THE VALUE OF RADIOGRAPHIC SCREENING FOR METALLIC PARTICLES IN THE EQUINE FOOT AND SIZE OF RELATED ARTIFACTS ON LOW-FIELD MRI
This study was supported by Hallmarq Veterinary Imaging Ltd, who provided the scanning system and technical support.
Parts of this study were presented at the Hallmarq annual MRI user meeting, January 9–10, 2010, Chamonix, France and the European Veterinary Diagnostic Imaging Annual Scientific Conference, July 20–24, 2010 in Giessen, Germany.
Abstract
Magnetic susceptibility artifacts as a result of metal debris from shoeing are a common problem in magnetic resonance imaging of the equine foot. Our purpose was to determine the suitability of radiography as a screening tool for the presence and location of metallic particles in the equine foot and to predict the size of the resultant magnetic susceptibility artifact. Radiography had 100% sensitivity for detection of metal particles ≥1 mm diameter. Metal particles of known diameter were placed within the hoof wall of 22 cadaver feet and scanned with a low-field strength MR imaging unit (0.21 T). Magnetic resonance images were characterized by a signal void with a hyperintense rim and adjacent image distortion at the level of the known metal location. T2* weighted sequences were the most and fast spin echo (FSE) sequences the least affected. For all four sequences (T1 gradient echo [GRE]; T2*W GRE; T2 FSE; and short tau inversion recovery FSE), linear relationships were observed between particle and resultant artifact size. Magnetic susceptibility artifact size, location and superimposition on clinically relevant anatomic structures can be predicted radiographically for particles larger than 1 mm. If metal debris cannot be removed, the least artifact-prone FSE sequences should be selected.
Introduction
Metal creates magnetic susceptibility artifacts in magnetic resonance (MR) imaging that are characterized by image distortion and large areas of signal void.1 This creates a problem In equine digit MR imaging due to metal debris related to shoeing, even after shoe removal.2 Current practice is to perform radiographic screening and to remove metal debris before MR imaging. However, this can be challenging if particles are located deep in the nail hole or at other locations. Radiography has not been validated for metallic screening, nor has the relationship between particle size and resulting artifact size been established.
Our purpose was to determine radiographic sensitivity for the presence of metal debris, ascertain its location in the foot radiographically and to predict the artifact magnitude.
Materials and Methods
Twenty-three unshod front and rear cadaver horse feet from horses undergoing euthanasia were harvested and radiographed immediately afterwards using computed radiography technology. Dorsoproximal-palmarodistal oblique [DPr-PaDiO] and lateromedial [LM] projections were acquired. Radiographs were reviewed for pre-existing metal fragments. If fragments were present, these would be attempted to be removed and the foot re-radiographed to confirm the absence of metal debris. Radiographic scoring (yes/no) for the absence of metal particles was performed again on all final radiographs. Then a standard foot MR imaging examination using a low-field unit (0.21 T) designed for standing equine imaging1 was performed. T1-weighted (T1W) gradient echo (GRE), T2* weighted (T2*W) GRE and short tau inversion recovery (STIR) fast spin echo (FSE) sequences were acquired in sagittal, transverse, and dorsal planes.3, 4 The T2W FSE sequence was obtained only in the transverse plane (Table 1). The images were also scored (yes/no) for presence of magnetic susceptibility artifacts.
| Sequence | S Thick/Gap (mm) | TR (ms) | TE (ms) | Flip Angle (°) | Acq. Time (Min:s) | TI | FOV (mm) |
|---|---|---|---|---|---|---|---|
| T1W GRE SAG | 5/1 | 120 | 8 | 75 | 02:58 | N/A | 180 × 180 |
| T2*W GRE SAG | 5/1 | 135 | 13 | 32 | 04:49 | N/A | 180 × 180 |
| STIR FSE SAG | 5/1 | 2910 | 27 | 90 | 03:18 | 67 ms | 180 × 180 |
| T1W GRE TRA | 5/1 | 120 | 8 | 75 | 02:14 | NA | 180 × 180 |
| T2*W GRE TRA | 5/1 | 135 | 13 | 32 | 03:36 | NA | 180 × 180 |
| T2W FSE TRA | 5/1 | 1920 | 84 | 90 | 03:47 | NA | 180 × 180 |
| STIR FSE TRA | 5/1 | 2910 | 27 | 90 | 03:18 | 67 ms | 180 × 180 |
| T1W GRE FRO | 5/1 | 120 | 8 | 75 | 02:14 | NA | 180 × 180 |
| T2*W GRE FRO | 5/1 | 135 | 13 | 32 | 03:36 | NA | 180 × 180 |
| STIR FSE FRO | 5/1 | 2910 | 27 | 90 | 03:18 | 67 ms | 180 × 180 |
- S Thick/Gap, slice thickness and gap between slices; TR, repetition time; TE, echo delay time; Acq. time, acquisition time; FOV, field of view; TI, time from inversion; MRI, magnetic resonance imaging; GRE, gradient echo; FSE, fast spin echo; STIR, short tau inversion recovery; NA, not applicable.
After the initial screening procedure, metal particles (stainless steel ball bearings, a ball made from a shoe nail, and shoe nail fragments) were placed in preexisting nail holes, at approximately 2 cm proximal to the sole. If no nail hole was present, one was created using a manual drill in a location consistent with shoeing nail holes. After drilling, the cavity was flushed thoroughly and cleaned to reduce metal burr fragments. Stainless steel ball bearings2 with known diameter (1, 2, 3, and 5 mm) were placed into the most dorsomedial and/or palmaro(plantaro)-lateral nail hole, and DPr-PaDiO and LM radiographs were obtained again. The radiographic and MR imaging protocol was then repeated six times for each metal particle. For each MR imaging examination, the metal particle that was present from the previous MR imaging examination was removed from the foot and then placed back either into a different foot or into the same foot in a different location. This selection was performed randomly. Then the radiographic and MR imaging protocol was performed six times with a 5 mm diameter ball that was specifically made by a goldsmith from one shoe nail,3 each time using a different leg. Five extra feet were imaged, each with one nail fragment in situ obtained from standard shoe nails of 2 mm width (standard nail distal width) cut at 2, 3, 4, and twice 10 mm length to demonstrate the artifact magnitude for nonspherical debris similar to a clinical setting.
Using a dedicated DICOM imaging workstation4 radiographs and MR images with inserted metal particles were evaluated by a board certified radiologist. Radiographic size calibration was performed to adjust for magnification. Radiographs were scored for presence, diameter, and location of metal particles using both projections. MR images were scored for the presence of a magnetic susceptibility artifact, defined as an area of signal void.5 In MR images, the maximum signal void dimension was measured in all sequences using a circle shaped region of interest tool which was applied in a consistent manner with predetermined window settings, making sure the visible sharp edges of the void area were used as ROI edges. All radiographic and MR image measurements were rounded up to the nearest millimeter. Measurements obtained with the 5 mm stainless steel ball bearings were compared to those obtained from the shoe nail ball of similar size.
Radiographic sensitivity for the detection of inserted round metal particles was calculated according to the standard formula (true positives/true positives+false negatives) using scoring results from the radiographs of feet with known size metallic particles.
Standard linear mixed-effect models of particle size against the resultant artifact size in individual feet were carried out for four sequences (T1W GRE, T2*W GRE, and STIR FSE in dorsal, sagittal and transverse planes, and T2W FSE in the transverse plane). The foot that the particle was inserted into was entered as a random effect to account for the fact that some feet were used more than once. In addition, a similar linear mixed-effect model was also run comparing the mean resultant artifact sizes between the four sequences. To compare the resultant artifact size from the 5 mm stainless steel ball bearing to that of the 5 mm shoe nail ball the same 6 ft were used and so standard paired t-tests were carried out on the four sequence data. All analyses were carried out in R.5 P<0.05 was taken to indicate statistical significance.
Results
Metal debris was not found on the initial radiographic screening examination in any of the 23 feet. Therefore a second radiographic screening was not necessary. A 10 mm magnetic susceptibility artifact was present in one foot, which was not visible radiographically. This foot was discarded. The 22 artifact-free feet were used for the remainder of the study. Radiographic identification of all placed metal particles was achieved on all projections, resulting in a 100% radiographic sensitivity (95% CI: 84–100%) for the detection of round metal particles ≥1 mm. Radiographic measurements of all metal particles corresponded to their known diameter within a millimeter range. Radiographic fragment localization corresponded well to the known location of the fragment using the LM projection for the proximodistal and the DPr-PaDiO projection for the lateromedial fragment position determination (Fig. 1).

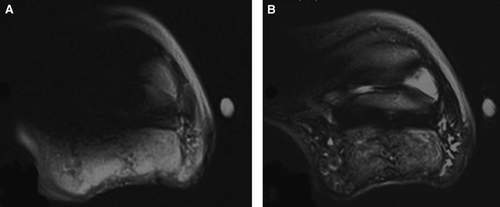
A magnetic susceptibility artifact was created by all placed metal particles (Fig. 2). In FSE images there was distortion in adjacent areas (Fig. 3). The artifact size was dependent on the image sequence and particle diameter (Table 2). For nonspherical particles, the artifact size depended on the maximum particle dimension and the sequence. For all metal particle sizes, the largest void signal diameter was observed in T2*W GRE and T1W GRE sequences (Fig. 4). In contrast STIR FSE sequences always had the smallest void signal diameter, with a statistically significant difference between STIR FSE and T2*W GRE sequences (P=0.023, Fig. 4). Furthermore, the relationship between particle and artifact size followed a statistically significant positive linear relationships for all sequences (Table 3, Fig. 5).



| Ball Diameter | Sequence | Median | Mean |
|---|---|---|---|
| 1 mm | T1W GRE | 15 | 12.22 |
| T2*W GRE | 10 | 11.39 | |
| T2W FSE | 5 | 4.50 | |
| STIR FSE | 1 | 2.78 | |
| 2 mm | T1W GRE | 20 | 18.61 |
| T2*W GRE | 25 | 22.22 | |
| T2W FSE | 15 | 14.17 | |
| STIR FSE | 15 | 15.28 | |
| 3 mm | T1W GRE | 40 | 39.72 |
| T2*W GRE | 45 | 43.61 | |
| T2W FSE | 32.5 | 32.50 | |
| STIR FSE | 30 | 29.17 | |
| 5 mm | T1W GRE | 62.5 | 64.17 |
| T2*W GRE | 75 | 72.22 | |
| T2W FSE | 60 | 62.50 | |
| STIR FSE | 50 | 51.39 | |
| 5 mm shoe nail | T1W GRE | 60 | 59.72 |
| T2*W GRE | 70 | 66.94 | |
| T2W FSE | 47.5 | 51.67 | |
| STIR FSE | 45 | 45.56 |
| Imaging Sequence | Formula | P-value |
|---|---|---|
| T1 W GRE | A= −4.26+13.6φ | 0.020 |
| T2* W GRE | A= −6.02+15.5φ | 0.001 |
| T2W FSE (transverse only) | A= −12.52+14.9φ | 0.001 |
| STIR FSE | A= −7.38+11.5φ | 0.001 |
- A, maximal artifact dimension; φ, maximal metal particle dimension; P, P-value from the linear mixed-effect analyses; GRE, gradient echo; FSE, fast spin echo; STIR, short tau inversion recovery.
Comparing the 5 mm stainless steel ball bearing and 5 mm shoe nail ball images for artifact size, the artifact magnitude and MRI sequence dependent minima and maxima were not statistically different (P>0.057 for all four sequences, Table 2, Fig. 5).
Comparing artifact magnitude between the different image planes, there was no difference in artifact size for any ball-shaped metal particle. For the nonspherical shoe nail fragments, the largest void signal was always in the transverse plane on all sequences. The T2W FSE sequences were not assessed as they were only obtained in the transverse plane.
Discussion
As expected, radiography is a very sensitive tool to detect or rule out the presence of metallic debris larger than 1 mm, which would create a visible artifact on MR images. Specificity could not be determined with our methodology. Our results indicate that two orthogonal radiographic views are necessary to correctly assess metallic debris for its artifact potential. Whereas the DPr-PaDiO view may be sufficient to localize a nail fragment to a specific nail hole, the LM radiograph allows determination of the depth of the fragment in the nail hole which is essential for removal. Two views are also necessary to assess the maximum dimension of a nail fragment as in a clinical setting fragments would rarely be spherical. To predict the artifact size accurately, radiographs should be size-calibrated.
Magnetic susceptibility is the ratio of induced internal magnetization in a tissue to the external magnetic field strength. The internal magnetic field distorts the linear magnetic field gradient and causes signal void and image distortion. Metallic objects induce artifacts in MR images via two mechanisms, ferromagnetism and eddy currents. Ferromagnetic elements such as iron disturb the static magnetic field through their own magnetic properties. Steel is only weakly ferromagnetic but it is a conductive electric medium. The changing magnetic field from the gradient coils, as they are switched on and off, induces a current on the surface of the metallic object which in turn creates a small magnetic field that opposes and distorts the gradient field.6 The signal void from shoe nail fragments is at least partially created by eddy currents. The presence and appearance of magnetic susceptibility artifacts in our study is similar to other metal induced artifacts.7-9 The artifact magnitude is strongly correlated to field strength5, 10 and therefore our results only apply to units of similar field strength and design. However, the MR unit used in this study is used widely in horses.
T2*W GRE sequences were characterized by the largest signal void as these sequences are most susceptible to artifacts. This is due to the T2*W relaxation process where signal decay depends entirely on the magnetic field inhomogeneities.11 Fast spin echo sequences are less susceptible to magnetic field inhomogeneity by applying multiple 180° flip angles and thus minimizing the effect of field inhomogeneities.11
Based on our results, a relatively simple calculation can be performed to predict the size of a magnetic susceptibility artifact from a small metallic particle with low-field MR imaging. In general, one can expect an approximate 10 × larger artifact from a metal particle within this size range in T2*GRE gradient echo sequences. Furthermore, particle sizes of greater than 5 mm diameter are likely to result in a signal void encompassing all of the foot, resulting in a nondiagnostic study. As the depth was approximately the same, the effect of proximodistal location was not observed. To determine the particle-to-artifact size relationship irrespective of the random orientation of the particle to the selected image plane, spherical particles were used. Stainless steel ball bearings were used because of their known diameter available in sizes smaller than 5 mm. However, since these ball bearings may not have the same ferromagnetic properties as equine shoe nails the experiment was repeated with original shoe nail fragments, one of which was made as a ball. Our results indicate that steel balls have artifact-inducing properties similar to shoe nail fragments and are thus representative of the clinical setting. The fact that transversely oriented images consistently had the largest artifact is probably related to the fact that in this orientation the metal particle was more likely to be fully included in the selected slice width. Adaptations in the standardized imaging protocol for a horse with a nonremovable metallic particle should be considered to reduce the artifact magnitude.
In conclusion, magnetic susceptibility artifacts of diagnostic relevance (≥1 and ≤5 mm in diameter) can be predicted reliably with screening radiography. A metallic artifact does not always rule out a diagnostic MR examination. Clinicians should determine the size and position of nail debris radiographically and attempt to remove the particle. If this is unsuccessful the anatomic area of interest can be mapped radiographically against the expected artifact location and size. Fast spin echo sequences will minimize the magnitude of magnetic susceptibility artifacts.
ACKNOWLEDGMENTS
We would like to thank Roy W. Macdonald for creating a ball from a shoe nail and Hallmarq Veterinary Imaging Ltd. for their continuous support for this study.