Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction
ABSTRACT
Introduction. Significant scientific advances during the past 3 decades have deepened our understanding of the physiology and pathophysiology of penile erection. A critical evaluation of the current state of knowledge is essential to provide perspective for future research and development of new therapies.
Aim. To develop an evidence-based, state-of-the-art consensus report on the anatomy, physiology, and pathophysiology of erectile dysfunction (ED).
Methods. Consensus process over a period of 16 months, representing the opinions of 12 experts from seven countries.
Main Outcome Measure. Expert opinion was based on the grading of scientific and evidence-based medical literature, internal committee discussion, public presentation, and debate.
Results. ED occurs from multifaceted, complex mechanisms that can involve disruptions in neural, vascular, and hormonal signaling. Research on central neural regulation of penile erection is progressing rapidly with the identification of key neurotransmitters and the association of neural structures with both spinal and supraspinal pathways that regulate sexual function. In parallel to advances in cardiovascular physiology, the most extensive efforts in the physiology of penile erection have focused on elucidating mechanisms that regulate the functions of the endothelium and vascular smooth muscle of the corpus cavernosum. Major health concerns such as atherosclerosis, hyperlipidemia, hypertension, diabetes, and metabolic syndrome (MetS) have become well integrated into the investigation of ED.
Conclusions. Despite the efficacy of current therapies, they remain insufficient to address growing patient populations, such as those with diabetes and MetS. In addition, increasing awareness of the adverse side effects of commonly prescribed medications on sexual function provides a rationale for developing new treatment strategies that minimize the likelihood of causing sexual dysfunction. Many basic questions with regard to erectile function remain unanswered and further laboratory and clinical studies are necessary. Gratzke C, Angulo J, Chitaley K, Dai Y-T, Kim NN, Paick J-S, Simonsen U, Ückert S, Wespes E, Andersson KE, Lue TF, and Stief CG. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 2010;7:445–475.
Brain, Autonomic Nervous System, and Neurotransmission
Penile erection is initiated after central processing and integration of tactile, visual, olfactory, and imaginative stimuli. Signals to the peripheral tissues involved are generated, and the final response is mediated by coordinated spinal activity in the autonomic pathways to the penis and in the somatic pathways to the perineal striated muscles. The central regulation of penile erection involves many transmitters and transmitter systems, the details of which are still incompletely known. Some of the anatomical areas of the brain that relate to sexual function have been defined, including the medial amygdala, medial preoptic area (MPOA), paraventricular nucleus, the periaqueductal gray, and ventral tegmentum [1–3]. In rats, electrical stimulation of the MPOA [4], the paraventricular nucleus [5], or the hippocampal formation [6] can elicit an erectile response.
Spinally, there seems to be a network consisting of primary afferents from the genitals, spinal interneurons, and sympathetic, parasympathetic, and somatic nuclei. This network appears capable of integrating information from the periphery and eliciting reflexive erections, and also to be the recipient of supraspinal information [7]. The degree of preservation of sensory function in the T11-L2 dermatomes could be used to determine the potential for psychogenic erectile responses in men with spinal cord injury [8].
Peripherally, the balance between factors that control the degree of contraction of the smooth muscle of the corpora cavernosa determines the functional state of the penis. Many details of neurotransmission, impulse propagation, and intracellular transduction of signals in penile smooth muscles remain to be elucidated. However, the information on central control mechanisms involved in erection is rapidly expanding, and new details are continuously added [1,9–16].
Central Neuromediation
The central mechanisms controlling erection include supraspinal as well as spinal pathways. The current knowledge about these mechanisms is largely based on experimental data from animals (mainly, rats).
Dopamine
Central dopaminergic neurons project to the MPOA and the paraventricular nucleus [17]. Furthermore, dopaminergic neurons have been identified that travel from the caudal hypothalamus to innervate the autonomic and somatic nuclei in the lumbosacral spinal cord [18,19]. Thus, dopamine can be expected to participate in the regulation of both the autonomic and somatic components of the penile reflexes.
Both the two major families of dopamine receptors, D1-like (D1, D5) and D2-like (D2, D3, D4) receptors [20], have been associated with central erectile functions; however, the D2-like receptor subtype seems to have the predominating effect. The nonselective dopamine receptor agonist, apomorphine, when administered systemically to male rats, was found to induce penile erection [21], simultaneously producing yawning and seminal emission. Similarly, low-dose systemic administration of other dopamine agonists initiates erection [1]. The effects of these agonists can be attenuated by centrally but not peripherally acting dopamine receptor antagonists.
Oxytocin
Oxytocinergic spinal projections from the supraoptic and paraventricular nuclei of the hypothalamus are likely to influence the sacral autonomic outflow more than the somatic outflow [22,23]. The finding that immunoreactive oxytocin-containing spinal neurons associate with sacral preganglionic neurons supports the idea that oxytocin has an important role in the autonomic spinal circuitry that mediates penile erection [24,25]. Oxytocin is a potent inducer of penile erection when injected into the lateral cerebral ventricle, the paraventricular nucleus, or the hippocampus of laboratory animals; intrathecal oxytocin can also initiate an erection. These erections can be blocked by the administration of oxytocin antagonists given intracerebroventricularly (i.c.v.) or intrathecally, or by electrolytic lesion of the paraventricular nucleus. Additionally, noncontact erections can be reduced by a selective oxytocin receptor antagonist administered into the lateral ventricles, which supports the view that oxytocin mediates this response [26].
Adrenocorticotropic Hormones (ACTH) and Related Peptides
Administered i.c.v., the ACTH and α-melanocyte-stimulating hormones (α-MSH) are able to induce penile erection, along with grooming, stretching, and yawning [1,2,27]. These effects are most probably mediated via stimulation of melanocortin (MC) receptors, of which five different subtypes have been cloned and characterized [28,29]. Alpha-MSH/ACTH seem to act in the hypothalamic periventricular region, and grooming, stretching, and yawning, but not penile erection, was reported to be mediated by MC4 receptors [27,30]. It is unclear, however, what MC receptor subtype(s) can be linked to the erectile responses. For example, the MC3 receptor is found in high density in the hypothalamus and limbic systems [31], regions known to be important for erectile functions. The site and mechanism of action responsible of α-MSH/ACTH seem to be different from those involving dopamine or oxytocin [15].
Martin et al. [32] concluded that current evidence indicates that the MC4 receptor subtype contributes to the pro-erectile effects observed with MC pan-receptor agonists. However, the putative receptor subtypes, pathways, and mechanisms implicated in mediating the pro-erectile effects of MCs remain to be fully elucidated. Melanotan II, a synthetic analogue of α-MSH, when given subcutaneously, was shown to have pro-erectile effects in men with psychogenic impotence [33]. Still, the therapeutic potential of α-MSH analogues remains to be established [34–36].
Nitric Oxide (NO)
Several investigators have shown that within the central nervous system, NO can modulate sexual behavior and penile erection [37–41]. NO may act in several discrete brain regions, e.g., in the MPOA [40,41] and the paraventricular nucleus [5,30]. NO production increases in the paraventricular nucleus of male rats during noncontact penile erections and copulation, confirming that NO is a physiological mediator of penile erection at the level of the paraventricular nucleus [39].
As mentioned previously, injection of NO synthase (NOS) inhibitors i.c.v. or in the paraventricular nucleus prevents penile erectile responses induced by dopamine agonists, oxytocin, and N-methyl-D-aspartate (NMDA) in rats. NO may also mediate the actions of ACTH/α-MSH and 5-HT2C agonists, which elicit erections when injected into the intracerebroventricular system, according to mechanisms unrelated to oxytocinergic neurotransmission [38]. The inhibitory effect of NOS inhibitors was not observed when these compounds were injected concomitantly with L-arginine, the substrate for NO [38].
Excitatory Amino Acids
Microinjections of L-glutamate into the MPOA elicits an increase in intracavernous pressure [4], and behavioral studies have shown that NMDA increases the number of penile erections when injected into the paraventricular nucleus [42–44]. Furthermore, NMDA, amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid, or trans-1-amino-1,3-cyclo-pentadicarboxylic acid increases intracavernosal pressures (ICPs) when injected into the paraventricular nucleus [45]. The effect of NMDA was prevented by intracerebroventricular administration of an oxytocin antagonist [42]. The NOS signal transduction pathway is considered to mediate the effect of NMDA. Injection of the amino acid leads to an increased concentration of NO metabolites in the paraventricular nucleus [46], and the administration of NOS inhibitors into the paraventricular nucleus and i.c.v. blocked the NMDA effect [42,47].
Serotonin
Neurons containing serotonin (5-hydroxytryptamine, 5-HT) can be found in the medullary raphe nuclei and ventral medulla reticular formation, including the rostral nucleus paragigantocellularis, and bulbospinal neurons containing 5-HT project to the lumbar spinal cord in the rat and cat [1]. Some serotonergic fibers occur in close apposition with sacral preganglionic neurons and motoneurons, and synapses were demonstrated at the ultrastructural level [25]. These morphological findings support the involvement of 5-HT in both the supraspinal and spinal pharmacology of erection, with participation in both the sympathetic and parasympathetic outflow mechanisms.
In animals, 5-HT seems to exert a general inhibitory effect on male sexual behavior [48], although the amine may be inhibitory or facilitatory depending upon its action at different sites and at different 5-HT receptors within the central nervous system [49,50]. This may explain conflicting reports of 5-HT agonists either enhancing or depressing sexual function. Yonezawa et al. [51] found that p-chloroamphetamine, an indirect serotonin (5-HT) agonist, elicited both penile erection and ejaculation simultaneously in anesthetized rats. It was suggested that these effects were mainly produced by the release of 5-HT as limited to the lower spinal cord and/or peripheral sites. The use of selective 5-HT receptor agonists and antagonists can reveal different components of male copulatory behavior [9].
Gamma-Aminobutyric Acid (GABA)
Cumulative data resulting from investigations on the role of GABA in penile erection indicate that this neurotransmitter may function as an inhibitory modulator in the autonomic and somatic reflex pathways involved in penile erection [52]. Activation of GABAA receptors in the paraventricular nucleus of male rats reduced penile erection and yawning in response to apomorphine, N-methyl-D-aspartate (NMDA), and oxytocin [52]. Dorfman et al. examined age-related changes of the GABAB receptor in the lumbar spinal cord of Sprague Dawley rats of different ages using quantitative autoradiography [53]. GABAB receptor affinity showed significant age-dependent and regional increases. The GABAB receptor decrease in aged rats did not seem, however, to be related to the inhibitory function in penile erection.
Cannabinoids
Administration of endogenous and exogenous cannabinoids was shown to be associated with changes in penile erection and modulation of male sexual behavior [54,55]. The cannabinoid CB1 receptor antagonist SR 141716A was shown to potentiate the penile erection responses to apomorphine in rats [56]. Also, it was shown that cannabinoid CB1 receptors present in the paraventricular nucleus may influence erectile function and sexual activity possibly by modulating paraventricular oxytocinergic neurons mediating erectile function [57]. It was also demonstrated that SR 141716 induced penile erection by a mechanism involving excitatory amino acid neurotransmission causing activation of neuronal NOS (nNOS) in paraventricular oxytocinergic neurons [58].
Opioid Peptides
Available information supports the hypothesis that opioid µ-receptor stimulation centrally prevents penile erection by inhibiting mechanisms that converge upon central oxytocinergic neurotransmission [1]. In rats, morphine injected into the paraventricular nucleus prevents noncontact penile erections (i.e., when penile erection is induced in the male by the presence of an inaccessible receptive female) and impaired copulation. These morphine effects are apparently mediated by prevention of the increased NO production that occurs in the paraventricular nucleus during sexual activity [59]. Morphine also prevents apomorphine-, oxytocin-, NMDA- and noncontact-induced penile erection and yawning by inhibiting NOS activity in the paraventricular nucleus [60–62].
Prolactin
Long-term hyperprolactinemia can depress sexual behavior, reduce sexual potency in men, and depress genital reflexes in rats [50,63]. Acute and chronic central prolactin treatment in rats, however, may have stimulatory and inhibitory effects on male sexual behavior, respectively [64]. Correspondingly, striatal dopaminergic activity was shown to be increased and decreased by acute and 5-day central prolactin treatment [64], supporting the view that the effects of prolactin are associated with changes in striatal dopaminergic activity. Prolactin has been shown to inhibit the dopaminergic incerto-hypothalamic pathway to the MPOA [65]. In humans, it is still unclear whether the negative effects of hyperprolactinemia on erectile function are mediated centrally by way of reduction in sexual interest and sex drive [66], or through a direct effect of prolactin on corpus cavernosum smooth muscle contractility. In dogs, a direct effect on the corpus cavernosum was suggested [67]. In any case, the effect seems independent of circulating testosterone levels and gonadal axis function [68].
Sexual Hormones
Androgens, particularly testosterone, are necessary (although not sufficient) for sexual desire in men. They are essential in the maintenance of libido and have an important role in regulating erectile capacity [69–73]. In men with normal gonadal function, however, there is no correlation between circulating testosterone levels and measures of sexual interest, activity, or erectile function [74]. Following castration in the male (which may reduce plasma testosterone levels by 90% [75]), or other causes leading to a reduction in androgen levels, there is generally a decline in libido, and sometimes in erectile and ejaculatory functions. Testosterone administration restores sexual interest and associated sexual activity in hypogonadal or castrated adult men [76–78]. The testosterone dose–response relationships for sexual function and visuospatial cognition differ in older and young men, higher testosterone doses needed in the elderly for normal sexual functioning [72]. El-Sakka et al. [79] assessed the pattern of age-related testosterone depletion in patients with erectile dysfunction (ED). They found a significant decrease in testosterone level throughout the 4-year follow-up in patients with ED. Patients with decreasing testosterone were older than patients with a steady testosterone level. When castration has been performed in humans, the resultant sexual function may range from a complete loss of libido to continued normal sexual activity. Thus, the role of androgens in erectile function is complex, and androgen deprivation may not always cause erectile impotence, either in man [80], or in rats [81].
Perspectives and Conclusions
Ongoing and future studies assessing the efficacy and tolerability of centrally acting agents for male sexual dysfunction will reveal which targets are the most promising. Based on current literature, clinical trials have shown benefits for some drugs acting on mediator systems discussed above. However, today, none of the available agents can be regarded as a major player in the practical treatment of ED. In light of the relatively large fraction of the ED patients that does not respond to or does not tolerate phosphodiesterase type 5 (PDE5) inhibitors, additional centrally acting drugs modulating sexual responses may be a potential solution. It cannot be expected that these drugs will work in patients with severe end-organ damage; however, they may potentially add to existing therapy by modifying arousability and sexual desire. The central regulation of the erectile process is still only partly known. Central transmitter systems, which seem to be dependent on androgens as well as NO, may be the targets of future drugs aimed at the treatment of ED. Increased knowledge of the central (and peripheral) changes associated with ED may lead to an increased understanding of these pathogenetic mechanisms and therefore new treatments and possibly even prevention of the disorder.
Regulation of Smooth Muscle Function
The penile corpora cavernosa are highly specialized vascular structures that are morphologically adapted to their function of becoming engorged during sexual arousal. The trabecular smooth muscle constitutes approximately 40–50% of tissue cross-sectional area, as assessed by histomorphometric analysis [82,83]. Most of the remaining cavernosal tissue area is occupied by extracellular matrix, which provides a fibro-elastic framework and consists predominantly of collagen types I, III, and IV, and elastin [84–87]. Collagen types V and XI are also synthesized by the cavernosal smooth muscle at detectable levels. Although smaller in number, endothelial cells and neurons play critical roles in maintaining and regulating vascular smooth muscle cell (VSMC) tone.
This complex architecture is maintained by the active and dynamic expression of numerous growth factors. Well established as a regulator of limb morphogenesis, sonic hedgehog (Shh) has also been identified in the penis [88,89]. Studies indicate that inhibition of Shh in the adult leads to rapid atrophy and disorganization of the corpus cavernosum [88,89], suggesting that Shh is a critical protein in the development and maintenance of penile cavernosal tissue structure. In addition, Shh has been shown to stimulate the expression of vascular endothelial growth factor (VEGF) and NOS in the penis [89]. Numerous other growth factors are expressed in the penis and are also likely to play important roles in maintaining cavernosal tissue structure and function. However, most studies have examined the use of exogenously applied growth factors in therapeutic capacities, rather than investigating the roles of endogenously produced growth factors.
As a major constituent and primary effector of the vascular structures in the genitals, the VSMC is highly adaptable and multifunctional. The two primary functions of VSMCs are contraction and synthesis/maintenance of extracellular matrix. However, these two categories are considered to be extremes that are manifested under in vitro conditions and it is likely that a range of intermediary phenotypes exist in any given tissue in vivo [90,91].
Mechanism of Smooth Muscle Contraction
Changes in smooth muscle tone are crucial for regulating erectile function. Unlike striated muscle, the molecular contractile units of interdigitating actin (thin) and myosin (thick) filaments are not regularly aligned with one another and can be oriented in multiple directions [92–94]. Smooth muscle myosin is a large hexameric protein, consisting of two heavy chains and four light chains. The heavy chains are identical and have both globular and linear domains. The linear domains form coiled structures that result in the tail of the myosin molecule, while the globular domains possess actin-binding sites and ATPase catalytic activity. Actin filaments are composed of two long strands of globular actin that intertwine into a double helical arrangement.
The contractile response of the smooth muscle cell is tightly associated with the intracellular concentration of free Ca2+ and its regulatory action through calmodulin. Calmodulin-activated myosin stimulates phosphorylation events that initiate cross-bridge movement along the actin filament and generation of force. Myosin light chain phosphatase (MLCP) dephosphorylates myosin and inactivates cross-bridge movement. At any given level of intracellular Ca2+, the contractile apparatus may become further sensitized by the inhibition of MLCP, increasing the efficiency of myosin phosphorylation by myosin light chain kinase.
The activity of MLCP can be modulated by a variety of factors. A well-recognized mechanism involves the Rho/Rho kinase pathway. Rho proteins are small GTPases and can be activated by the binding of agonists to G-protein-coupled receptors [95]. Activated Rho can in turn activate Rho kinase, a serine/threonine kinase. Rho kinase can then phosphorylate multiple substrates including MLCP, the 17 kD protein kinase C-potentiated inhibitor protein, and myosin light chain (MLC20) [96,97]. In genital smooth muscle, the RhoA/ROK pathway and its effects on MLCP have been shown to play an important role in regulating smooth muscle contractility in both male and female genital smooth muscle, whereas the importance of MLC20 phosphorylation by Rho kinase remains unclear [98–101].
Pathways Regulating VSMC Tone
Pathways that regulate smooth muscle contractility ultimately influence intracellular Ca2+ levels and/or alter the calcium sensitivity of the contractile proteins (Figure 1). Vasoactive substances induce changes in smooth muscle tone by pharmacomechanical coupling and/or changes in cell membrane potential via electromechanical coupling.
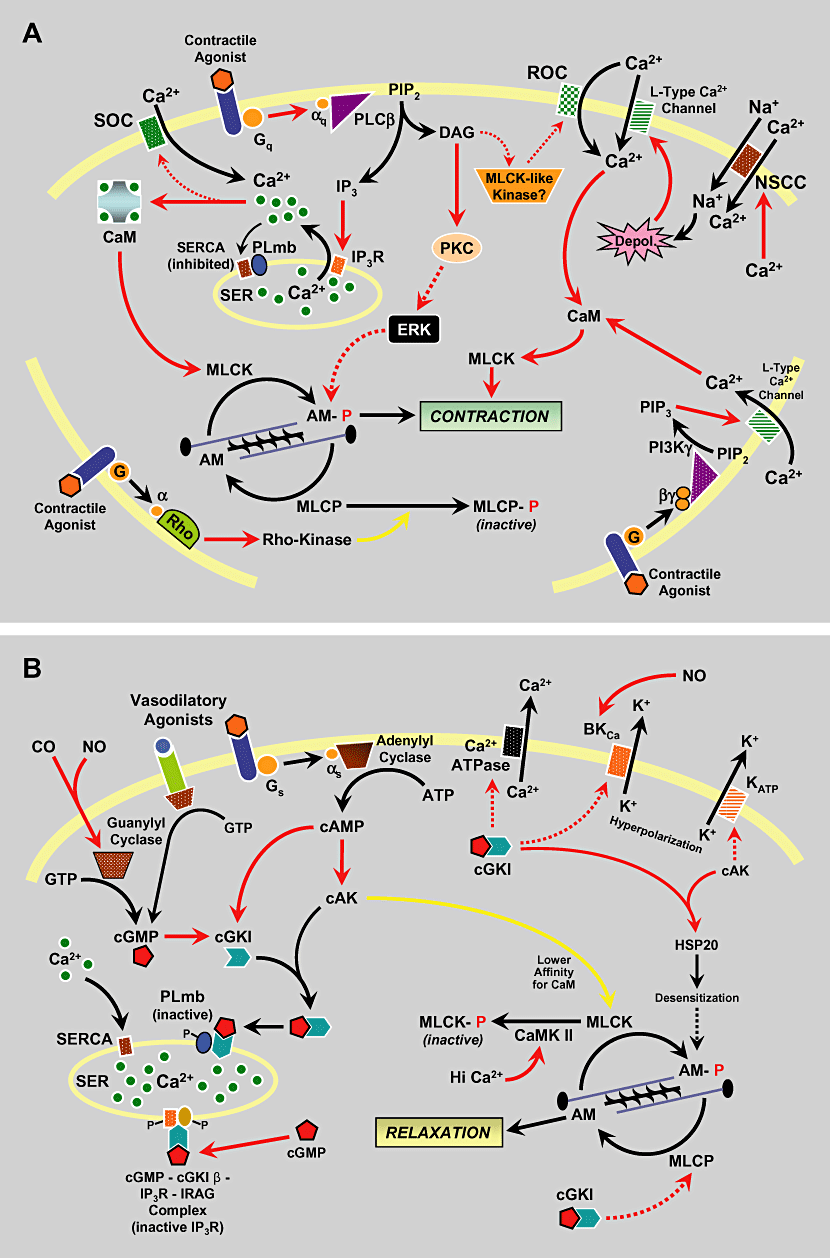
Signal transduction pathways regulating smooth muscle tone. Pathways mediating contraction are shown in panel A and pathways mediating relaxation are shown in panel B. Red arrows indicate association, binding, and/or activation, whereas yellow arrows indicate inhibitory regulation. Indirect or putative interactions are indicated by dashed arrows. AM = actin-myosin contractile apparatus; BKCa = calcium-activated maxi-K+ channel; CaM = calmodulin; CaMK = calmodulin-dependent kinase; cAK = cAMP-dependent protein kinase; cGK = cGMP-dependent protein kinase; CO = carbon monoxide; DAG = 1,2-diacylglycerol; IP3 = inositol 1,4,5-trisphosphate; IP3R = IP3 receptor; IRAG = IP3R-associated cGK substrate; KATP = ATP-dependent K+ channel; MLCP = myosin light chain phosphatase; MLCK = myosin light chain kinase; NO = nitric oxide; PI3K = phosphoinositide 3-kinase; PIP2 = phosphatidylinositol 4,5-bisphosphate; PIP3 = phosphatidylinositol 3,4,5-trisphosphate; PKC = protein kinase C; PLCβ = phospholipase C beta; PLmb = phospholamban; ROC = receptor-operated channel; SER = smooth endoplasmic reticulum; SERCA = SER calcium ATPase. Adapted from Kim NN. Vascular physiology of erectile function. In: Carson CC, Kirby RS, Goldstein I, Wyllie MG, eds. Textbook of erectile dysfunction, 2nd edition. New York: Informa Healthcare; 2009.
Pharmacomechanical Coupling
Inositol 1,4,5-trisphosphate (IP3), 1,2-diacylglycerol (DAG), and protein kinase C (PKC)
The binding of vasoconstrictor agonists, such as norepinephrine (NE), endothelin-1 (ET-1), angiotensin II (AT-II), prostaglandin (PG) F2α and thromboxane (Tx) A2, to their respective receptors stimulates phospholipase C beta (PLC-β). This membrane-bound enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to liberate IP3 and DAG. IP3 binds to specific receptors (IP3R) on the smooth endoplasmic reticulum (SER) to stimulate the release of Ca2+ from intracellular stores. IP3Rs act as Ca2+-activated Ca2+ channels. Binding of IP3 to these receptors not only activates the channel, but also increases the sensitivity of the IP3R to Ca2+ and facilitates Ca2+-induced release of Ca2+[102]. Upon dissociation of agonists from their receptors, free Ca2+ is recycled back into the SER by the SER Ca2+-ATPase pump. It restores intracellular Ca2+ levels to the basal state and thereby is an important mechanism of signal termination.
DAG is also an important intracellular second messenger generated by PLC. DAG directly activates PKC. With regard to smooth muscle tone, PKC can regulate ion channels or phosphorylate multiple substrates to facilitate contraction [93,103]. PKC may also mediate Ca2+-independent contraction, since several of the PKC isoforms are insensitive to Ca2+ while still being activated by DAG. In VSMCs, termination of DAG/PKC signaling is accomplished predominantly by hydrolysis of DAG by lipases to yield free fatty acids and glycerol [103].
Cyclic Nucleotides
Generation of cyclic nucleotides (cyclic guanosine monophosphate [cGMP] and cyclic adenosine monophosphate [cAMP]) by guanylyl and adenylyl cyclases is a primary mode of mediating penile vascular and nonvascular smooth muscle relaxation. Vasodilators such as vasoactive intestinal polypeptide (VIP) and PGs E and D activate G-protein (Gs)-coupled receptors that can stimulate plasma membrane-associated adenylyl cyclase, whereas soluble guanylyl cyclase (sGC) can be directly activated by NO or carbon monoxide (CO) by binding to the heme moiety of the enzyme. Increased levels of intracellular cAMP and cGMP cause the activation of cAMP-dependent and cGMP-dependent protein kinases (cAK and cGK) [104–106]. Thus, in addition to specific activation, there is potential cross-activation of cAK (also called protein kinase A) and cGK (also called protein kinase G), which may be a mechanistic basis for signal cross talk. However, it has been postulated that activation of cGK by cGMP is the main mechanism to mediate relaxation of penile erectile smooth muscle. cGK are derived from two different genes that encode type I (cGKI) and type II (cGKII). In smooth muscle, only cGKI is expressed and exists as two splice variants (cGKI alpha and cGKI beta). While specific roles of the two different cGKI isoforms are an area of continuing investigation, there is evidence that both isoforms differ considerably in their functional properties [107–109]. Immunoprecipitation studies indicate that in smooth muscle cells, cGKI is associated with IP3R and a protein known as IP3R-associated cGK substrate (IRAG), both of which act as substrates for the kinase [110]. Phosphorylation of IP3R and IRAG decreases agonist-induced Ca2+ release from the SER [111]. In addition, cGKI is known to phosphorylate phospholamban, a small membrane-associated protein that constitutively inhibits the SER Ca2+-ATPase. Phosphorylation of phospholamban inactivates its inhibitory control of ATPase activity and increases Ca2+ reuptake into the SER, where Ca2+ is bound to proteins such as calsequestrin. Protein kinase A can also phosphorylate phospholamban to increase Ca2+ reuptake [112]. Thus, the combined actions of cGKI and cAK can inhibit Ca2+ release from intracellular stores or stimulate Ca2+ re-uptake.
Additional and perhaps equally important pharmacomechanical mechanisms by which cGMP and/or cGKI may cause relaxation also involve inhibition of Rho kinase and stimulation of MLCP [113]. Some studies suggest that Rho kinase and MLCP can both be phosphorylated by cGKI to antagonize Rho kinase activity and stimulate MLCP. In the penis, immunostaining for cGKI alpha and cGKI beta has been observed within the smooth musculature and the endothelium of cavernous arteries and sinusoids. Double-staining protocols revealed the colocalization of alpha-actin, cGMP, endothelial NOS (eNOS), and cGKI isoforms [114]. Findings from in vitro functional studies are also in support of a significance of the cGKI in the control of human penile erectile tissue [115,116].
PDEs
One of the main mechanisms by which cyclic nucleotide signaling is terminated is by the action of PDEs, a heterogenous group of hydrolytic enzymes. PDEs are classified according to their preference or affinity for cAMP and/or cGMP, kinetic parameters of cyclic nucleotide hydrolysis, relative sensitivity to inhibition by various compounds, allosteric regulation by other molecules, and chromatographic behavior on anion exchange columns. Out of 11 families of PDEs consisting of more than 50 isoenzymes identified to date [117–119], six (PDE 1, 2, 3, 4, 5, and 11) have been proven to be of pharmacological importance. Since the distribution and functional significance of PDE isoenzymes varies in different tissues, isoenzyme-selective inhibitors have the potential to exert specific effects on the target tissue. Preferential expression of PDE5 in the corpus cavernosum and the cGMP-mediated relaxation of the cavernous smooth muscle during sexual stimulation have made inhibition of this enzyme by sildenafil, vardenafil, or tadalafil a clinical benefit in the management of ED. The purified protein is a homodimer of 99.6 kDa subunits and binds two zinc atoms per monomer which are necessary for catalysis.
Since PDEs form a biochemically and structurally diverse family of proteins, there might be more than one PDE isoenzyme or isogene serving as potential drug target in the treatment of ED. In the 1990s, the presence of PDE isoenzymes 2, 3, 4, and 5 was reported in cytosolic supernatants of human erectile tissue [120]. In addition, the expression of mRNA transcripts specifically encoding for 14 different human PDE isoenzymes and isoforms in human cavernous tissue was shown by means of real-time polymerase chain reaction (RT-PCR) and Northern blot analysis: PDE1A, PDE1B, PDE1C, PDE2A, and PDE10A, which hydrolyze both cAMP and cGMP; the cAMP-specific PDE isoenzymes PDE3A, PDE4 (A-D), PDE7A, and PDE8A, and the cGMP-specific PDEs PDE5A and PDE9A [121]. The intracellular level of cAMP in human erectile tissue is mainly regulated by the cAMP-degrading PDE isoenzymes 3 and 4. Results obtained in vitro suggest that PDE3 and PDE4 might be the predominant isoenzymes in the human corpus cavernosum [122]. Interestingly, it has also been shown that the reversion of tension mediated by an alpha1-adrenoceptor of isolated human corpus cavernosum induced by sildenafil and tadalafil was reversed by the cAK inhibitor Rp-8-CPT-cAMPS, suggesting an involvement of cAMP-mediated mechanisms in the action of PDE5 inhibitors [115]. On the basis of these observations, an important complementary role might be considered for the adenylyl cyclase/cAMP/cAK pathway in the control of cavernous smooth muscle tone.
Electromechanical Coupling
Pathways that regulate VSMC tone and that are associated with changes in membrane potential are defined as electromechanical coupling mechanisms. The primary electromechanical mechanism of contraction in VSMCs involves depolarization and the opening of voltage-gated L-type Ca2+ channels to allow influx of extracellular Ca2+. Contractile responses caused by NE, ET-1, and AT-II are partly mediated by L-type Ca2+ channels [123–125]. Recent studies indicate that L-type Ca2+ channels can be activated by phosphatidylinositol 3,4,5-trisphosphate (PIP3) which is derived from PIP2 through the action of phosphoinositide 3-kinases (PI3K) [123]. PI3Ks are associated with the plasma membrane and can be activated by G-protein-coupled receptors or tyrosine kinases.
A major mechanism of VSMC relaxation is the activation of K+ channels. Activation of cGK and cAK has been associated with the opening of Ca2+-activated maxi K+ (BKCa) channels in the plasma membrane, causing hyperpolarization. Various mechanisms involving direct and indirect phosphorylation/dephosphorylation events mediated by cGK or cAK have been postulated for the activation of BKCa channels in smooth muscle from different vascular beds. However, the precise mechanisms remain undefined. Hyperpolarization mediated by BKCa channels has been shown to be an important mechanism of NO-cGK-dependent relaxation in the cavernosal smooth muscle of the penis [126]. Some vasodilators that stimulate cAMP production have also been shown to activate ATP-sensitive K+ channels in penile cavernosal tissue and resistance arteries [127,128]. Collectively, changes in membrane potential due to increased K+ efflux inactivate L-type Ca2+ channels to inhibit Ca2+ influx. NO may also cause VSMC hyperpolarization independent of cGMP and cGK. In aortic smooth muscle cells, NO has been shown to directly activate BKCa channels [129].
Endogenous Regulators of Penile Cavernosal VSMC Contractility
NO
NO is the primary mediator of nonadrenergic, noncholinergic (NANC) parasympathetic input and endothelium-dependent relaxation in the corpus cavernosum [1]. NO can regulate a wide array of physiological functions in mammals. It is synthesized on demand from the amino acid L-arginine and molecular oxygen by a family of enzymes known as NOS. Three distinct isoforms of NOS have been identified which were originally named after the tissues in which they were first described. nNOS (NOS type I) and eNOS (or NOS type III) are Ca2+/calmodulin-dependent, constitutive isoforms. Inducible NOS (iNOS or NOS type II) is a Ca2+-independent isoform that is mainly expressed in macrophages and tissues following an immunological stimulus [130]. NO can readily cross plasma membranes to enter target cells and promote the synthesis and accumulation of cGMP by the activation of the sGC. sGC is a heterodimeric protein that contains a prosthetic heme attached to a histidine residue of the β-subunit, which is required for the activation of the enzyme. Although the binding of NO occurs in the β-subunit, both subunits are required for the stimulation of enzyme activity [131,132].
PGs
PGs are produced by the action of cyclooxygenases on the common precursor arachidonic acid [133]. Human corpus cavernosum smooth muscle cells in culture have been shown to produce prostaglandin E (PGE2) and PGF2α. It has been demonstrated that prostanoids can induce both relaxation and contraction in penile corpus cavernosum. PGE is the only endogenous PG that appears to elicit relaxation of human trabecular smooth muscle, the others causing constriction or having no effect on smooth muscle tone. Exogenous PGE1 and PGE2 relax isolated cavernosal tissue at submicromolar concentrations, while PGE2 causes contraction at concentrations of 10 µM or greater. Prostaglandin E receptor (EP) receptors, which mediate the response to PGE, have been the most extensively studied and are categorized into four pharmacologic subclasses (EP1–EP4) [134,135]. Several different isoforms of the EP3 receptor have been identified and arise from alternative splicing of a single gene product. In general, the EP1, EP3I, and EP3III receptors mediate smooth muscle contraction by stimulating phosphatidylinositol hydrolysis or inhibiting adenylyl cyclase, while EP2, EP3II, and EP4 receptors mediate smooth muscle relaxation by coupling to Gs protein and stimulating adenylyl cyclase to increase intracellular cAMP [136].
Peptide Regulators—VIP and ET
The density and distribution of VIPergic nerves within the penis has led many to postulate that VIP, in addition to NO, is an important NANC neurotransmitter regulating penile erection. VIP-immunoreactive nerves are widely distributed throughout the male genitourinary system, and VIP and NOS containing nerves are often colocalized in penile tissue [1]. Isolated corpus cavernosum tissue from various species, including human, exhibits relaxant responses to VIP. These effects are accompanied by an increase in tissue levels of cAMP. The role of endogenous VIP in mediating penile erection is further supported by the observations that anti-VIP antibodies and VIP receptor antagonists inhibit nerve-mediated relaxation in isolated cavernosal tissue strips [1]. However, the role of VIP as a modulator of trabecular smooth muscle tone remains inconclusive since the effects of the peptide in vivo are not necessarily consistent with ex vivo or in vitro findings. Intracavernosal administration of VIP in humans has yielded varying results, ranging from no effect to partial tumescence to full erection. Thus, while descriptive studies are supportive of VIP's potential role in mediating or enhancing the onset and maintenance of penile erection, the mechanisms underlying its regulation and action have yet to be completely elucidated.
ET-1 is one of the most potent vasoconstrictors yet described [137,138]. This peptide has also been shown to have growth factor activity, stimulating mitogenesis in fibroblasts, smooth muscle, and endothelial cells. Similar to NO, ET release from the intimal lining of blood vessels can be induced by shear stress. In human corpus cavernosum, ET-1 is synthesized by the endothelium and elicits strong, sustained contractions of corpus cavernosum smooth muscle [139,140]. Both ET receptor subtypes (ETA and ETB) have been identified in penile corpus cavernosum. They are distributed on both the endothelium and the smooth muscle and are distinguished by their binding affinity for ET-3 [141]. Exogenous ET-1 or ET-2 cause equipotent contraction in isolated cavernosal tissue strips, whereas ET-3 induces much weaker contraction in corpus cavernosum. While the receptor-binding affinity of ET-1 and ET-2 is not necessarily greater than that of other contractile factors, the rate of dissociation is significantly slower than many ligands [141]. This may account for the unique ability of ETs to maintain long-lasting, sustained contraction in corpus cavernosum smooth muscle. It has been suggested that ET may also exert vasodilatory effects at low concentrations through a “super-high” affinity form of the ETB receptor. Although this vasodilatory role of ET in penile erection remains unclear, it has been demonstrated in the rat that ET-3 and submaximal doses of ET-1 increase ICP, potentially by stimulating NO production [142].
Noncontractile Responses in VSMCs
Changes in VSMC growth and extracellular matrix production can have a profound impact on the function of genital tissues. The extracellular matrix itself is a dynamic structure that plays an important role in modulating cell morphology, movement, growth, differentiation, and survival by regulating cell adhesion, cytoskeletal machinery, and intracellular signaling. It has been postulated that smooth muscle cells may transform from contractile to synthetic cells (or vice versa) in response to changes in their environment (e.g., chronic disease states or acute injury). Alternatively, there may be an inherently heterogeneous population of VSMCs in a given vascular tissue at any one time. In addition to growth factors and cytokines, vasoactive factors have also been shown to have trophic effects in the vasculature, suggesting that many of the same intracellular mediators that cause contraction or relaxation are also involved in trophic responses in VSMCs.
Synthetic VSMCs are primarily characterized by a significantly decreased expression of contractile proteins. Thus, activation of signaling pathways that may have mediated tonic responses in contractile VSMCs can modulate cell growth or matrix production in a synthetic VSMC. However, the specific mechanisms regulating gene expression and cell growth remain, in large part, to be elucidated. For example, the NO-cGK pathway and its effects on gene expression is an area of active study. While it appears that cGKI modulates the exracellular signal-regulated kinase (ERK) pathway (also called mitogen-activated protein kinase or MAP kinase) to modulate proliferation and migration of VSMCs, the molecular targets of ERK that eventually control gene transcription have not been clearly defined [143].
Many growth factors stimulate cell surface receptors with intrinsic tyrosine kinase activity in their cytoplasmic domains. This tyrosine kinase activity is considered essential to regulating cell growth. Several of these receptors have been linked with the activation PLC-γ. Also, phospholipase D (PLD) may be more important for mediating trophic responses than contractile responses. Some variations in responses to growth factors and vasoactive substances may be due to the different mechanisms of activation for different PLC isoforms. Stimulation of PKC has been shown to have both proliferative and antiproliferative effects to platelet-derived growth factor, epidermal growth factor, and AT-II [103]. While the reasons for this variability remain unclear, it must be stressed that multiple isoforms of PKC exist and each isoform has numerous substrates. PKC has also been shown to modulate DNA synthesis, potentially through the phosphorylation of transcription factors [103].
Summary and Perspective
An impressive amount of knowledge has been accumulated regarding smooth muscle biology and vascular physiology. Future concepts in genital tissue pharmacology will benefit from these insights. To date, it is widely accepted that several disorders of the male sexual response, such as male ED and orgasmic dysfunctions, can be therapeutically approached by influencing the function of the vascular and nonvascular smooth musculature of the genital tract. In order to achieve a pronounced drug effect without significant adverse events, especially on the cardiovascular system, a certain degree of tissue selectivity is mandatory. Selective intervention in intracellular pathways regulating smooth muscle tone has become a promising strategy to modulate tissue function.
Diabetes and MetS
Diabetics are at increased risk for maladies, including retinopathy, neuropathy, nephropathy, and vascular disease. ED is often characterized in part by insufficient NANC nerve stimulus, and/or an inability to dilate feeder arterioles of the penis resultant from vascular disease. As the diabetic population is susceptible to these changes, ED is indeed prevalent in this cohort. Although PDE5 inhibitors have revolutionized the field of ED treatment, these drugs are less effective in certain subsets of the population, including diabetics. For the sake of this amended chapter, the work outlined will review mainly type 2 diabetic ED and the MetS, highlighting studies of type 1 diabetic ED when relevant. Some valuable reviews include: Hidalgo-Tamola et al. [144], Moore et al. [145], Vrentzos et al. [146], Musicki et al. [147], and DiSanto [148].
Epidemiolgic Data
Diabetes Mellitus
Diabetes mellitus is a common chronic disease, affecting 0.5–2% worldwide. The prevalence of diabetes as a comorbidity has remained at 20–25%, irrespective of whether treating clinics are endocrine based or andrology based [149,150]. ED in diabetics is more common than retinopathy or nephropathy [151]. The Massachusetts Male Aging Study reported that up to 75% of men with diabetes have a lifetime risk of developing ED, much higher rates than 52% (40–70 years of age) [152–154]. The onset of ED occurs in the earlier age for those with diabetes, presenting within 10 years of the diabetic onset in more than 50% of patients with any type of diabetes [155].
MetS
MetS, which is also called insulin resistance syndrome or syndrome X, includes glucose intolerance, insulin resistance, obesity, dyslipidemia, and hypertension. Several epidemiological data have identified MetS as potential risk factors of ED. Grover et al. [156] evaluated the effect of various cardiovascular risk factors on ED in a primary care setting. ED was found in 49.4% according to a score of less than 26 on the International Index of Erectile Function-erectile function (IIEF-EF) domain in a cross-sectional survey of 3,921 Canadian men. The presence of diabetes (odds ratio 3.13), undiagnosed hyperglycemia (odds ratio 1.46), impaired fasting glucose (odds ratio 1.26), and MetS (odds ratio 1.45) were identified as independent risk factors for ED.
Clinical Findings
Diabetes Mellitus
In 12% of type 1 diabetic men, ED was the first symptom of diabetes [157]. The prevalence of coronary artery disease (CAD) (20%) and peripheral vascular disease (5%) among men with diabetes is far higher than in the general population. Pathologic changes in the cavernous arteries [158], ultrastructural changes in the cavernous smooth muscle [159], and impaired endothelium-dependent relaxation of the corporeal smooth muscle [160] have also been noted in penile specimens from diabetic men with ED.
The presence of ED in diabetic patients could be the harbinger of fatal cardiovascular disease. Gazzaruso et al. [161] demonstrated a higher prevalence of ED in diabetic patients with silent CAD than those without any evidence of myocardial ischemia. ED was associated with more than 14 times higher risk for silent CAD in diabetic men. In a subsequent study, ED was associated with higher major cardiovascular morbidity and mortality in diabetic patients with silent CAD [161].
Hemoglobin (Hb)A1c levels have been shown to increase with the severity of ED [162,163] and was found to be an independent predictor of the EF score in 78 men with type 2 diabetes [164]. However, there have been conflicting results about the beneficial effects of strict glycemic control on erectile function. Some studies reported improved erectile function following the reduction of HbA1c, while others reported no significant change despite the aggressive blood sugar control [165,166].
Hypogonadism is often associated with diabetic ED patients. Corona et al. found hypogonadism in 24.5% of men with diabetes and ED vs. 12.6% in the rest of the men with ED [167]. Testosterone supplementation in human diabetics with ED receiving pharmacological treatment might be advantageous in the diabetic men in whom PDE5 inhibitors given for ED do not work [168].
MetS
MetS and increased waist-to-hip ratio have been associated with a higher proportion of moderate-to-severe ED in men older than 50 years [169]. Conversely, ED may be predictive of MetS presence in men with a body mass index (BMI) of <25 kg/m [145,170]. These interesting findings suggest that ED may be a warning sign for MetS in men otherwise considered at low cardiovascular risk.
Studies indicated the possible role of inflammation and endothelial dysfunction in the development of ED for patients with MetS. Men with MetS had an increased prevalence of ED, reduced endothelial function score, and higher circulating concentrations of high sensitivity C-reactive protein (CRP) compared with men without metabolic disorders [171]. This and other studies clearly showed the relationship between MetS, the inflammatory endothelial activation, and the prevalence of ED [171,172].
As mentioned earlier, low circulating androgen levels are clearly a risk factor for MetS and the reverse relationship is true as well. ED might occur as a possible consequence of hypogonadism and MetS. A recent study by Zhody et al. [173] elegantly related hypogonadism to ED and MetS by analyzing BMI measurements in 158 obese men. With increasing BMI, the frequency of hypogonadism and ED increased, while total serum T showed a strong negative correlation. To assess the effect of BMI on vasculogenic ED, the authors examined this relationship in the absence of other risk factors and found that for a BMI <25, three out of 13 men (23.1%) had vasculogenic ED as compared with 32 out of 54 men (59.3%) with a BMI ≥25.
Currently, no direct pharmacologic therapy for MetS is available. Esposito et al. [174] assessed the effect of weight loss and increased physical activity on men with ED associated with obesity. BMI decreased significantly in the intervention group, and was associated with a decrease in serum concentrations of interleukin-6 and CRP. Erectile function scores improved significantly with lifestyle intervention but remained stable in the control group. In multivariate analyses, changes in BMI, physical activity, and CRP were independently associated with erectile function improvement. Thus, lifestyle changes are associated with improved sexual function and lowered inflammation in obese men with ED.
Basic Science Mechanisms
The majority of basic science studies, to date, that examine mechanisms of diabetic ED have done so using animal models of type I diabetes. Available studies outlining ED in animal models of type 2 diabetes and MetS have recently been reviewed [144].
Nitrergic Dysfunction
Erection is activated by NO release from nNOS at NANC nerve terminals. Maintenance of cavernosal vasodilation is thought to occur through the activation of eNOS in endothelial cells, presumably in response to shear stress. Impaired vasodilator signaling often results from NANC nerve dysfunction and/or endothelial dysfunction, leading to ED. Numerous studies have demonstrated type I diabetic animals to have impaired cavernosal relaxation to electrical field stimulation as well as decreased ICP following electrical cavernosal nerve stimulus, indicative of nitrergic dysfunction [175–181]. Decreased penile nNOS content was detected in various rodent models of type 2 diabetes [182–184]. Impaired nitrergic-mediated relaxation in type 2 diabetic mice has also been reported; however, the extent of the impaired relaxant response was modest, leading the authors to question the true pathophysiologic relevance of this finding to the ED phenotype [185].
Endothelial Dysfunction
Endothelial dysfunction is characterized by lowered NO bioavailability resulting from decreased eNOS expression or activity, or increased NO scavenging. It is clear that an attenuation of endothelium-dependent vasodilation of cavernosal tissue is present in several animal models of type 1 and type 2 diabetes [144,147,184–186]. The activation of eNOS can occur by hemodynamic stimuli, such as shear stress, as well as through protein signaling, such as by VEGF, leading to eNOS phosphorylation on serine 1177 [147,187]. In addition to phosphorylation events, eNOS activity and subsequent NO production are regulated by substrate concentration, cofactor availability, and enzyme coupling. Relevance of dysfunctional eNOS enzyme regulation remains speculative in regard to diabetic ED (see citation [147] for review).
Oxidative Stress
Chronic hyperglycemia induces free radical production through formation of advance glycation end-products (AGE), lipid peroxidation, polyol pathway activation, superoxide production, and activation of PKC [188]. Increased penile and serum AGE and reactive oxygen species (ROS) levels have been detected in type I diabetic rodents [189,190]. Impairments in NO-mediated cavernosal relaxation in these rodents are prevented with superoxide dismutase or a peroxynitrite decomposition catalyst, supporting a delirious role of ROS in type I diabetic ED [191–193].
Studies examining oxidative stress in animal models of type 2 diabetes or MetS are scant. Decreased antioxidant levels, such as glutathione (GSH), may result in elevated ROS/oxidative stress in type 2 diabetic men. Kovanecz et al. found prolonged treatment with pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist said to have anti-inflammatory effects and to improve the glutathione/glutathione disulphide (GSH/GSSH) ratio [194], suggesting that glycemic-stabilizing agents may also have benefit in decreasing damaging ROS.
Cavernosal Hypercontractility
Increased contractile function of the cavernosum can result from heightened sympathetic activation or potentiated intracellular contractile signaling of smooth muscle cells. Many animal models of diabetic ED have pointed to cavernosal hypercontractility as a pertinent mechanism underlying the disease phenotype. A recent review extensively outlines potential pro-signaling pathways in the penile smooth muscle cell that may contribute to diabetic ED [148].
Studies by Carneiro et al. and Luttrell et al. have suggested the presence of increased contraction in response to sympathetic activation in type II diabetic ED [185,195]. Wingard et al. found the heightened contractile signaling in the type 2 diabetic rodent in response to phenylephrine and ET-1 to be mediated by overactivity of PKC and Rho kinase, two primary kinases mediating smooth muscle cell tone [186].
Veno-occlusive Dysfunction
The limiting of blood outflow through mechanical compression of the emissary veins against the tunica albuginea is essential for the maintenance of elevated corporal pressures and a rigid erection. Studies in animal models of type 2 diabetes have suggested that a veno-occlusive disorder may underlie the ED phenotype. Kovanecz et al. found Zucker diabetic fat rats to have an inability to sustain adequate intracorporal pressure after the cessation of penile saline infusion, suggesting the presence of a veno-occlusive disorder [194]. These studies have recently been validated in the db/db mouse model of type 2 diabetes [185].
Conclusions
The numbers of patients with type 2 diabetes and MetS continue to rise. Current pharmacologic treatments remain insufficient for these populations, and the need for improved therapeutics is evident. Organic ED in these cohorts is underlined by multifaceted, complex mechanisms, involving nerve, vascular, and hormonal signaling at its core. It is clear that more clinical and basic science studies are warranted.
ED and Cardiovascular Disease
The vascular system is responsible for providing adequate blood supply to the erectile tissue facilitating the corporo-veno-occlusive mechanism required for erection. Thus, any alteration of the vascular system may compromise erectile function. Vascular disease in arteries supplying blood to the penis obviously impedes erectile function by limiting blood flow, but systemic vascular dysfunction is also intimately related to ED. Cardiovascular disease shares with ED the same risk factors, namely hypertension, hypercholesterolemia, diabetes, and smoking [152,196].
Atherosclerosis/Vascular Ischemia and ED
Association of ED to systemic vascular diseases is clear. On one hand, there is a high prevalence of ED in patients having CAD [197,198], peripheral arterial disease [199], and cerebrovascular disease [200]. In addition, the prevalence of ED seems to be increased as the severity of vascular disease augments [201]. Patients with lesions in two or more coronary arteries had worse erectile function than patients with normal coronary arteries or single-vessel CAD [202].
On the other hand, cardiovascular diseases are prevalent among patients with ED. In fact, CAD has been revealed in patients reporting ED without any other symptomatology of vascular disease [203]. ED has also been associated to the presence of peripheral atherosclerotic lesions. Among patients with ED, 66.4% presented atherosclerotic lesions, while lesions were only present in 36.5% of patients without ED [204]. In most cases, ED symptoms preceded CAD symptoms [197,201]. Thus, ED would be a sentinel symptom that warns of a probable underlying systemic vascular disease [205].
Chronic ischemia provoked by atherosclerotic stenosis of the proximal iliac artery in rabbits is also associated with functional changes in the distal part of the penile vasculature such as decreased NOS activity, increased production of contractile Tx, and PG formation. Neurogenic contractions were potentiated, while endothelium-dependent and neurogenic NO-mediated relaxations were reduced in cavernosal tissue [206,207]. A time-dependent reduction of the expression of nNOS and eNOS in the cavernosal tissue from these animals, with a parallel increase of the expression of iNOS, was demonstrated [208]. Reduced NOS activity and impaired endothelium-dependent and neurogenic NO-mediated relaxation of cavernosal tissue have been confirmed in a rabbit model of cavernosal ischemia without hyperlipidemia [209]. An elevation of the cavernosal content of endogenous inhibitors of NOS was proposed to be responsible for these effects [209]. The apolipoprotein E knockout mouse, a known experimental model of atherosclerosis, shows reduced erectile responses and impaired NO/cGMP pathway [210,211].
Hyperlipidemia and ED
Association of ED to hyperlipidemia has been found in several clinical studies. High concentrations of low-density lipoprotein seem to be related to ED [212], although low levels of high-density lipoproteins have been shown to be predictive of ED [213]. Hypercholesterolemia at baseline was also shown as a predictor of ED 25 years later [214]. In contrast, a survey of 1,899 men aged 30–79 years in Boston area revealed the absence of association of untreated hyperlipidemia and ED [215].
Chronic hypercholesterolemia reduces endothelium-dependent relaxations, but not the endothelium-independent relaxations in rabbit corpus cavernosum [216,217]. In contrast, the neuronal vasodilation does not appear affected in these animals [207]. The selective action of the endothelial NO/cGMP pathway in hypercholesterolemia could be due to increased superoxide production [217] by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [218] or to increased plasma levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOS [219]. VEGF and VEGF receptor 2 are downregulated in corporal tissue of rats' high-cholesterol diet [220]. Intracavernosal administration of VEGF and fibroblast growth factor-2 improved endothelial function, increased the expression of nNOS, and activated eNOS [221,222].
Hypertension and ED
The analysis of a representative care claims database identifying 273,325 patients with ED in the United States revealed that the prevalence of hypertension in this ED population was as high as 41.6% [223]. Conversely, a high prevalence of ED is generally observed in hypertensive patient populations [224–226]. However, hypertension is a risk factor not only for ED but also for cardiovascular disease. Then, the impact of hypertension on erectile function is contributed by the cardiovascular complications following hypertension are associated to even higher prevalence of ED [152,227]. Some studies have detected lower levels of serum testosterone in hypertensive patients [228,229], a fact that could be relevant to the development of ED in these patients. In hypertensive population, ED was associated to older age, longer duration of hypertension, and a more severe hypertension. ED was also related to the antihypertensive therapy [224].
Erectile responses were markedly inhibited in spontaneously hypertensive rats (SHR) after normalization by mean arterial pressure (MAP), although moderately reduced absolute increases in ICP were observed [230,231]. Reduction of ICP/MAP response in SHR preceded the development of hypertension. The impairment of cavernosal endothelium-dependent and NO donor-induced relaxations also occurred before systemic vascular alterations were manifested [231]. This suggests that erectile tissue is at the front line of the development of endothelial dysfunction and would be an early target end organ. Oxidative stress may play a significant role in the alterations caused by hypertension on erectile function. In fact, an augmented production of superoxide anions could result from increased activity of NADPH oxidase driven by AT-II [232].
Cigarette Smoking and ED
Cigarette smoking is clearly related to ED. Active and passive smokers are at higher risk for ED than men not exposed to smoke, and the risk increases as the exposure increases [233,234]. Some studies suggest that smoking is associated to ED independently of cardiovascular disease [235,236]. Although a causative effect cannot be proven in cross-sectional studies, this hypothesis is supported by the dose–response shown in several studies [234,236,237] and the fact that erectile function is improved after smoking cessation [238,239].
A reduction of penile NOS activity and nNOS expression was observed after passive smoking in rats, although erectile responses were not reduced [240]. Cigarette smoking would be related to downregulation of NO/cGMP pathway in penile tissue, probably related to increased oxidative stress [241,242], although an elevated activity and expression of arginase together with an increase of the content of the endogenous NOS inhibitor, ADMA, could participate [243]. On the other hand, acute nicotine administration caused a significant reduction of physiological erectile responses to erotic films in healthy nonsmoker men [244].
Pathophysiological Mechanisms in Vascular ED
Increased Vasoconstriction
RhoA/Rho kinase pathway plays an important role in calcium sensitization and tonic contraction of smooth muscle. Cardiovascular diseases are associated with an enhancement of RhoA/Rho kinase activity [245]. RhoA and Rho kinase expression is elevated in the cavernosal tissue from hypertensive rats, possibly contributing to reduced erectile function in these rats [246]. ET-1 levels are elevated in plasma from patients with hypertension and hypercholesterolemia [247,248]. Patients with organic ED also show higher venous and cavernosal ET-1 levels [249]. In cavernosal tissue from DOCA-salt hypertensive rats (specific animal model), contractile responses to ET-1 were increased and relaxation caused by ETB activation was reduced [250]. A decrease in ETB receptors in cavernosal tissue from hypercholesterolemic rabbits was also detected [251]. AT-II has been detected in endothelial and smooth muscle cells of human corpus cavernosum [252] and caused its contraction [253]. AT-II converting enzyme expression is upregulated in rats with arteriogenic ED [254]. In fact, administration of an AT1 antagonist has been shown to improve erectile function in hypertensive patients [255].
Impaired Neurogenic Vasodilatation
Impaired neurogenic relaxation of cavernosal tissue has been observed in a rabbit model of cavernosal ischemia [209], in SHR, affecting both NO neurotransmission and CO neurotransmission [256] and after cigarette extract administration [241] while was unaffected in a rabbit model of hypercholesterolemia [207].
Endothelial Dysfunction
Coronary endothelial dysfunction has been associated with the presence of ED [257]. Endothelium-dependent flow-mediated dilation (FMD) of the brachial artery was significantly reduced in ED patients, although nitroglycerine-induced dilation was also impaired [258,259]. The impairment of FMD correlates to the severity of ED [260]. Endothelium-dependent penile blood flow increases generated by reactive hyperemia were reduced in patients with ED. At the same time, endothelial function in the forearm vasculature was not significantly altered in ED patients [261].
Penile Structural Alterations
Objective reduction of smooth muscle cells has been demonstrated in patients with organic ED [262]. A decrease in cavernous trabecular smooth muscle and an increase in connective tissue are correlated with diffuse venous leakage and a failure of the veno-occlusive mechanism, hence resulting in ED [263,264]. SHR show hyperproliferation of smooth muscle in cavernosal tissue and penile vasculature, which correlates with blood pressure values. Increased fibrosis was also observed [265]. Hypertension-induced alterations were improved after antagonism of type 1 AT-II receptors (AT1) [266].
Drugs Causing ED
ED is a common symptom among older men and will inevitably coexist with other physical conditions prevalent in this population such as depression, diabetes, and cardiovascular disease which are themselves risk factors for ED [152,227,267]. In addition, sexual symptoms related to medication can involve a combination of complaints concerning sexual desire, arousal, and orgasm rather than being concentrated on ED alone. Self-reported and questionnaire data concerning ED as a side effect of medication should therefore be interpreted with caution.
Cardiovascular Drugs and Erectile Function
ED and heart disease have common risk factors [214,227], but comparing untreated and treated patients with heart disease or hypertension revealed that medication increases the relative risk for development of ED [268].
Treatment of Hypertension and ED
Current recommendations for treatment of hypertension suggest thiazide diuretics as first-line therapy, while angiotensin-converting enzyme (ACE) inhibitors, AT1 receptor antagonists, calcium channel blockers, and beta-adrenoceptor antagonists are indicated as first-line agents in specific high-risk conditions [269]. Often, two or more antihypertensive medications will be required to achieve a blood pressure <140/90 mm Hg (or <130/80 mm Hg in diabetic patients) in hypertensive patients [269]. All drugs have ED listed as a potential side effect, but well-designed controlled clinical trials give conflicting results concerning causative relationships [270]. Animal studies do suggest possible mechanisms using in vitro and in vivo methodology [271].
Diuretics
This class of drug has been extensively studied following early trials which showed a high prevalence of self-reported ED. Possible mechanisms include decreased vascular resistance and lowered zinc levels leading to reduced androgen production. Appropriate controlled studies with ED as an end point give consistent results despite trends toward lower-dosage schedules [225]. Similar findings were documented from the Treatment of Mild Hypertension Study (TOMHS) where the prevalence of ED at 2 years in men taking a low-dose thiazide was twice that of both the placebo group and those on alternative agents [272]. Interestingly, after 4 years of treatment, prevalence of ED in the placebo group approached that of the thiazide group, a finding not fully explained by dropouts. It may be that thiazide therapy unmasks latent ED at an earlier stage rather then being directly causal. Thus, it is likely that thiazide diuretics are associated with ED in men with hypertension, although this may represent unmasking of an existing problem and the effect can be reduced by lifestyle changes.
β- and α-Adrenoceptor Antagonists
In penile tissue, activation of β-adrenoceptors leads to corporal relaxation [1] and vasodilation of penile arteries [273]. This response is attenuated in vitro by nonselective drugs such as propanolol, possibly by blocking postjunctional β2-adrenoceptors [273,274], but not by cardiac selective agents such as practolol and atenolol. Depending on the lipophilicity of the β-adrenoceptor antagonists (e.g., propranolol is hydrophobic, while atenolol is hydrophilic), they may also exert an inhibitory effect within the central nervous system, perhaps leading to lowered sex hormone levels [275]. The effect of β-adrenoceptor antagonists on erectile function to a large degree can be explained by their mechanisms of action (e.g., they are either general β-adrenoceptor antagonists, selective β1-adrenoceptor antagonists, or also possess vasodilatatory properties) (Figure 2). Nonselective drugs such as propanolol were associated with higher prevalence of ED compared with patients treated with placebo or ACE inhibitors [276,277]. Later trials using agents with higher selectivity for the β1-adrenoceptor such as acebutolol have shown a substantial reduction in ED as a side effect with no difference being found against the placebo and ACE inhibitor groups [272]. This also applies to the use of selective β1-adrenoceptor antagonists in the prophylaxis of angina [278]. The general β-adrenoceptor antagonists which also cause vasodilation by blocking α1-adrenoceptors, e.g., carvedilol, have, in a crossover study, been reported to be associated with worsening in sexual function [279]. Some of the recently introduced β1-adrenoceptor antagonists such as nebivolol have also vasodilatatory effects mediated by release of NO. In crossover studies, nebivolol, in contrast to the selective β1-adrenoceptor antagonists, metoprolol, and atenolol, did not decrease sexual intercourse activity in hypertensive men, and may even have positive effects on erectile function [280,281].

β-adrenoceptor antagonists and erectile function. Propranolol is a general β-adrenoceptor antagonist, while carvedilol and labetalol also block α1-adrenoceptors. Metoprolol, bisoprolol, and atenolol are selective β1-adrenoceptor antagonists, and nebivolol, in addition, leads to release of nitric oxide (NO).
In clinical trials, α1-adrenoceptor antagonists (e.g., doxazosin) used to treat hypertension [272] or lower urinary tract symptoms [282] are not associated with complaints of ED and indeed had lower rates than placebo groups. Drugs stimulatory to the α2-adrenoceptors such as clonidine result in diminished erectile function both clinically and experimentally by peripheral and central mechanisms [274,283].
ACE Inhibitors and AT1 Receptor Antagonists
In addition to circulating AT-II, ACE and chymase are expressed in erectile tissue, and functional as well as binding studies suggest that AT-II induces contraction by activation of AT1 receptors [270]. Moreover, AT-II increases during the detumescence phase in man [284]. The ACE inhibitor captopril does not cause any significant adverse effect on sexual function in awake rats [274], and enalapril may even improve erectile function in SHR [285]. This is also supported by clinical studies comparing an ACE inhibitor with other agents and placebo. All three studies found either no difference compared with placebo or improved sexual function from baseline compared with other antihypertensive drugs [272,275,276,286].
In studies of hypertensive animals, AT1 receptor antagonists (e.g., losartan, valsartan, and candesartan) reverse structural changes in the penile vasculature and appear to conserve erectile function [285,287–289]. Moreover, in clinical cross-sectional studies, AT1 receptor antagonists, in contrast to other antihypertensive drugs, even tend to improve erectile function [225]. In direct comparison with the β-adrenoceptor antagonist carvedilol, valsartan has a beneficial effect on existing sexual dysfunction at baseline and has no adverse sexual effects during 12 months of treatment [279]. In the case of losartan, 3-month treatment was also reported to improve sexual function [290].
Calcium Channel Blockers
Smooth muscle contraction requires increased cytosolic calcium derived from internal stores and extracellular fluid. It would, therefore, be anticipated that calcium channel blockers would have a permissive effect on penile erection but might inhibit bulbospongiosal contraction during ejaculation. This is supported by findings of in vitro studies which demonstrated a modest relaxant effect on isolated cavernosal smooth muscle [283] and penile arteries [291]. Clinical studies have demonstrated no adverse effect on erection and ejaculatory complaints seem short-lived [275]. In the TOMHS, there was no significant excess risk of ED in the amlodipine group compared with placebo-treated patients [272]. Another study also showed no increase in the prevalence of ED when hypertension was treated with diltiazem alone or in combination with an ACE inhibitor [292]. A comparative study of two calcium channel antagonists showed that neither had any significant effect on sexual function, although two patients withdrew from the nifedipine arm because of reduced libido [293].
Treatment of Heart Disease and ED
ED is highly prevalent among patients with heart failure, because of neurohumeral changes, an imbalance of circulating vasomodulators, reduced cardiac capacity, depression, and potential adverse effects of heart failure medical treatment [294]. An array of drugs is applied for the treatment of heart disease. In most cases, a multiple drug regimen is applied for conditions such as chronic heart failure, where patients are treated with diuretics for removal of surplus liquid, ACE inhibitors and/or AT1 receptor antagonists to cause peripheral vasodilation, digoxin as positive inotropic agent, antithrombotics, antiarrhythmics, anticoagulants, and hypolipidemic drugs. In addition, the aldosterone receptor antagonists, spironolactone and eplerenone, and β-adrenoceptor antagonists such as metoprolol, bisaprolol, carvedilol, and recently, nebivolol have been found to enhance survival in patients suffering from heart failure [295–297]. Standard heart frequency therapy with β-adrenoceptor antagonists, digoxin, and thiazide diuretics may worsen sexual dysfunction owing to medication side effects, but evidence regarding the effect on erectile function of most of these drugs is sparse [270].
Lipid-Lowering Drugs (Fibrates, Statins)
ED was reported to be a frequent side effect of treatment of hyperlipidemic subjects using clofibrate [298] or gemfibrozil [299]. In patients referred to a clinic for primary hyperlipidemia, an increased risk of ED was also observed in patients treated with fibrates [300]. Fibrates interact with PPARs [301], which in the liver stimulates microsomal esterification of estradiol and testosterone [302]. That may explain the increased prevalence of ED reported in patients treated with fibrates.
In patients with hyperlipidemia and treated with statins such as simvastatin and pravastatin and referred to a clinic for primary hyperlipidemia, an increased risk for ED was reported [300,303]. It is controversial whether simvastatin is associated with ED [304–306], and so far, in patients treated with simvastatin, underlying diseased vasculature rather than the drug appears to be the cause of ED. In contrast to simvastatin, positive effects on erectile function was reported for atorvastatin on erectile function [307–310]. These reported positive effects of atorvastatin, in contrast to simvastatin, on erectile function suggest that the effect of statins is not a class effect of statins. Moreover, in an observational prospective study, it was reported that differences in dose, relative efficacy, or relative lipophilicity of statin did not show correlation with changes in IIEF score over a 6-month period [311]. However, statins are structurally a heterogenous group of compounds and, therefore, other mechanisms than the lipid-lowering effects may have significance for the effect of a statin on erection [246,312,313].
Aldosterone Receptor Antagonists
Treatment with spironolactone and eplerenone increased survival in systolic chronic heart failure. In addition to being an aldosterone receptor antagonist, spironolactone also blocks androgen receptors (ARs) and that may explain why it is associated with sexual dysfunction. In contrast, the newer aldosterone receptor antagonist, eplerenone, is devoid of effects on sex hormone receptors.
Psychotropic Medication
Antipsychotics
It is difficult to separate disease from drug effect, as well as to obtain reliable information from patients with psychotic illness. There is a paucity of controlled studies. Mainly, older antipsychotic drugs result in decreased erection and anorgasmia, while newer antipsychotics appear to have lower incidence of sexual dysfunction. Among newer antipsychotics, risperidone appears to have highest rate of sexual dysfunction, while there are insufficient data on aripiprazole and ziprasidone.
Antidepressive and Anxiolytic Drugs
Depression by itself makes it difficult to separate effect of illness from additive effect of drugs, as mood disorders may lead to lack of interest and emotional withdrawal from the sexual partner. Antidepressants can have numerous effects on sexual function including altered sexual desire, erection difficulties, and orgasm problems. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine can negatively affect all the steps of the male sexual response cycle (desire–arousal–excitement–orgasm). Bupropion, nefazodone, and mirtazapine have lower rates of sexual dysfunction than SSRIs. The evidence has been summarized in a recent Cochrane review of 15 randomized studies [314]. The main conclusion regarding comedication to correct ED was an effect of sildenafil in three of the clinical trials, while the available evidence was insufficient regarding other strategies for correction of ED. Anxiolytic agents such as bupropion, acting mainly by inhibiting dopamine reuptake, and buspirone, which acts on 5-HT1A receptors, are not associated with sexual side effects in placebo-controlled trials [315] and can be used to alleviate sexual symptoms caused by other antidepressant medication [316]. There are insufficient randomized data assessing effect of dose reduction, e.g., drug holidays, on sexual function in patients treated with antidepressants.
Opiates
Long-term intrathecal administration of opiates results in hypogonadotropic hypogonadism and associated sexual dysfunction that can be restored with appropriate supplementation [317]. Administration of opioid antagonists to older men with ED, however, did not improve erectile function measured objectively by nocturnal penile tumescence monitoring [318]. Opioids do have a generalized depressant effect on sexual function when directly administered to the MPOA in rat brain, but treatment with the opioid receptor antagonist, naloxone, had no sexual effect on healthy male volunteers [283].
Anti-Androgens
These drugs cause partial or near-complete blockade of circulating androgens by inhibiting production or antagonism at the AR. Nonsteroidal drugs such as flutamide and bicalutamide have relatively pure effects on the AR, while the steroidal antiandrogen, cyproterone acetate, also has inhibitory effects on the hypothalamus. Even at a low dose of 50 mg, bicalutamide therapy resulted in half the patients in one placebo-controlled study suffering loss of erectile function [319]. In summary, antiandrogen drugs produce the expected effects of sexual desire and erection commensurate with the degree of androgen ablation achieved.
Conflict of Interest: T.F. Lue, Speakers Bureau for Pfizer, Bayer, AMS, Medtronic and Lilly; J. Angulo, Speakers Bureau for Pfizer; N.N. Kim, Employee Alagin Research LLC, Research Funding from Pfizer, and Advisory Board for Boehringer-Ingelheim.




