Characterising the castration-resistant prostate cancer population: a systematic review
Disclosures: Professor Kirby has received funding for research, advice, lecturing and conference costs from the pharmaceutical industry. Dr Hirst is currently an employee of AstraZeneca R&D UK, and has previously worked as a consultant in epidemiology for several pharmaceutical companies. Professor Crawford has served as a consultant and speaker for GlaxoSmithKline, Sanofi-Aventis and AstraZeneca.
Summary
Background: Castration-resistant prostate cancer (CRPC) is an advanced form of prostate cancer associated with poor survival rates. However, characterisation of the disease epidemiology is hampered by use of varying terminology, definition and disease management. The aim of this review was to conduct a systematic review to provide greater clarity on the sum of the available epidemiologic evidence and to guide future research into the disease prevalence, progression, characteristics and outcome.
Methods: Systematic searches of PubMed and Embase were performed in March 2010 to identify relevant observational studies relating to the epidemiology, progression and outcomes of CRPC. Further studies were identified for inclusion in our review through manual searches of the authors’ bibliographical databases and the reference lists of the included articles.
Results: We identified 12 articles (10 full papers and 2 abstracts) reporting studies that included a total of 71,179 patients observed for up to 12 years for evaluation in our review. Five studies looked at the prevalence of CRPC in patients with prostate cancer. Together, the data indicate that 10–20% of prostate cancer patients develop CRPC within approximately 5 years of follow-up. Two studies reported the prevalence of bone metastases present at diagnosis of CRPC. Together, ≥ 84% were shown to have metastases at diagnosis. Of those patients with no metastases present at diagnosis of CRPC, 33% could expect to develop them within 2 years. The median survival of patients with CRPC was reported in five studies, with values varying from 9 to 30 months. A pooled, sample-weighted survival estimate calculated from the survival data included in this review is 14 months. Very few studies that met our inclusion criteria evaluated treatment patterns in CRPC. One study reported that only 37% of patients with CRPC received chemotherapy, with the remainder receiving only steroids and supportive care. The most common palliative therapies administered to patients with skeletal symptoms were radiotherapy, radionuclide therapy, bisphosphonates and opioids.
Conclusions: This review highlights the poor prognosis of patients with CRPC, and demonstrates a survival of 9–13 months in those patients with metastatic CRPC. Furthermore, progression to CRPC is associated with deterioration in quality of life, and few therapeutic options are currently available to patients with CRPC. However, epidemiologic study of these patients is hampered by differing terminology, definitions and treatment paradigms. Our review highlights the need for further well-designed, epidemiological studies of CRPC, using standardised definitions and methods.
Review Criteria
Observational studies reporting epidemiological data on CRPC were identified through systematic searches of literature on PubMed, Embase and authors own databases. Articles were selected using predefined inclusion/exclusion criteria, and data were abstracted in a structured manner. Where possible and appropriate, meta-analysis of data was performed to provide pooled weighted means.
Message for the Clinic
This review of real-world evidence demonstrates that CRPC is a common and highly morbid progression of prostate cancer, with around 10–20% of prostate cancer patients progressing to this state within 5 years. Metastases are present in over 84% of CRPC patients, and the mean survival is around 14 months from CRPC diagnosis. Bone pain occurs in most patients, and fractures, spinal cord compression and vertebral collapse are common. Variability in definitions and clinical practice hinder the comparison of research, and efforts should be made to improve consistency in future research.
Introduction
Cancer of the prostate is the most common cancer occurring in the men of the USA and Europe (1,2). In the minority of patients whose cancers are aggressive or advanced, therapeutic options include prostatectomy, radiation therapy and, more commonly, androgen-deprivation therapy (3). Castration-resistant prostate cancer (CRPC) is an advanced form of prostate cancer characterised by disease progression following surgical or pharmaceutical (androgen deprivation) castration. The process by which prostate cancer cells become castrate resistant is unclear, but it has been proposed that androgen ablation provides a selective advantage to androgen-independent cells, which grow and eventually repopulate the tumour (4). Compared with castration-sensitive prostate cancer, the prognosis for patients with CRPC is poor and survival is reduced. Treatment options have, until very recently, been limited mainly to symptomatic relief of bone metastases, which are more common in CRPC than in castration-sensitive disease (5–8).
Defining epidemiological parameters of disease is an essential component of understanding how, when and where the disease develops; knowledge of the natural history of the disease and the likely outcomes of disease enable effective targeting and development of treatments. To give a clear picture of the burden of CRPC, one must take into account the prevalence of the disease, relative timing of onset in relation to prostate cancer diagnosis, characteristics of the patients including demographics and comorbidity, onset of metastatic disease, and likely survival. There is, however, a paucity of epidemiological evidence specifically characterising CRPC outside of controlled trial settings in which patients may not represent the general population and normal disease progression. This may result in suboptimal disease management; for example, identifying patients with CRPC who are at risk of developing metastases is currently hindered by poor understanding of the epidemiology of CRPC.
Identifying individuals with CRPC may seem straightforward to treating physicians, who are responsible for managing this progression of the disease after castration treatment. Characterising the disease in epidemiological terms, for example incidence, prevalence and survival, is, however, less clear. This may be attributed at least in part to the difficulty in defining, and hence studying, the patient population. The varying terminology – CRPC, HRPC (hormone refractory), AIPC (androgen independent), ERPC (endocrine resistant) – reflect subtle differences in definition which may hinder comparison of research. Physicians may also use different methods in diagnosis: PSA testing, development of metastases or other factors may determine whether a patient is defined as CRPC. The recently published European Association of Urology (EAU) guidelines aim to standardise CRPC diagnosis, and include a list of five defining factors of CRPC (3). These are:
- •
Serum castration levels of testosterone.
- •
Three consecutive rises of PSA 2 weeks apart resulting in two 50% increases over the nadir.
- •
Anti-androgen withdrawal for at least 4 weeks.
- •
PSA progression despite secondary hormonal manipulations.
- •
Progression of osseous or soft tissue lesions.
CRPC is a heterogeneous disease, and despite the availability of such practical guides to diagnose CRPC, in practice, this may vary. Furthermore, treatment pathways and clinical practice, in particular, the stage in the disease at which androgen-deprivation therapy is initiated, vary markedly between geographical locations and even individual clinics. Therefore, establishing common epidemiological estimates for the CRPC population becomes highly complex and risks becoming less relevant to individual scenarios.
The aim of this review was to improve the clarity of epidemiological evidence around CRPC, by systematically identifying, evaluating and describing the most relevant studies that characterise the CRPC patient population using observational data. From this, we aim to provide clearer guidance on measurement of epidemiological estimates of disease prevalence, progression and outcome, and to guide future research into CRPC.
Methods
PubMed and Embase searches were performed in March 2010 using the search terms detailed in Figure 1. Searches were limited to journal articles published in the previous 10 years reporting studies in men. Observational epidemiological studies were sought, as they were considered the best source of real-world non-interventional data on disease epidemiology, and randomised controlled trials, in vitro studies, editorials, letters, practice guidelines, reviews, case reports and comments, were excluded.
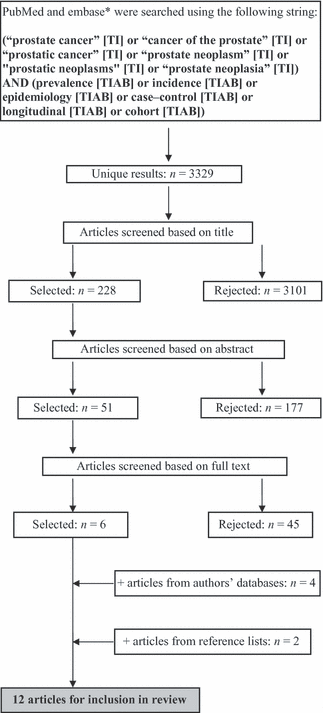
Search strategy. *The search string syntax was adapted for use in Embase
Relevant articles were screened, first, on the basis of the title and then on the abstract as outlined in Figure 1. Articles were then further screened based on the full text, and those that explored the epidemiology, time course and outcomes of CRPC were selected.
Further studies were identified for inclusion in our review through manual searches of the authors’ bibliographical databases and the reference lists of the included articles.
The definition of CRPC, prevalence, metastatic status and survival of patients with CRPC were evaluated for each of the included studies, and symptoms, quality of life, and treatment patterns were also described if reported in the studies. Where possible, data were pooled to provide estimates for each of the epidemiological parameters.
Results
The PubMed and Embase searches identified 3329 unique articles. From these, six relevant articles were selected. The main reasons for excluding articles were a main focus on drug trial data (interventional study), the role of gene polymorphisms, the epidemiology of prostate cancer, in general (not CRPC) or that the prevalence/survival/progression of CRPC was not reported. Four further articles were selected from the authors’ databases, and two were identified through searching of reference lists. This resulted in 12 articles (10 full papers and 2 abstracts) suitable for evaluation in our review.
Definition of CRPC used
Various diagnostic criteria were used by the 12 studies included in our review; none exactly matched with the EAU guidelines outlined above (3). Rising PSA levels were used to diagnose CRPC in nine (75%) of the studies (9–17). However, two of these also categorised patients who had a new lesion on a bone scan or growth of a lesion on a computed tomography (CT) scan as having CRPC (10,14). Another study relied upon observing worsening metastatic lesions by bone or CT scan in patients receiving hormone therapy (18). One study selected patients who had a diagnosis of symptomatic M1 metastatic CRPC using the Tumour Node Metastasis (TNM) staging criteria (19), and the final study assigned CRPC status to patients who failed to respond to postcastration hormone therapy and were switched to a third-line therapy (20).
The prevalence of CRPC in patients with prostate cancer
Five studies estimated the prevalence of CRPC in patients with prostate cancer (Table 1). Four of these evaluated patients were those who had been recently diagnosed with prostate cancer (9–11,20); the fifth study investigated patients with prostate cancer, who had undergone radical prostatectomy (12).
| Reference | Study sample | Country (years study conducted) | Patient age | Definition of CRPC | Follow-up period | Prevalence of CRPC | |
|---|---|---|---|---|---|---|---|
| Patients with PC | Alemayehu (9) | Retrospective study of data from 15,361 patients with PC from a US medical care claims database | USA (records from 2001 to 2007 were analysed) | 40 years or more at index date | At least two increases in PSA following surgical/medical castration. Furthermore, patients with CRPC were identified using a propensity score of multiple factors | Up to 6 years | 17.8% of castrate patients with PC |
| Cabrera (20) | Retrospective study of 44,791 patients with PC who had undergone castration, identified from a US medical care claims database | USA (records from 2000 to 2008 were analysed) | 18–97 years | CRPC status was assigned to patients who failed to respond to postcastration hormone therapy, defined as a switch from their second-line hormone treatment to a third hormonal therapy or chemotherapy | Followed until exitfrom database, mean ∼25 months | 9.5% of castrate patients with PC | |
| Morgan (11) | Retrospective study of 8678 patients with PC using data from a UK primary care database | UK (records from 1998 to 2008 were analysed) | 40 years or more at index date | A record of medical or surgical castration and temporal evidence of increasing levels of PSA | Up to 10 years | 11.2% of patients with PC | |
| Berruti (10) | 211 patients newly diagnosed with PC in secondary care | Italy (1996–2003) | Median: 70 (range: 47–87) years | Despite castrate levels of testosterone, two consecutive increases in PSA during androgen deprivation, a new lesion on a bone scan or growth of a lesion on a CT scan | Median: 55 months | 53% of patients with PC in secondary care | |
| Patients with PC who had RP | Bianco (12) | 1045 patients with PC who had undergone RP | USA (1990–1999) | NR | Serum PSA > 0.4 ng/ml with an increasing trend | Median: 55 (range: 1–145) months | 19% of patients with PC who have undergone RP |
- CRPC, castration-resistant prostate cancer; CT, computed tomography; NR, not reported; PC, prostate cancer; PSA, prostate-specific antigen; RP, radical prostatectomy.
A statistical, propensity score algorithm was used by Alemayehu et al. to identify patients with CRPC from a pool of prostate cancer patients identified from a large US medical claims database (10). During a 6-year period, 15,361 hormone-treated patients (aged 40 years or over) were diagnosed with prostate cancer. In the same period, 2740 developed CRPC, which suggests a prevalence of 17.8%. The largest study of CRPC conducted to date used another US claims database, MarketScan (20). In total, 44,791 medically or surgically castrated adult prostate cancer patients were followed up until their exit from the database. Of these, 4266 (9.5%) developed CRPC (mean follow-up of approximately 2.1 years per patient). A similar study was conducted using data from UK primary care patients recorded in The Health Improvement Network (THIN) database (11). The data reveal that, in a 5-year period, 8678 patients aged 40 years or over were diagnosed with prostate cancer. Of these, 969 developed CRPC, a prevalence of 11.2%.
An Italian study of 211 secondary care patients with prostate cancer demonstrated that, within the 55 months following diagnosis, 53% of patients (median age 70 years) were considered to have CRPC (10). A further study investigated patients with prostate cancer who had undergone radical prostatectomy (12). The authors reported that 19% of patients developed CRPC within a median 55-month follow-up period.
As discussed above, the available CRPC definition guidelines (3) were not routinely used by the studies included in this review. Despite the heterogeneity between studies, the results of four of these five different studies suggest that 10–20% of prostate cancer patients develop CRPC in approximately 5 years of follow-up. It is likely that if similar study populations and disease definitions were used, this estimated range would be even tighter. The greatest outlier in these data came from the study that categorised patients who had a new lesion or growth of a lesion on a CT scan as having CRPC (10), which probably explains why this study estimated a higher prevalence (53%) than the studies that characterised CRPC on the basis of increasing PSA levels or treatment patterns.
Metastatic CRPC
Bone scans were used to investigate the prevalence of metastases at the time of CRPC diagnosis in two small studies (13,14) (Table 2). A Japanese study reported that in a population of 151 patients with CRPC (defined as three consecutive increases in PSA after castration), 84% had bone metastases at diagnosis (13). A separate study conducted in Italy reported that, of 200 patients with CRPC, 95% had bone lesions at diagnosis; however, as bone lesions were a qualifying criterion for CRPC status in the study, this may be an overestimate.
| Reference | Study sample | Country (years study conducted) | Mean age at CRPC diagnosis (years) | Definition of CRPC | Percentage of patients who received chemotherapy | Prevalence of bone metastases at diagnosis of CRPC | Follow-up period | Progression to presence of bone metastases |
|---|---|---|---|---|---|---|---|---|
| Inoue (13) | 151 patients with CRPC | Japan (1990–2004) | 26% were age 60–6944% were age 70–79 | Three consecutive increases in PSA after combined anti-androgen therapy under surgical/medical castration | NR | 84% | ||
| Berruti (14) | 200 patients with CRPC in secondary care | Italy (1990–2003) | 73 (range: 52–92) | Two consecutive increases in PSA during androgen deprivation, a new lesion on a bone scan or growth of a lesion on a CT scan | 37% | Bone metastasis defined as EOD score ≥ 195% had bone lesions | ||
| Smith (17) | 201 patients with CRPC | NR (1999–2002) | 73 (range: 66–80) at start of study | Rising PSA despite androgen-deprivation therapy | 0% | NA | 48 months | 33% at 2 years |
- CRPC, castration-resistant prostate cancer; CT, computed tomography; EOD, extent of disease; NR, not reported; PSA, prostate-specific antigen.
The progression to development of metastases was shown in a further paper that evaluated patients with CRPC (mean age 73 years) who had no metastases present at CRPC diagnosis (defined as rising PSA levels despite androgen-deprivation therapy) (17) (Table 2). Between 1999 and 2002, 201 chemotherapy-naïve CRPC patients, were followed up for 24 months from CRPC diagnosis. Of those patients who had no metastases at CRPC diagnosis, 33% had developed one or more (identified by bone scanning and radiography) within 2 years of CRPC diagnosis.
Survival for patients with CRPC
The median survival of patients with CRPC was reported in five studies (13–16,18). Reported values varied from 9 to 30 months (Table 3). Again, there was heterogeneity between studies. The individual studies did not consistently report the mean patient age, and so evaluating the effect of age on survival is not possible from these data. The study populations also varied in terms of the proportion of patients with metastases and bone pain. Another factor affecting survival that was not comparable between studies was the use of chemotherapy. One study did not report the percentage of patients who received chemotherapy and the values reported by the other four studies ranged from 14% to 100%. Radiotherapy use was not consistently reported.
| Reference | Study sample | Country (years study conducted) | Mean age at CRPC diagnosis (years) | Definition of CRPC | Follow-up period | Percentage of patients who received chemotherapy | Survival |
|---|---|---|---|---|---|---|---|
| Soerdjbalie-Maikoe (15) | 84 patients with CRPC in secondary care | Netherlands (NR) | NR | Progressively worsening bone pain, rising serum PSA concentration, rising alkaline phosphatise activity and appearance of metastases on bone scintigraphy | NR | 23% | Mean survival was 8.6 ± 10.6 months from diagnosis of CRPC |
| Hwang (18) | Retrospective cohort of 89 patients with CRPC in secondary care | USA (1994–1999) | 73 (range: 52–96) | Worsening metastatic lesions by bone or CT scan while receiving hormone therapy | From documentation of CRPC until death | 14% | Median survival was 13 months (range: 0.6–67) from diagnosis of CRPC |
| Berruti (14) | 200 patients with CRPC in secondary care | Italy (1990–2003) | 73 (range: 52–92) | Despite castrate levels of testosterone, two consecutive increases in PSA during androgen deprivation, a new lesion on a bone scan or growth of a lesion on a CT scan | From documentation of CRPC until death or last follow-up (18 month after latest enrolment) | 37% | Median survival was 14.5 months from diagnosis of CRPC |
| Inoue (13) | 151 patients with CRPC | Japan (1990–2004) | 26% were age 60–6944% were age 70–79 | Three consecutive increases in PSA after combined anti-androgen therapy under surgical/medical castration | Mean follow-up period from initial PC diagnosis: 43 months and from CRPC diagnosis: 18 months | NR | Median survival in the 65 patients with bone pain at diagnosis of CRPC was 18 months compared with 30 months in those without bone pain at diagnosis (n = 81) |
| Chin (16) | 88 patients with CRPC in secondary care | Canada (August 2005–June 2007) | Median: 71 years | Increasing PSA levels or symptomatic progression despite castrate levels of testosterone | Until February 2008 | 100% | Median survival was 15.9 (range: 12.4–20.5) months from first chemotherapy following a CRPC diagnosis |
- CRPC, castration-resistant prostate cancer; CT, computed tomography; NR, not reported; PSA, prostate-specific antigen.
Two studies included the presence of metastatic lesions as one of their criteria for defining CRPC (15,18), and these studies reported the shortest survival estimates. In the first study, in which CRPC was defined as rising serum PSA concentrations and serum alkaline phosphatase activity, progressively worsening bone pain or the appearance or re-appearance of skeletal metastases on bone scintigraphy despite being androgen deprived, the mean survival after the development of CRPC in 84 patients was 8.6 months (15). The second study of 89 US patients with CRPC (mean age 73 years) reported the mean survival after the diagnosis of CRPC (defined as worsening metastatic lesions while receiving hormone therapy) to be 13 months (18).
The study populations investigated by Berruti et al. and Inoue et al. included patients with and without metastases at CRPC diagnosis. Survival estimates were reported to be 15 and 30 months respectively (13,14). Inoue et al. reported a positive impact of irradiation administered for bone pain on the survival estimates in their study. This may go some way to explaining their longer survival estimate.
One of the longest survival estimates (15.9 months [range: 12.4–20.5]) was reported by a retrospective study of Canadian secondary care patients (median age 71 years) (16). This was the only study in which all patients had received chemotherapy following their CRPC diagnosis, which may indicate a fitter population who were able to tolerate chemotherapy.
A pooled, sample-weighted survival estimate calculated from the survival data included in this review is 14.0 months.
Symptoms and quality of life in patients with CRPC
Primary symptoms of prostate cancer were not evaluated in any of the studies of CRPC selected for inclusion in our review. However, bone pain and skeletal events were evaluated in three papers (13–15) (Table 4). In the study by Inoue et al., 45% of the population were experiencing bone pain at the time of CRPC diagnosis (13). This increased to 80% during the follow-up period (mean: 18 months). It was also reported that 14% of the study population experienced bone fractures during the follow-up period. Of 200 patients with CRPC (37% of whom received chemotherapy) in the study by Berruti et al., 89% experienced bone pain during an 18-month follow-up period (14). Other reported skeletal events included vertebral collapse (21%), fractures (13%) and spinal cord compression (10%). In the study by Soerdjbalie-Maikoe et al., the presence of progressively worsening bone pain was used as one of the criteria for diagnosing CRPC (15). Of 84 patients, 19 (23%) received chemotherapy. The authors reported that 24% of their patient population developed spinal cord compression 3 days to 10 months after the establishment of CRPC.
| Reference | Study sample | Country (years study conducted) | Mean age at CRPC diagnosis (years) | Definition of CRPC | Follow-up period | Percentage of patients who received chemotherapy | Outcome |
|---|---|---|---|---|---|---|---|
| Inoue (13) | 151 patients with CRPC | Japan (1990–2004) | 26% were age 60–6944% were age 70–79 | Three consecutive increases in PSA after combined anti-androgen therapy under surgical/medical castration | Mean 18 months from CRPC diagnosis | NR | 45% experienced bone pain at time of CRPC diagnosis. 80% experienced bone pain and 14% experienced bone fractures during the follow-up period |
| Berruti (14) | 200 patients with CRPC in secondary care | Italy (1990–2003) | 73 (range: 52–92) | Despite castrate levels of testosterone, two consecutive increases in PSA during androgen deprivation, a new lesion on a bone scan or growth of a lesion on a CT scan | 18 months | 37% | 89% experienced bone pain during the follow-up period |
| Soerdjbalie-Maikoe (15) | 84 patients with CRPC in secondary care | The Netherlands (NR) | NR | Progressively worsening bone pain, rising serum PSA concentration, rising alkaline phosphatise activity and appearance of metastases on bone scintigraphy | NR | 23% | 24% developed spinal cord compression 3 days–10 months after diagnosis |
| Sullivan (19) | 280 patients with metastatic CRPC | Multinational (NR) | 72 years | Diagnosis of symptomatic M1 metastatic CRPC was by TNM staging criteria | 9 months | ∼12% | HRQoL symptoms assessed in the FACT-P and the EQ-5D questionnaires, and the majority of those in EORTC showed a rapid and significant deterioration over the study period. The most sensitive domains were pain, nausea and vomiting, dyspnoea and appetite loss |
- CRPC, castration-resistant prostate cancer; CT, computed tomography; EORTC, European Organization for Research and Treatment of Cancer Quality of Life; EQ-5D, 5-dimension EuroQol questionnaire; FACT-P, Functional Assessment of Cancer Therapy-Prostate; HRQoL, health-related quality of life; NR, not reported; PSA, prostate-specific antigen; TNM, Tumour Node Metastasis.
Health-related quality of life findings for a population of 280 patients with metastatic CRPC (approximately 12% of whom received chemotherapy) were reported from an observational, multinational cohort study (19) (Table 4). Health-related quality of life was assessed over a 9-month follow-up period using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC), the Functional Assessment of Cancer Therapy-Prostate (FACT-P) and the five-dimension EuroQol questionnaire (EQ-5D). The results of the study highlighted a rapid and significant deterioration in FACT-P and EQ-5D scores and in most items evaluated by EORTC during the 9-month observation period. The most sensitive domains were pain, nausea and vomiting, dyspnoea and appetite loss.
Treatment patterns in CRPC
Very few of the included observational studies evaluated treatment patterns in CRPC. One study reported the treatment received by patients with CRPC (14) and highlighted the high proportion of patients with CRPC who do not receive radical therapy, which may be because of the limited options available to these patients. Of 200 patients (95% of whom had bone metastases), 37% received chemotherapy, with the remaining 63% receiving only steroids and supportive care (14).
Chin et al. (16) performed a retrospective chart review of 88 patients with CRPC in secondary care who received docetaxel as a first-line chemotherapy. Of these, 36 patients (41%) progressed to require a second-line chemotherapy agent. Third-line therapy was used by eight patients (9%) and fourth-line in one patient (1%).
On entry to the observational health-related quality of life study described earlier (19), the treatments used by 280 patients with metastatic CRPC were reported to include bone-seeking radioisotope (8% of patients), external beam therapy of prostate or prostate bed (9%), transurethral resection of the prostate/laser ablation of prostate (14%) and other external beam therapy (44%). Docetaxel was the most commonly used chemotherapeutic agent used before baseline (7% of patients).
The therapeutic choices for patients in the end stages of disease reported in a separate study were quite different. Hwang et al. reported the hospital resources used by 87 patients with CRPC in the last 6 months of life (18). In total, 49% of the patients had received radiation for symptom palliation and 52% received physical therapy. Opioids were prescribed to 90% of patients in the last 6 months of life. Three studies reported the palliative therapy administered to patients with bone pain or other skeletal events (13–15). The most common therapies used were radiotherapy, radionuclide therapy, bisphosphonates and opioids.
Discussion
Summary of results
Data from retrospective and prospective observational studies involving a total of 71,179 patients observed for up to 12 years demonstrate that CRPC is associated with frequent bone metastases, reduced survival and a poor quality of life (Table 5). The epidemiologic data described here add to and complement the body of evidence from controlled clinical trial settings (21). Data from a study of 200 patients with CRPC revealed that only 37% of patients received chemotherapy, with the remaining 63% receiving only steroids and supportive care, perhaps an indicator of the limited treatment options for patients with CRPC (14).
| Prevalence | 10–20% of prostate cancer patients develop CRPC within approximately 5 years of follow-up |
| Metastases | ≥ 84% of patients have metastases present at the time of CRPC diagnosis |
| In those without metastases at diagnosis, 33% of patients with CRPC develop metastases within 2 years of their diagnosis | |
| Survival | The median survival from CRPC diagnosis is 14 months |
Disease progression
Progression to CRPC
Our study identified five papers that evaluated the prevalence of CRPC in patients with prostate cancer. Estimates of the proportion of prostate cancer patients who develop CRPC varied from 9.5% overall to 53% within 5 years of follow-up from diagnosis (10,20). However, when we consider only those studies that defined CRPC in terms of a rise in PSA levels following castration, the prevalence is in the range from 10% to 20% over an approximate 5-year period. However, the data in the included studies do not allow a thorough evaluation of time-to-castration-resistance after the initiation of androgen suppression, or factors, such as metastases and treatment pathway, which may influence this. The results of our review do suggest that the prevalence is similar in those who have undergone prostatectomy and those who have not, and a previous study has also reported that prior prostatectomy does not confer a survival advantage in men with CRPC (22). However, further epidemiological research into the impact of disease stage at time of castration and treatment paradigm is required.
Metastatic CRPC
Despite the advances in PSA screening, and consequent increases in early detection, many patients still experience metastatic progression of the disease or have metastases present at the time of CRPC diagnosis. There is no clear temporal relationship between the development of metastases and the development of castration-resistance – either can occur first – and this is very dependent on treatment practice, because early commencement of castration therapy may result in resistance before the development of metastases, whereas late initiation of castration therapy may mean that patients are already further in disease progression and hence more likely to have metastatic disease at the point of CRPC. Given the impact that the presence of metastasis has on survival in CRPC, understanding the development and process of metastatic progression in patients with prostate cancer is important. Despite the paucity of data, the studies included in our review give an insight into the progression to metastatic disease.
The high prevalence (≥ 84%) of bone metastases at the point of CRPC diagnosis was revealed by two studies that met our inclusion criteria (13,14), although one of these used the presence of metastases as a defining characteristic of CRPC. Of patients who do not present with metastases at the time of CRPC diagnosis, Smith et al. showed that 33% of patients had developed bone metastases within 2 years (17). Similarly, Abouassaly et al. evaluated 4003 patients with prostate cancer (mean age 70 years) recorded in the US CAPSURE database, and found that 4.8% were shown to have progressed to metastatic disease at a median of 18 months from the initiation of androgen-deprivation therapy (23). A database study of more than 23,000 Danish patients with prostate cancer showed that 97% were free from metastases at prostate cancer diagnosis. However, within a median 2.2 year follow-up period, 11.5% of the patients had developed metastases (24). In an analysis of the UK THIN database, rate of first metastases was 34.4 per 1000 patient years for CRPC compared with 24.8 for non-CRPC patients (11).
Survival
This review confirms the poor survival associated with CRPC. Data from the US population-based SEER registries demonstrate that compared with patients who are diagnosed with early, localised disease, in whom 5-year survival approaches 100% (25), those with advanced prostate cancer at the time of diagnosis have a median survival of 24.5 months (26). In the CRPC populations included in this review, the median survival ranged from 9 to 30 months, and for those with metastatic disease this was reduced to 9–13 months, confirming the impact of metastases on survival, as also demonstrated in large randomised controlled trials (21).
Although inconsistent reporting on the use of chemotherapy prevents useful comparisons, our data indicate that the population in which chemotherapy use was most prevalent appeared to have the longest survival estimate (16). This may, however, be a reflection of a generally fitter population who were able to tolerate chemotherapy. The impact of age at diagnosis on the survival estimates could not clearly be evaluated from these data because of incomplete reporting of patient age.
Clinical implications
The data included in this review highlight the rapid and significant deterioration in quality of life experienced by patients after diagnosis with CRPC. Although CRPC is incurable, it is not untreatable, and the therapeutic options available to patients should be considered carefully.
Data from Sullivan et al. show the importance of palliative radiotherapy as a treatment approach in patients with metastatic CRPC (19), and one included study suggests that approximately two-third of patients with CRPC did not receive treatment beyond steroids and supportive care (14), perhaps reflecting a lack of acceptable therapeutic options for patients with CRPC. The extent of care and resources used by patients for whom only palliative options were given was highlighted in a study by Hwang et al. (18).
Secondary symptoms of prostate cancer were evaluated in three studies included in our review (13–15). Skeletal events, which may comprise metastatic disease or osteoporotic effects of hormone treatments, occurred in up to 89% of patients (27,28). The treatment recommendations from the EAU highlight that although chemotherapeutic options are available for patients with metastatic CRPC, there is a lack of cytotoxic therapy approved for use in patients with non-metastatic CRPC, and that such patients should be encouraged to participate in clinical trials (8). NICE recommends only treatment with docetaxel for CRPC, and only in those with a Karnofsky performance-status score over 60%, but docetaxel is associated with significant side effects, and its success is limited without careful management of these effects (6,29). While observational data from studies included in our review show that bisphosphonates are used palliatively in 16–49% of European patients (14,15), NICE recommends these only along with Stronium-89 for pain relief. Recently, the FDA approved the chemotherapy agent, cabazitaxel, for use in conjunction with prednisone for second-line CRPC therapy after docetaxel. Abiraterone has also recently been approved in combination with docetaxel in CRPC. The deteriorating quality of life and lack of desirable treatment available to patients with CRPC highlight the need for new therapeutic options.
Strengths and limitations
This is the first systematic review to explore the epidemiology of CRPC in real-world settings, and to characterise the progression of the patient population through a number of stages from prostate cancer diagnosis until death. It shows lack of epidemiological research that specifically characterises patients with CRPC, and the inconsistencies in methods and definitions used by such studies. Although practical guidelines for defining CRPC are now available, these have not been used consistently in the literature to date, and are difficult to apply retrospectively because of data limitations (3).
Our analysis was also limited by the inconsistent reporting of data between studies, for example, in terms of point prevalence vs. period prevalence, varying follow-up periods and index points (e.g. diagnosis of prostate cancer, date of prostatectomy, data of castration, to date of CRPC diagnosis). Incidence estimates were not reported by the studies included in this review. This highlights the need for consistent epidemiological reporting in future studies.
Conclusions and future work
This review demonstrates the high prevalence of CRPC in men with castrate prostate cancer, and the poor prognosis of these patients in terms of increased risk of bone metastases and shorter survival compared with castration-sensitive patients. As new therapeutic options are becoming available the inconsistencies and gaps in evidence highlighted here also emphasise the need for further well-designed, epidemiological studies of CRPC, using standardized methods and definition of CRPC and of metastatic disease, in order that this disease can be better understood and thus treated.
Author contributions
The review concept and design were developed jointly by all authors. All authors were involved in the data interpretation, and all provided input in each draft of the manuscript.
Acknowledgements
We thank Dr Catriona Turnbull, from Oxford PharmaGenesis™ Ltd, who provided writing support funded by AstraZeneca R&D, Alderley Park, UK.




