Brachmann-de Lange syndrome with congenital diaphragmatic hernia and NIPBL gene mutation
ABSTRACT
We report herein a case of Brachmann-de Lange syndrome complicated with congenital diaphragmatic hernia in which a NIPBL gene mutation was identified. A female infant born at 37 weeks of gestation died 134 min after delivery, even though endotracheal intubation and resuscitation were performed immediately after the scheduled caesarean operation. We diagnosed the infant with Brachmann-de Lange syndrome from her physical characteristics. An abnormal peak at the 29th exon in the translation area of the NIPBL gene was detected using denaturing high-performance liquid chromatography. In addition, a mutation of cytosine to thymine (nonsense mutation) at the 5524th base was identified using the direct sequence method. This variation was likely the cause of the syndrome.
INTRODUCTION
Brachmann-de Lange syndrome (BDLS) is a multiple congenital anomaly syndrome characterized by growth and mental retardation, variable anomalies of the upper limbs and a peculiar face with hypertrichosis. A pediatrician named de Lange (1933) reported two cases of this disease while working at Amsterdam University in the Netherlands, and termed the disease Cornelia de Lange syndrome. It was subsequently revealed that Brachmann (1916) had reported on a patient exhibiting the same symptoms. As a result of these two reports, the condition is currently known as Brachmann-de Lange syndrome (Opitz 1985).
Brachmann-de Lange syndrome was originally thought to be related to 3q partial trisomic syndrome, as the clinical manifestations of the two diseases are relatively similar. More recently, Krantz et al. (2004) and Tonkin et al. (2004) reported a variation in the NIPBL gene in a BDLS patient, allowing the two diseases to be more easily distinguished.
We report herein a case of BDLS with congenital diaphragmatic hernia caused by a mutation in the NIPBL gene that was identified using denaturing high-performance liquid chromatography.
Case report
A 21-year-old woman delivered a female infant at 37 weeks and 2 days of gestation by scheduled caesarean operation due to intrauterine growth retardation and congenital diaphragmatic hernia diagnosed by fetal echography at a gestational age of 30 weeks and 2 days. The infant's birthweight was 1766 g (–2.6 SD) and her Apgar score was 1 at 1 min and 3 at 5 min. When the infant was born, her entire body was pale and she did not demonstrate spontaneous breathing patterns. Endotracheal intubation was immediately performed and artificial ventilation with high frequency oscillation (HFO) and nitric oxide inhalation therapy was initiated. Unfortunately, there was no improvement in her condition, even following the administration of resuscitative medication, including adrenaline and surfactant, and she died 134 min after birth.
We considered that the patient had BDLS due to her characteristic facial features, including synophrys, brachyrrhinia, long philtrum, thin lip, small mandible and short cervix, and the presence of hirsutism and a congenital diaphragmatic hernia. Although her limbs were small and short, and a bilateral single transverse palmar crease was recognized on each hand, the BDLS characteristics of syndactyly and limb reduction defects were not observed (Fig. 1).
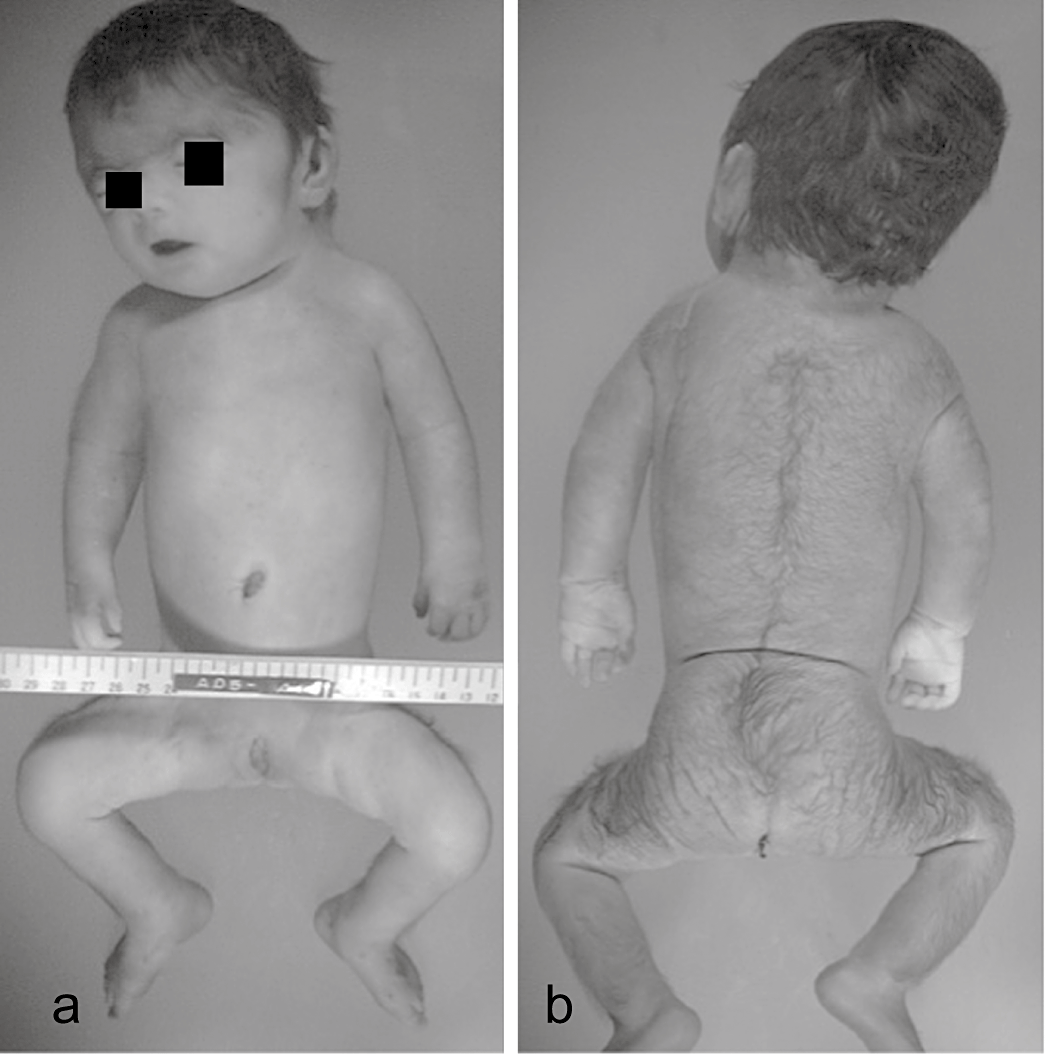
Photographs highlighting the patient's symptoms (a,b) including hypertrichosis, short extremities, hypoplasia of the nipple and umbilicus, synophrys of the face, short and upturned nose or anteverted nostrils, long philtrum, thin lip, small mandible, short cervix and single bimanual palmar flexion curve without syndactyly or defects of the fingers.
At laboratory examination at birth, we identified slight acidosis; however, significant abnormal findings, including anemia and electrolyte imbalance in the cord blood were not observed. The infant's blood gas (venous blood) at 47 min after birth was also recognized as mixed acidosis of pH 6.763, PCO2 188.0 mmHg, PO2 3.2 mmHg and BE –16.5 mmol/L. Her hemoglobin was 6.8 g/dL, her C-reactive protein (CRP) was negative and there was no elevation in liver enzyme levels. Hypernatremia was observed in her electrolytes (Table 1). Amniotic fluid chromosomes were of a normal karyotype of 46, XX. X-ray of the entire body revealed a hanging bell-shaped thoracic cage, low pneumatization in the bilateral lungs and a stomach bubble in the middle thorax (Fig. 2).
| Cord blood | Patient's blood (47 min after birth) | |
|---|---|---|
| Blood gas analysis | ||
| pH | 7.276 | 6.763 |
| pCO2 | 48.9 mmHg | 188.0 mmHg |
| pO2 | 20.5 mmHg | 3.2 mmHg |
| Base excess | −4.3 mmol/L | −16.5 mmol/L |
| Blood cell counts | ||
| White blood count | 5900/µL | 4900/µL |
| Platelet count | 221 000/µL | 93000/µL |
| Chemistry | ||
| C-reactive protein | <0.05 mg/dL | <0.1 mg/dL |
| Sodium | 140 mmol/L | 183 mmol/L |
| Potassium | 4.5 mmol/L | 5.3 mmol/L |
| Calcium | 9.5 mg/dL | 8.3 mg/dL |
| Hemoglobin | 13.6 g/dL | 6.8 g/dL |
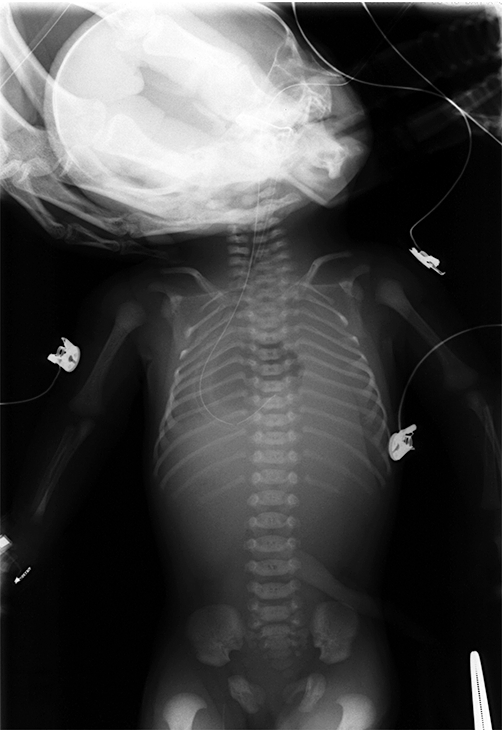
X-ray of the entire body showing the hanging bell-shaped thoracic cage, low pneumatization in the bilateral lungs and a stomach bubble located in the middle thorax.
Pathological autopsy of the infant was undertaken after we obtained informed consent from her parents. The placental weight was 190 g, which was small for the number of gestational weeks (our center average is 514 g), villi were immature and the umbilical cord contained a single umbilical artery. The left diaphragm was almost entirely defective and the liver, stomach, spleen, pancreas, small intestine and large intestine protruded into the intrathoracic area. Marked hypoplasia of the lungs was also recognized with a pulmonary weight ratio of 0.003 (normal is 0.012). In addition, the lungs were histologically immature. Bilateral hydroureter, annular pancreas and atrial septal defect were also observed. We did not examine the brain, as the parents did not consent to craniotomy.
After we obtained written informed consent from the parents for gene diagnosis, we extracted genomic DNA from the patient's blood and amplified the coding region (extending from the 2nd exon to the 47th exon) of the NIPBL gene using polymerase chain reaction (PCR). An abnormal peak in exon 29 was detected when analyzed using denaturing high-performance liquid chromatography (Fig. 3). Within the translation area of the NIPBL gene, a mutation of cytosine (C) to thymine (T) (nonsense mutation) at the 5524th base was identified using the direct sequence method. This amino acid change formed a stop codon, a result that we hypothesized would influence the complications in this patient.
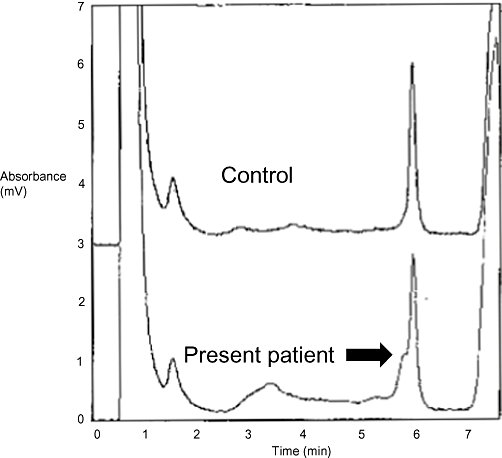
Denaturing high-performance liquid chromatography of the 29th exon of the NIPBL gene (upper panel: control, lower panel: patient). Arrow shows the abnormal peak in the translation area (29th exon) of the NIPBL gene.
DISCUSSION
Cornelia de Lange (1933) identified 10 traits, such as mental retardation, low birthweight, dwarfism, microbrachycephaly, heavy eyebrows meeting at the midline, long eyelashes, low-set ears, small hands and feet, proximal placed thumb and syndactyly of the toes in two patients while working at Amsterdam University. Beck (1976) later reported the original diagnostic standards of BDLS (Table 2) and suggested that patients with BDLS could be diagnosed if they exhibited eight of these 10 traits. In the current case, BDLS was not diagnosed in the fetal period, but was diagnosed after birth. The infant demonstrated nine of the traits described by de Lange and five of the traits described in the Beck standards. After confirming our findings with both the de Lange and Beck standards, we finally made a diagnosis based on the baby's physical characteristics.
| This patient's findings | |
|---|---|
| Cornelia de Lange (1933) | |
| Mental retardation | ? |
| Low birthweight | + |
| Dwarfism | ? |
| Microbrachycephaly | + |
| Heavy eyebrows meeting at the midline | + |
| Long eyelashes | + |
| Low ear insertion | − |
| Small hands and feet | + |
| Proximally placed thumb | − |
| Syndactyly of the toes | − |
| Beck (1976) | |
| Low hair line on forehead | + |
| Low hair line on neck | + |
| Long philtrum | + |
| Bushy eyebrows | + |
| Confluent eyebrows | + |
| Thick eyelashes | + |
| Antimongoloid eye slanting | − |
| Anteverted nostrils | + |
| Crescent-shaped mouth | + |
| Thin prolabium | + |
- +, present; ?, not detected due to early death.
This patient was also diagnosed based on the presence of intrauterine growth retardation and diaphragmatic hernia during the fetal period. Limb shortening was also observed. In the absence of abnormal karyotype or altered bone structures with limb shortening, BDLS is generally considered as a differential diagnosis (Beck and Fenger 1985; Kenneth 1988). Further, the placenta weighed only 190 g, which was low for the gestational period. This finding was consistent with the hypothesis that growth of not only the fetus, but also of the placenta is inadequate in cases of BDLS.
There have been only a few reports of BDLS with congenital diaphragmatic hernia in Japan (Kuroiwa et al. 1990; Suzuki et al. 1999). A small number of reports (e.g. Cunniff et al. (1993), Russel et al. (1993) and Marino et al. (2002)) have been described in other countries. The reports by these groups suggested that the prognosis was worse when the patient also exhibited congenital diaphragmatic hernia. The precise causes of congenital diaphragmatic hernia remain unknown. BDLS, Fryns syndrome, Goltz syndrome and Smith-Lemli-Opitz syndrome are all associated with congenital diaphragmatic hernia (Tibboel and Gaag 1996; Bianch et al. 2000). Recently, gene analysis of these various multiple malformation syndromes has been undertaken (Holder et al. 2007). Further gene analyses in the various multiple malformation syndromes specifically associated with congenital diaphragmatic hernia are likely to shed light on which anomalies lead to diaphragmatic hernia.
In the present case, a mutation of C to T (nonsense mutation) at the 5524th base in the translation area of the NIPBL gene was identified. As a result, we concluded that this variation was likely to be the cause of the BDLS with diaphragmatic hernia. The NIPBL gene is located at 5p13.1 and contains 47 exons, and its transcription is thought to be related to Notch signal transmission. There have been many confirmed gene mutations, including deletion and insertion mutations, that are associated with BDLS (Gillis et al. 2004; Bhuiyan et al. 2006; Schoumans et al. 2007). Further, Musio et al. (2006) and Deardorff et al. (2007) have presented reports relating BDLS to both SMC1 and SMC3 gene mutations.
DNA analysis is important for confirming BDLS diagnosis. Analysis of gene mutations in genes such as NIPBL also represents a useful diagnostic method. With the accumulation of cases such as ours, further description of this disease will be possible.




