Lercanidipine is an effective and well tolerated antihypertensive drug regardless the cardiovascular risk profile: the LAURA Study
Summary
To determine whether the antihypertensive effectiveness of lercanidipine was independent of the different cardiovascular risk levels.
Patients with treated or untreated mild-to-moderate essential hypertension were included in a multicentre, prospective, non-comparative, open-label study. Patients received lercanidipine (10 mg/day, uptitrated to 20 mg/day) during 6 months.
A total of 3175 patients, age 63 ± 10 years, 51% women, were included. The cardiovascular risk was low in 237 patients, medium in 1396, high in 722, and very high in 820. At baseline, blood pressure (BP) was 159.5 ± 11.7/95.2 ± 7.4 mmHg. BP was progressively higher according to increase in cardiovascular risk. After 6 months of treatment, BP was 136.0 ± 9.7/79.7 ± 6.8 mmHg. The decrease in systolic BP and diastolic BP at each follow-up visit compared with baseline was statistically significant both in the intergroup and intragroup comparisons (p < 0.001). Mean decreases of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were −18.5/−13.8 mmHg in the low risk group, −23/−15.2 mmHg in the medium risk group, −24.4/−16.1 mmHg in the high risk group, and −27.4/−17.4 mmHg in the very high risk group. Most frequent side effects were oedema (5.1%), headache (3.3%), flushes (2.5%), and asthenia (1%). Only 1.7% of patients discontinued antihypertensive medication because of adverse events. Tolerability of lercanidipine was independent of the cardiovascular risk group.
Lercanidipine was effective and well-tolerated in patients with mild-to-moderate hypertension in the daily practice. The effectiveness and safety of the drug were independent of the degree of cardiovascular risk.
Introduction
Cardiovascular disease is responsible for a large and increasing proportion of death and disability worldwide. There is evidence that cardiovascular mortality decrease observed in industrialised countries in the second half of the 20th century has occurred concomitantly with an improved control of hypertension (1–3). However, > 70% of hypertensive patients have their blood pressure (BP) uncontrolled what may result a significant increase in cardiovascular risk (4,5). Even small elevations above optimal BP values increase the likelihood of developing hypertension and incurring target organ damage (6,7). Therefore, BP lowering is critical to help reduce the risk of cardiovascular disease and prevent major coronary events. Nevertheless, although the control of BP is important, clinical practice guidelines agree that the aim of treatment in hypertensive patients should be not only to control BP, but also protect the target organs affected by hypertension and reduce associated morbidity and mortality (8,9). The latest European guidelines also emphasises that the global assessment of cardiovascular risk in the hypertensive patient includes the identification of lesions caused by hypertension in the target organs. According to the clinical guidelines, the cardiovascular risk is defined by the presence of cardiovascular risk factors, target organ damage and associated clinical conditions (9).
Calcium channel blockers (CCB) clearly have a place within the therapeutic tools aimed to reduce cardiovascular risk. While earlier research were focused on increasing potency and selectivity, the most recent developments have brought dihydropyridinic CCB with a particularly slow onset and long duration of action which may result a better tolerability (10). Among them, lercanidipine is a third-generation vasoselective dihydropyridine (DHP) acting through the blockade of the L-type calcium channels in cell membranes (11–13). This drug has a high lipophilicity, which enables a slower and smooth onset and longer duration of action than other DHP (14). In some studies, lercanidipine appears to be a well-tolerated drug with a low adverse events rate because of its long-lasting and vascular-selective calcium entry-blocking activity, while sympathetic activation and reflex tachycardia is not induced (11,12). The overall side effects rate is lower than observed with other DHP (15,16). The efficacy of lercanidipine has been evaluated in non-comparative (17,18) and in comparative studies with other CCB and different antihypertensive drugs (19–23) showing a comparable effect with all of the cases. The effect of lercanidipine has also been successfully evaluated in severe or resistant hypertension, elderly subjects and diabetics (23,24).
From a clinical point of view, it should be of interest to know whether a BP-lowering drug is effective and well tolerated along the different cardiovascular risk profiles. This would facilitate its use in overall hypertensive population in daily practice. Thus, the LAURA study was aimed to assess whether the effectiveness and tolerability of lercanidipine may be different according to the cardiovascular risk level in a wide sample of a hypertensive population.
Patients and methods
The LAURA study (estudio del comportamiento de LercAnidipino segUn niveles de riesgo cArdiovascular) was a multicentre, prospective, observational, non-comparative, open-label study, designed to determine the effectiveness and safety of antihypertensive treatment with lercanidipine in patients drawn from the general population, with different cardiovascular risk profiles. The purpose of the study was to evaluate the drug in conditions of daily clinical practice, to determine whether its effect was independent of the cardiovascular risk level in a hypertensive population. The study was conducted in the Spanish primary healthcare setting in the summer time. A total of 500 family physicians were invited to recruit patients with mild-to-moderate essential hypertension (eight patients each) who according to their criteria were candidates to be treated with lercanidipine. The expected study cohort included 4000 patients. The duration of the study was 6 months.
Eligible patients were male and female aged ≥ 18 years with newly diagnosed hypertension, defined as systolic blood pressure (SBP) ≥ 140 (≥ 130 in diabetics) and < 180 mmHg, and diastolic blood pressure (DBP) ≥ 90 (≥ 80 in diabetics) and < 110 mmHg, or previously treated hypertensives in whom the physician had decided to switch the current therapy as a result of side effects or lack of control were eligible. The exclusion criteria were severe hypertension (SBP ≥ 180 mmHg or DBP ≥ 110 mmHg), known hypersensitivity or history of severe adverse events to any DHP, evidence of unstable angina or decompensated congestive heart failure, myocardial infarction within the previous 30 days, left ventricular outflow obstruction, liver dysfunction (serum aminotransferases > 2-fold increase or serum bilirubin > 1.5-fold increase above upper limit of normal), and renal insufficiency [serum creatinine concentration > 1.5 mg/dl (> 133 μmol/l) in men and > 1.4 mg/dl (> 124 μmol/l) in women], as well as any contraindication for prescribing lercanidipine as stated in the technical form of the product. Pregnant women, nursing mothers, or women of childbearing potential not using adequate methods of contraception were also excluded.
Blood pressure readings were taken with a mercury sphygmomanometer with the patient in a seated position and the back supported, and after resting 5 min. The patients were advised to avoid smoking or drinking coffee within 30 min before BP assessment. The visit BP was the average of two separate measurements taken by the examining physician (a third measure was obtained when there was a difference ≥ 5 mmHg between the two readings). Adequate BP control was defined as SBP < 140 mmHg and DBP < 90 mmHg (< 130 and < 80 mmHg for diabetics) (9).
All patients underwent a complete physical examination and investigation of other cardiovascular risk factors. With the available information about BP levels, associated cardiovascular risk factors, target organ damage and associated clinical conditions the patients were classified according to the ESH/ESC guidelines 2003 in the different added cardiovascular risk groups: low, medium, high or very high. According to the ESH/ESC guidelines 2003 (9), the following data were recorded: (a) cardiovascular risk factors: levels of SBP and DBP, age (men > 55 years, women > 65 years), smoking, dyslipidaemia [total cholesterol > 250 mg/dl (> 6.5 mmol/l) or LDL-cholesterol > 155 mg/dl (> 4.0 mmol/l) or HDL-cholesterol < 40 mg/dl (< 1.0 mmol/l) in men and < 48 mg/dl (< 1.2 mmol/l) in women], family history of premature cardiovascular disease (at age < 55 years in men and < 65 years in women), abdominal obesity (abdominal circumference ≥ 102 cm in men and ≥ 88 cm in women) and C-reactive protein ≥ 1 mg/dl; (b) target organ damage: left ventricular hypertertrophy (electrocardiogram: Sokolow–Lyons > 38 mm; Cornell > 2440 mm ms; echocardiogram: left ventricular mass index ≥ 125 in men and ≥ 110 g/m2 in women), ultrasound evidence of arterial wall thickening (carotid IMT ≥ 0.9 mm) or atherosclerotic plaque, slight increase in serum creatinine [1.3–1.5 mg/dl (115–133 μmol/l) in men and 1.2–1.4 mg/dl (107–124 μmol/l) in women], microalbuminuria [30–300 mg/24 h; albumin–creatinine ratio ≥ 22 mg/g (≥ 2.5 mg/mmol) in men and ≥ 31 mg/g (≥ 3.5 mg/mmol) in women]; (c) diabetes mellitus (fasting plasma glucose > 126 mg/dl (> 7.0 mmol/l) or postprandial plasma glucose > 198 mg/dl (> 11.0 mmol/l)]; (d) associated clinical conditions: cerebrovascular disease (ischaemic stroke, cerebral haemorrhage or transient ischaemic attack), heart disease (myocardial infarction, angina, coronary revascularisation or congestive heart failure), renal disease [diabetic nephropaty, serum creatinine > 1.5 mg/dl (> 133 μmol/l) in men and 1.4 mg/dl (124 μmol/l) in women or proteinuria (> 300 mg/24 h)], peripheral vascular disease, advanced retinopathy (haemorrhages or exudates, papilloedema).
The study medication was dispensed at the baseline visit. The daily dose was one tablet of lercanidipine 10 mg, taken in the morning, immediately after wake up. In previously treated hypertensive patients, a washout period of 7–10 days was required. Patients were followed at 4, 12, and 24 weeks after beginning of treatment with lercanidipine. At each visit, BP and heart rate were measured, treatment compliance was checked, and patients were interviewed for the occurrence of adverse events. Lercanidipine could be uptitrated to 20 mg/day if BP control was not attained at any visit. If BP was still uncontrolled after 20 mg other antihypertensive medication could be added. The recommendation of a reduced calorie diet and the prescription of hypocholesterolemic and hypoglycemic agents was left at the discretion of the physician. All adverse events were designated by the investigator as either drug or not drug-related. At the study end, effectiveness and tolerability of treatment with lercanidipine was assessed by the patients and the investigators as ‘poor’, ‘regular’, ‘good’ and ‘very good’. The study protocol is shown in Table 1.
| Procedure | Visit 0: baseline | Visit 1: 4 weeks | Visit 2: 12 weeks | Visit 3: 24 weeks |
|---|---|---|---|---|
| SBP, DBP, heart rate | X | X | X | X |
| Eligibility criteria | X | |||
| Bio-demographic data | X | |||
| Anamnesis | X | |||
| Physical examination | X | |||
| Blood tests | X | X | ||
| Assessment of cardiovascular risk | X | |||
| Study medication (lercanidipine) supplied | X | X | X | X |
| Adverse events | X | X | X | |
| Compliance with treatment | X | X | X |
- SBP, systolic blood pressure; DBP, diastolic blood pressure.
Statistical analysis
Categorical data are expressed as numbers and percentages and continuous data as mean and standard deviation (SD). The Student's t-test for paired and unpaired data was used to assess treatment effects on continuous variables. Categorical variables were analysed with the chi-square (χ2) test. To study differences in the quantitative variables over time as well as progression, or between group differences, the analysis of variance (ANOVA) for repeated or independent measurements was used. The analysis of covariance (ANCOVA) was used to assess the effect of lercanidipine in subsets of the study population divided according to low, medium, high and very high cardiovascular risk groups. Statistical significance was set at p < 0.05. The SPSS statistical software package for Windows (version 9.1) was used to analyse the data.
Results
A total of 3175 patients with a mean age of 63 ± 10 years were included in the study. 51% of patients were women. Table 2 shows the baseline characteristics of the overall study population. Remarkably, grade I hypertension was diagnosed in 43% of patients and grade II in 57% and baseline BP levels were SBP 159.5 ± 11.7 mmHg and DBP 95.2 ± 7.4 mmHg. With regard to the cardiovascular risk factors, the most frequent, after hypertension, was hypercholesterolemia (32% of patients). The most prevalent target organ damage was left ventricular hypertrophy (18% of patients). Finally, the most prevalent associated clinical conditions was ischaemic heart disease (10% of patients). The patients were stratified as follows: 237 patients (7.5%) at low cardiovascular risk, 1396 (44%) medium, 722 (22.7%) high, and 820 (25.8%) at very high risk.
| Baseline characteristics (n = 3175) | |
|---|---|
| Variables | |
| Biodemographic data | |
| Sex (male/female) | M: 49F: 51 |
| Age (years), mean ± SD | 63 ± 10 |
| BMI (kg/m2), mean ± SD | M: 28.2 ± 3.8F: 28.3 ± 4.2 |
| Waist circumference (cm), mean ± SD | M: 101.3 ± 12.2F: 96.8 ± 14.1 |
| Cardiovascular risk factors | |
| Grade I hypertension Grade II hypertension | 4357 |
| Hypercholesterolemia | 32 |
| Current smoking | 30 |
| Family history cardiovascular disease | 16 |
| Diabetes mellitus | 15 |
| Target organ damage | |
| Left ventricular hypertrophy | 18 |
| Atherosclerotic plaques | 7 |
| Mild renal impairment/microalbuminuria | 5 |
| Associated clinical conditions | |
| Ischaemic heart disease | 10 |
| Peripheral arterial disease | 6 |
| Congestive heart failure | 4 |
| Renal failure | 4 |
| Cerebrovascular disease | 3 |
| Advanced retinopathy | 1 |
| Cardiovascular risk | |
| Low risk | 7.5 |
| Medium risk | 44 |
| High | 22.7 |
| Very high | 25.8 |
| Clinical data | |
| SBP (mmHg), mean ± SD | 159.5 ± 11.7 |
| DBP (mmHg), mean ± SD | 95.2 ± 7.4 |
| Heart rate (bpm), mean ± SD | 68.4 ± 4.2 |
- M, male; F, female; BMI, body mass index; SBP, systolic blood pressure; DPB, diastolic blood pressure.
Table 3 shows the previous drugs and reasons for the use of lercanidipine. Treatment with lercanidipine was indicated by the investigators because of poorly controlled hypertension with previous agents in 46% of patients, as first therapy in naïve hypertensives in 38%, and resulting from adverse events related to antihypertensive drugs in 13%. Previous antihypertensive medications are indicated in Table 3.
| Data | Number of patients | Per cent |
|---|---|---|
| Total patients | 3175 | 100 |
| Naïve patients (newly treated) | 1207 | 38 |
| Previously treated with antihypertensive drugs | 1968 | 62 |
| Previous antihypertensive medication | ||
| Diuretics | 946 | 29.8 |
| Angiotensin converting enzyme (ACE) inhibitors | 914 | 28.8 |
| Beta-blockers | 268 | 8.4 |
| Calcium channel antagonists | 213 | 6.7 |
| Reasons to start treatment with lercanidipine | ||
| Poorly controlled blood pressure | 1461 | 46 |
| Adverse events | 413 | 13 |
| Other | 94 | 3 |
Changes in SBP and DBP during the study period in the overall population as well as in the different cardiovascular risk groups are shown in Table 4. At baseline, mean SBP was 159.5 ± 11.7 and DBP 95.2 ± 7.4 mmHg. Baseline BP was progressively higher in parallel with higher cardiovascular risk profile. After 6 months of treatment, mean SBP was 136.0 ± 9.7 and DBP 79.7 ± 6.8 mmHg. The decrease in SBP and DBP at each follow-up visit compared with baseline was statistically significant both in the intergroup and intragroup comparisons (p < 0.001, one-way ANOVA). The higher the cardiovascular risk level, the greater the BP reductions. Mean decreases in SBP and DBP were 18.5 ± 3.3 and 13.8 ± 2.3 mmHg in the low risk group, 23 ± 3.9 and 15.2 ± 2.7 mmHg in the medium risk group, 24.4 ± 4.0 and 16.1 ± 3.1 mmHg in the high risk group, and 27.4 ± 4.2 and 17.4 ± 3.2 mmHg in the very high risk group. Decreases in SBP and DBP in each cardiovascular risk group at the follow-up compared with baseline are shown in 1, 2. BP was controlled in 55% of patients treated with 10 mg/day of lercanidipine, while systolic BP was controlled in 60.7% of patients, and diastolic BP in 71.0%. In consequence, 45% of patients were uptitrated to 20 mg/day of lercanidipine. After having uptitrated to 20 mg/day of lercanidipine, BP was controlled in 82% of patients, while systolic BP was controlled in 85.4% of patients, and diastolic BP in 89.1%. 18% of patients needed to add other antihypertensive drugs to achieve BP goal.
| Blood pressure, mean ± SD | All patients, n = 3175 | Cardiovascular disease risk groups | |||
|---|---|---|---|---|---|
| Low, n = 237 | Medium, n = 1396 | High, n = 722 | Very high, n = 820 | ||
| Visit 0 (baseline) | |||||
| SBP, mmHg | 159.5 ± 11.7 | 149.5 ± 5.4 | 158.3 ± 9.7 | 159.7 ± 10.9 | 164.2 ± 14.5 |
| DBP, mmHg | 95.2 ± 7.4 | 92.0 ± 4.8 | 94.8 ± 6.7 | 95.3 ± 7.3 | 96.7 ± 8.6 |
| Visit 1 (4 weeks) | |||||
| SBP, mmHg | 144.9 ± 11.5 | 136.8 ± 7.7 | 144.4 ± 10.5 | 145.5 ± 11.5 | 147.9 ± 12.8 |
| DBP, mmHg | 85.6 ± 7.5 | 82.9 ± 6.1 | 85.6 ± 7.2 | 85.8 ± 7.6 | 86.3 ± 8.1 |
| Visit 2 (12 weeks) | |||||
| SBP, mmHg | 138.9 ± 10.1 | 133.4 ± 6.7 | 138.5 ± 9.4 | 139.3 ± 10.1 | 140.9 ± 11.5 |
| DBP, mmHg | 81.9 ± 6.9 | 80.3 ± 6.4 | 81.9 ± 6.6 | 81.9 ± 6.8 | 82.3 ± 7.8 |
| Visit 3 (24 weeks) | |||||
| SBP, mmHg | 136.0 ± 9.7 | 130.7 ± 6.9 | 135.9 ± 9.1 | 136.1 ± 8.7 | 137.5 ± 11.3 |
| DBP, mmHg | 79.7 ± 6.8 | 78.3 ± 6.8 | 79.7 ± 6.5 | 79.8 ± 6.3 | 79.8 ± 7.5 |
- SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure.
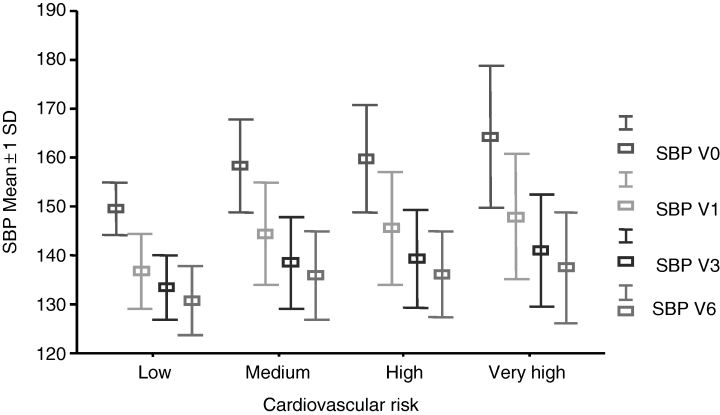
Mean ± 1 SD decreases in systolic blood pressure (SBP) in the four risk groups for cardiovascular disease during the study period when compared with baseline
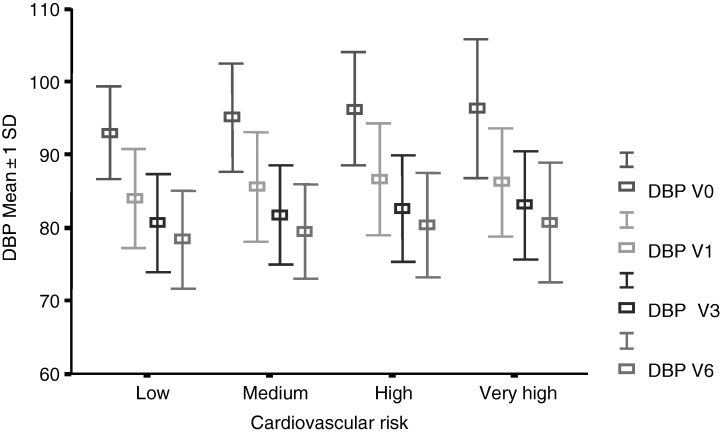
Mean ± 1 SD decreases in diastolic blood pressure (DBP) in the four risk groups for cardiovascular disease during the study period when compared with baseline
Ninety-four per cent of patients completed the 6-month treatment period with lercanidipine. The incidence of adverse events is shown in Table 5. As much as 11.5% of patients presented adverse events, being the most frequent the oedema (5.1%, more frequent with the 20 mg dose). But only 1.7% of patients discontinued the medication because of adverse events. No significant differences in the percent of patients suffering from adverse events according to stratification in the different risk groups were observed. As much as 91% of physicians and 84% of patients considered that tolerability of antihypertensive treatment with lercanidipine was ‘good’ or ‘very good’.
| Adverse events | Per cent |
|---|---|
| Total adverse events | 11.5 |
| Oedema | 5.1 |
| Headache | 3.3 |
| Flushing | 2.5 |
| Asthenia | 1 |
Discussion
The present results obtained in a cohort of patients with mild-to-moderate essential hypertension recruited in actual conditions of daily clinical practice confirm the effectiveness and favourable tolerability profile of lercanidipine. These findings are consistent with data previously reported in randomised trials (10,24) and in surveillance studies such as the ELYPSE study (17).
Previous studies have shown that the majority of hypertensive patients daily attended in Primary Care setting in Spain belong to the medium or high coronary risk groups (25,26). This point is relevant, because these patients are normally polymedicated and they have an increased risk of presenting side effects. The efficacy of an antihypertensive drugs does not only depend on BP control, but in its tolerability too. The presence of adverse events may be one of the main causes for the poor patient compliance of the prescribed therapy. Thus, the use of well tolerated drugs may result in a better patient adherence and probably in a better BP control (27–29). On the other hand, in usual care the different antihypertensive drugs very rarely achieve BP control in > 30–40% when used in monotherapy, and these figures are much lower when considering BP control in high coronary risk groups (25,26).
Our results indicates that lercanidipine has a good antihypertensive effectiveness among the different degrees of cardiovascular risk. In fact, lercanidipine showed to be more effective in patients with higher cardiovascular risk levels, most likely to be in relation to higher SBP and DBP values at baseline. Mean decreases of SBP and DPB were −18.5/−13.8 mmHg in the low risk group compared with −27.4/−17.4 mmHg in the very high risk group. Moreover, the majority of patients were controlled only with lercanidipine (18% of patients needed to add another antihypertensive drug to achieve a good BP control) including high risk group. In this large study, the efficacy of this drug has therefore proven to be similar to the previously published papers (17–23).
On the other hand, although the BP lowering effect of lercanidipine was greater in patients with higher cardiovascular risk levels, tolerability of the drug was independent of the cardiovascular risk profile. Therefore, lercanidipine was found to be an effective and well-tolerated antihypertensive drug for any hypertensive patient in daily clinical practice, regardless the cardiovascular risk profile. This represents an added value at the time of prescribing this antihypertensive medication in all kind of hypertensive patients, from low to high coronary risk. This good tolerability profile implies a low withdrawal rate, indicating a satisfactory patient compliance.
Lercanidipine appears to be associated with a better tolerability profile and less risk of vasodilation-related adverse reactions compared with other DHPs in the clinical practice setting (15,16,19–21). However, the percentage of patients with oedema was superior in our patients compared with others treated with lercanidipine, probably because our study was performed in the summer time. As it is known, the presence of oedema is more frequent during this season because of a bigger trend to the vasodilatation.
In conclusion, despite the limitations that this kind of observational intervention studies which are open and non-comparative, the LAURA study demonstrates that Lercanidipine is an a effective and well-tolerated antihypertensive agent in daily clinical practice, regardless the cardiovascular risk profile. These results confirm the previous findings from randomised controlled trials, and support that this drug is a fair option to be considered in the antihypertensive armamentarium.
Disclosures
Dr A. Navarro is the medical director of Recodarti Spain, the pharmaceutical industry proprietary of the study drug lercanidipine. The affiliation of this authors is clearly disclosed in the by line. All data have been recorded and analysed independently to prevent bias.
Acknowledgement
The assistance of Marta Pulido, MD, in writing and editing this article is appreciated.




