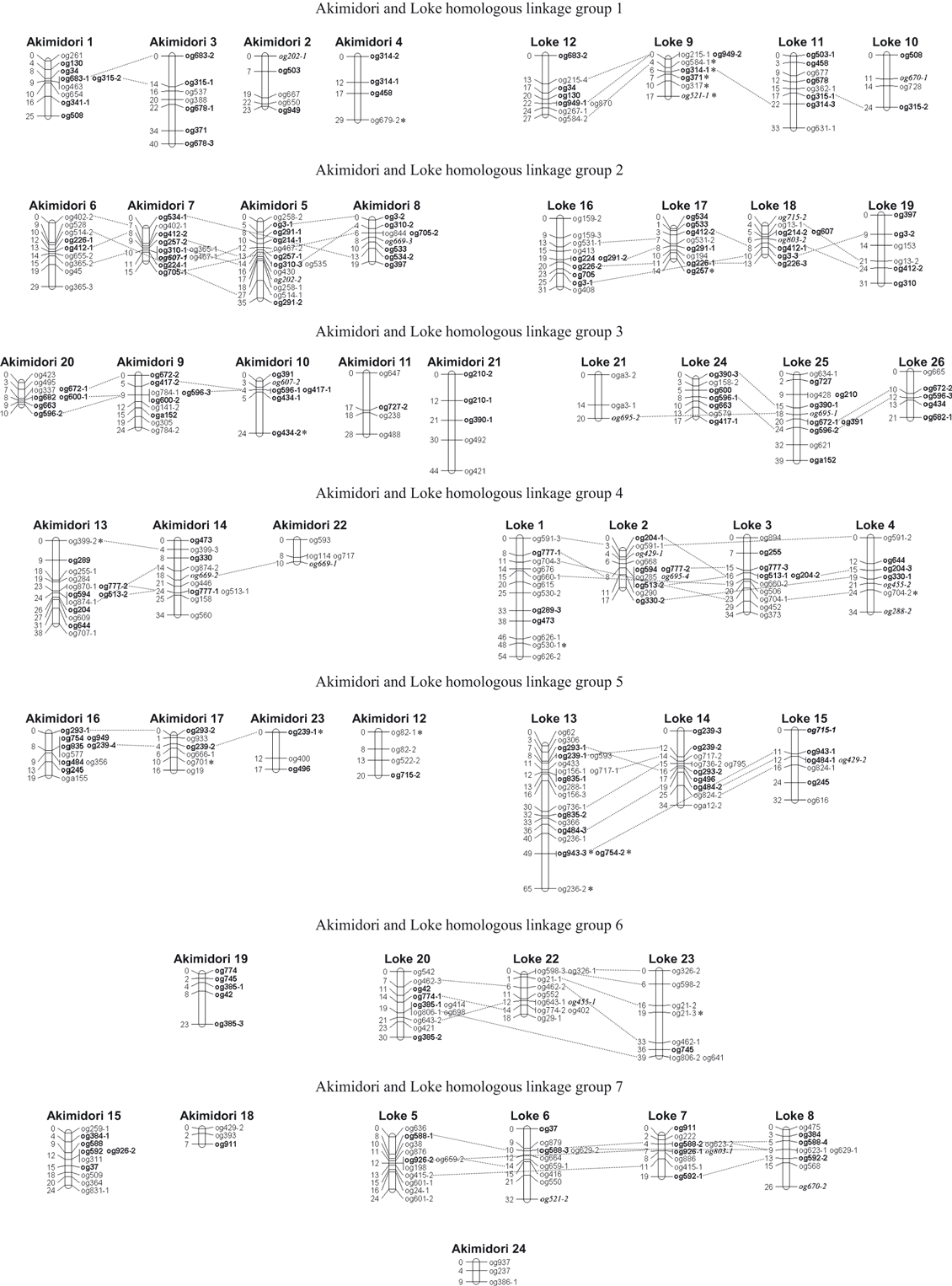
The tetraploid Dactylis glomerata simple sequence repeat (SSR)-based linkages of the two parental maps (Akimidori and Loke), showing homologous linkage groups. Linkage groups are represented as vertical bars, with the names of the markers at the right and the distances (cM) between markers on the left. Markers in italics are multi-copy markers, asterisked markers are distortion-segregated markers, and markers in bold are markers connecting the Akimidori and Loke homologous linkage groups.
On the Loke map, 227 loci were mapped to 26 LGs, among which seven HLGs were detected. All of the Loke LGs were connected with three or four other LGs. The seven HLGs were HLG1 (Loke 9, 10, 11 and 12); HLG2 (Loke 16, 17, 18 and 19); HLG3 (Loke 21, 24, 25, Loke 26); HLG4 (Loke 1, 2, 3 and 4); HLG5 (Loke 13, 14 and 15); HLG6 (Loke 20, 22 and 23); and HLG7 (Loke 5, 6, 7 and 8) (Figure 1).
Homologous linkage groups detected between the parental maps
After comparing products from the same SSR primers, we also detected locus groups homologous between the two parental maps. Akimidori LGs 1, 3, 2 and 4 were homologous to Loke HLG1; Akimidori LGs 5, 6, 7 and 8 to HLG2; Akimidori LGs 9, 10, 11, 20 and 21 to HLG3; Akimidori LGs 13, 14 and 22 to HLG4; Akimidori LGs 12, 16, 17 and 23 to HLG5; Akimidori LG 19 to HLG6; and Akimidori LGs 15 and 18 to HLG7. Akimidori LG 24 did not belong to any HLG; the three loci mapped on this LG (og937, og237 and og386-1) were mapped only on the Akimidori parental map (Figure 1). Akimidori 18 was not in HLG 4 or 7, because marker og429-1 was in Loke 2 and marker og911 was in Loke 7.
In our mapping results, 28 multi-copy markers were found and mapped either in the same LG or in different HLGs. There were 10 multi-copy markers on the Akimidori map: og82-1 and og82-2 (Akimidori [A]12); og210-1 and og210-2 (A21); og258-1 and og258-2 (A5); og291-1 and og291-2 (A5); og314-1 and og314-2 (A4); og365-2 and og365-3 (A6); og385-1 and og385-3 (A19); og434-1 and og434-2 (A10); og678-1 and og678-3 (A3); og784-1 and og784-2 (A9). There were eight pairs on the Loke map: og21-2 and og21-3 (Loke [L]23); og156-1 and og156-3 (L13); og159-2 and og159-3 (L16); og239-2 and og239-3 (L14); og385-1 and og385-2 (L20); og626-1 and og626-2 (L1); og835-1 and og835-2 (L13); oga3-1 and oga3-2 (L21). Eight markers were mapped on different HLGs: og202, og429, og607, og669, og670, og695, og717 and og715. For example, og607-1 in Akimidori LG 7 was in HLG2, but og607-2 in Akimidori LG 10 belonged to HLG3. Marker og202-1 in Akimidori LG 2 was in HLG1, but markers og202-2 in Akimidori LG 5 belonged to HLG 2. Marker og715-1 in Loke LG 15 belonged to HLG5 and marker og715-2 in Loke LG 18 belonged to HLG2.
Consensus linkage maps of two parental lines using TetraploidMap
The Akimidori and Loke parental consensus linkage maps consisted of seven linkage groups (LGs) that spanned a total of 461 and 549 cM, respectively, with an average of 65.9 (Akimidori) and 78.4 (Loke) cM per LG, ranging from 51 cM in LG Akimidori-H6 and Loke-H7 to 115 cM in LG Loke-H4 (Figure 2 and Table 3). The Akimidori consensus map has 223 loci including 21 double-simplex and 20 duplex markers, it had an average of 31.9 markers per LG ranged from 13 (H6) to 48 (H2). The density of the Akimidori parental map ranged from 1.5 to 3.9 cM between flanking markers, with an average of 2.1 cM. The Loke consensus map has 290 loci including 23 double-simplex and 14 duplex markers, it had an average of 41.4 markers per LG ranged from 33 (H3) to 49 (H4). The density of the Loke parental map ranged from 1.1 to 2.4 cM between flanking markers, with an average of 1.9 cM.
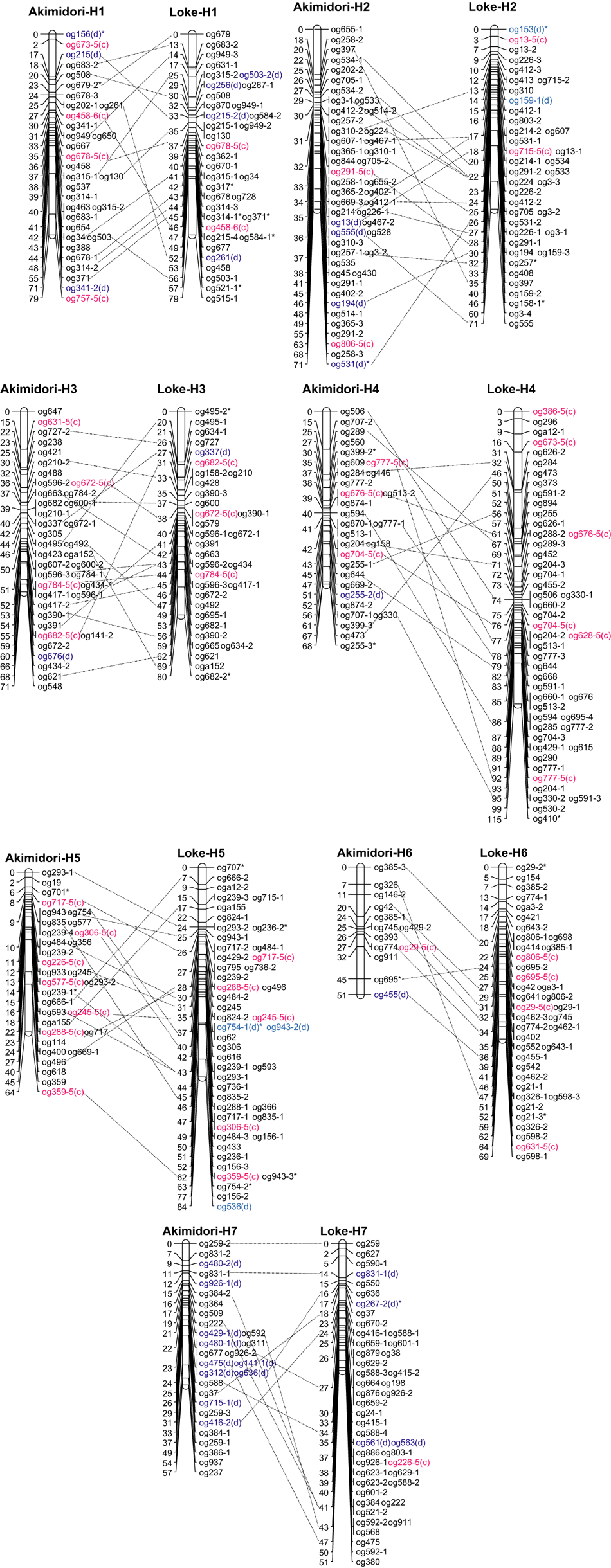
Relationships between two consensus parental maps of the tetraploid Dactylis glomerata. Asterisked markers are distortion-segregated markers, and markers with c or d in parenthesis are double-simplex (co-present in two parents and segregated as 3:1) and duplex (segregated as 5:1) markers.
| Homologous linkage group | Map length (cM) | No. loci | Map density (cM) | Inter-locus gap distance (cM) | No. double-simplex markers | No. duplex markers |
|---|---|---|---|---|---|---|
| Akimidori-H1 | 79 | 32 | 2.5 | 2.5 | 4 | 3 |
| Akimidori-H2 | 71 | 48 | 1.5 | 1.5 | 2 | 4 |
| Akimidori-H3 | 71 | 39 | 1.8 | 1.9 | 4 | 1 |
| Akimidori-H4 | 68 | 30 | 2.3 | 2.3 | 3 | 1 |
| Akimidori-H5 | 64 | 32 | 2.0 | 2.1 | 7 | 0 |
| Akimidori-H6 | 51 | 13 | 3.9 | 4.3 | 1 | 1 |
| Akimidori-H7 | 57 | 29 | 2.0 | 2.0 | 0 | 10 |
| Total | 461 | 223 | – | – | 21 | 20 |
| Mean | 65.9 | 31.9 | 2.1 | 2.1 | 3.0 | 2.9 |
| Loke-H1 | 79 | 36 | 2.2 | 2.3 | 2 | 4 |
| Loke-H2 | 71 | 39 | 1.8 | 1.9 | 2 | 2 |
| Loke-H3 | 80 | 33 | 2.4 | 2.5 | 3 | 1 |
| Loke-H4 | 115 | 49 | 2.3 | 2.4 | 6 | 0 |
| Loke-H5 | 84 | 48 | 1.8 | 1.8 | 5 | 3 |
| Loke-H6 | 69 | 39 | 1.8 | 1.8 | 4 | 0 |
| Loke-H7 | 51 | 46 | 1.1 | 1.1 | 1 | 4 |
| Total | 549 | 290 | – | – | 23 | 14 |
| Mean | 78.4 | 41.4 | 1.9 | 1.9 | 3.3 | 2.0 |
Two consensus parental linkage maps were aligned using double-simplex and the markers having different alleles of the same primer (Figure 2). A total of 99 markers (11–18 markers per LG) were used in the alignment including 11 double-simplex markers. As shown in the figure, the marker orders in two parental consensus maps were different, for reasons that still need to be identified.
Comparison between the linkage map construction and homologous linkage groups detected using Joinmap and TetraploidMap software
Two methods were used to construct the parental linkage map and detect homologous linkage groups: (i) construction of the linkage map with Joinmap and detection of homologous linkage groups based on the markers having different alleles of the same primer; and (ii) construction of the linkage map with TetraploidMap and detection of homologous linkage groups using double-simplex and the markers having different alleles of the same primer. The results from the two methods are generally the same except for some minor difference (Table 4 and Figure S1). For example, in Loke linkage groups, L21 was aligned into homologous group (HLG) 4 when using Joinmap but into HLG6 when using TetraploidMap, in Akimidori linkage groups, A22 was aligned into HLG 4 when using Joinmap but into HLG5 when using TetraploidMap.
| Homologous linkage group | TetraploidMap | Joinmap | ||
|---|---|---|---|---|
| No. LGs | LGs | No. LGs | LGs | |
| Akimidori | ||||
| H1 | 4 | A1–A4 | 4 | A1–A4 |
| H2 | 4 | A5–A8 | 4 | A5–A8 |
| H3 | 4 | A9, A10 + A11, A20, A21 | 5 | A9–A11, A20, A21 |
| H4 | 2 | A13, A14 | 3 | A13, A14, A22 |
| H5 | 4 | A16, A17, A23, A22 | 4 | A12, A16, A17, A23 |
| H6 | 2 | A19, A18 | 1 | A19 |
| H7 | 4 | A15, A24, A25 (new), A26 (new) | 2 | A18, A15 |
| Unknown | – | A12 | A24 | |
| Loke | ||||
| H1 | 4 | L9–L12 | 4 | L9–L12 |
| H2 | 4 | L16–L19 | 4 | L16–L19 |
| H3 | 4 | L24–L26, L28 (new) | 4 | L21, L24–L26 |
| H4 | 4 | L1–L4 | 4 | L1–L4 |
| H5 | 4 | L13–L15 | 3 | L13–L15 |
| H6 | 4 | L20, L21, L22, L23 | 3 | L20, L22, L23 |
| H7 | 4 | L5–L8 | 4 | L5–L8 |
Relationship between the diploid and tetraploid orchardgrass linkage map
From the diploid map of orchardgrass constructed by Xie et al. (2011), we were able to screen their 150 SSR primer pairs for polymorphism between our two tetraploid parents. Although 14 SSR markers in each of Xie’s diploid parental map were common to our tetraploid map, about half of them were mapped on different LG. This may be attributed to the following three factors. First, our relatively small mapping population may have caused some position errors when mapping SSR markers. Second, more complex chromosomal behavior during the meiosis in a tetraploid than in a diploid may have caused differences between the tetraploid and diploid maps. Third, we used a LI-COR sequencer to obtain the genotypes, whereas Xie et al. used PAGE and silver staining, which show more complex bands than the LI-COR sequencer. The map lengths of diploid orchardgrass were 866.7 and 772.0 cM in male and female parental maps, respectively, while our tetraploid orchardgrass maps were 562.0 cM (461.0 cM when using TetraploidMap) and 745.0 cM (549.0 cM when using Tetraploidmap) in the female parent (Akimidori) and male parent (Loke), respectively; shorter than the diploid orchardgrass linkage map. The limited mapping population size used in our study may be the reason, because some crossover could not be detected when using a smaller mapping population.
Comparative analysis with other graminaceous species
In all of the 969 Dactylis SSRs primer pairs (Hirata et al. 2010) that were used to survey polymorphism between our two parents, 63 of the sequences showed significant homology with sequences from other graminaceous crop plant species (rice, wheat, barley, oats). 314 SSR markers showing polymorphism were used in the map construction, 16 of the above sequences showing significant homology with sequences from other plant species, but only six showed homology with rice while the others were homologous with oats, wheat, barley and other species. Overall there were no clear relationships between orchardgrass chromosomes and other species (data not shown). Recently, Bushman et al. (2011) reported 1162 SSR markers from D. glomerata expressed sequence tag (EST) libraries and they found 870 SSR sequences that showed significant homology with rice chromosomes, of which 695 SSR primers can work. Mapping of these SSR markers will allow us to indicate the relationships between D. glomerata and rice in more detail.
Discussion
Marker segregation and linkage map
Almost all linkage maps of the polyploid species have been constructed using the single-dose restriction fragment (SDRF) markers after Wu et al. (1992) first proposed the concept of SDRF for constructing molecular linkage maps in autoploid species. Examples include sugarcane (Al-Janabi et al. 1993), alfalfa (Julier et al. 2003) and potato (Meyer et al. 1998). Here, we detected a total of 269 and 326 SDRF SSR markers in the two parents, only 17 and 29 of which, respectively, showed segregation distortion. The detected markers were used successfully in map construction.
The chromosome number of orchardgrass is 2n = 4x = 28 and we detected almost all LG, 24 LGs in Akimidori and 26 LGs in Loke. The inability to detect all LGs may have been due to the limited numbers of markers used (168 in Akimidori and 227 in Loke). The difference between the total lengths of the two parental maps (576 cM vs. 745 cM) was also affected by the limited numbers of mapped markers in Akimidori. These results were similar to the RAPD marker-based linkage map construction for sugarcane, which is considered to be auto-octaploid (2n = 8x = 64) (Al-Janabi et al. 1993), 176 (84.6%), where 208 single-dose markers were mapped and 41 tentative LGs were identified. Our results mapped much lower ratios of single-dose markers (69.7% in Akimidori and in 73.7% Loke) probably because we used LOD = 8.0, whereas a value of LOD = 6.0 was used for sugarcane.
Markers derived from the same SSR locus or RFLP probe can identify co-segregation groups corresponding to homologous chromosomes, and the number of homology groups is associated with the basic number of chromosomes in the genus (Grivet and Arruda 2002). We detected seven and five HLGs in Loke and Akimidori, respectively, and from the results of our mapping of products amplified by the same primer pairs, we could detect another two HLGs in Akimidori. The results were also confirmed by mapping of double-simplex and duplex markers using TetraploidMap.
Some of the SSR primer pairs showed multi-copy amplification; this has also been found in alfalfa (Julier et al. 2003) and cotton (Rong et al. 2004). The multi-copy amplification status may be caused by the duplication of genes in different chromosomes, but it will need to be confirmed in other mapping populations.
Our population size is smaller compared with other studies, although Wu et al. (1992) indicated that a population size of 75 is required to identify SDRFs with a 98% level of confidence for the four ploidy levels (2n = 4X, 6X, 8X and 10X), in our study, we used SDRF markers to construct the frame map firstly, and then added other two kinds of markers, but larger number of individuals in a mapping population allows us more precise results in linkage analysis. In fact, when compared to the diploid orchardgrass linkage map, our linkage map was somewhat different in the total map lengths and some marker positions.
Genomic constitution of orchardgrass
The taxonomic interpretation within the genus Dactylis varies with most researchers considering Dactylis glomerata to be a single high variable species in the early stages process of speciation, although some regional taxomists interpret their regional forms as different species (Stebbins and Zohary 1959; Schönfelder and Ludwig 1996). It is clear though that Dactylis originated as a diploid, which today has a very limited and discontinuous distribution. At least 17 diploids are described either as species, subspecies or regional populations (Stewart and Ellison 2011). Hexaploids are rare with only one regional population known in Tunisia and Egypt. Tetraploids have developed quite frequently from diploids, sometimes from a single diploid population, but more generally from crosses between two regional diploid populations. Tetraploids based on single diploid largely have a distribution concurrent with the diploid progenitor while those inter-populational tetraploids have had an evolutionary advantage and are distributed very widely and represent the forms used in agriculture (Stebbins and Zohary 1959; Lumaret 1988; Stewart and Ellison 2011).
Despite the differing origins of various tetraploids they are all generally considered to be cytologically autopolyploid (Jones 1962; Hu and Timothy 1971; Borrill 1978), and this opinion is supported by the results of genetic studies of various morphological and physiological features and isozymes (see the review by Lumaret 1988).
Our mapping results reconfirm this auto-tetraploid status of orchardgrass; as the segregation types of simplex (segregated as 1:1), double-simplex (segregated as 3:1), duplex (segregated as 5:1), duplex × simplex (segregated as 11:1) and double-duplex (segregated as 35:1) were detected in the mapping population. The proportion of multiple-dose markers vs. single-dose markers can be used to test the chromosome assortment type, as in sugarcane (Al-Janabi et al. 1993), where according to their report, in the case of type I markers, an auto-tetraploid species should have 1/5 non-simplex markers and an allo-tetraploid species 1/4 non-simplex markers. We found that the non-simplex type I markers in the two parents were all present at rates of <1/5, but because the proportions were all significantly different from both 1/5 and 1/4, the reason needs to be studied.
In conclusion, we have developed the first SSR marker-based genetic linkage map of tetraploid orchardgrass (D. glomerata), and confirmed the auto-ploidy nature of tetraploid orchardgrass in molecular marker level. The SSR linkage map will be useful for quantitative trait locus analysis, marker-assisted selection and breeding for important traits in orchardgrass in the future.
Acknowledgments
This study was funded by a grant for Scientific and Technical Supporting Programs from the Ministry of Science and Technology of China (No. 2008BADB3B03). We thank Dr. Alan V. Stewart (PGG Wrightson Seeds, Lincoln, New Zealand) and three other reviewers for their valuable comments on this manuscript. We also would like to thank Xiaodong Dai and Ying Huo for their technical assistances.




