STATISTICAL BRIEFING: OBSERVATIONAL STUDY DESIGNS
Studies done to assess how well a diagnostic test discriminates affected vs. nonaffected patients usually involve observing the differences between a group of patients that has the condition under study and a group without the condition (the control group). Such studies are known as observational studies.1,2 Observational studies include cohort, case–control, and cross-sectional designs (Table 1). Observational studies may be contrasted with experimental studies (clinical trials), in which subjects receive a treatment or exposure that is under the control of the investigator.
| Design | Definition | Effectmeasure |
|---|---|---|
| Cohort | Comparison of incidence of outcomes that occur over a period of time in patients with variable under study and patients without variable (may be prospective or retrospective) | Relative risk (risk ratio) |
| Case-control | Study to compare the relative proportions of affected patients and control patients that have the variable under study Retrospective only | Odds ratioLikelihood ratio |
| Cross-sectional | Comparison of prevalence of variables under study in a single group of patients subsequently determined to either have disease/exposure or not (may be prospective or retrospective) | Odds ratioLikelihood ratio |
In cohort, case–control, and cross-sectional designs, a representative sample of patients is studied as a basis for reaching conclusions that should be applicable to the population from which the sample is drawn. In cohort studies, subjects at risk for the outcome under study are identified, data are collected to determine the presence or absence or magnitude of the variable(s), and all the subjects are monitored over a period of time to identify any associations between the variables and outcome. It is important that none of the subjects already has the outcome at the start of the study. For example, in a recent cohort study of dogs with renal disease,3 dogs had their urine protein:creatinine ratio measured when first referred, and then all dogs were followed up until they had a uremic crisis, died, or a minimum of 225 days had elapsed. Kaplan–Meier survival analysis4 was used to determine if initial protein:creatinine ratio >1.0 was associated with time to uremic crisis or death. It was found that dogs with urine protein:creatinine ratio >1 at the time of initial diagnosis had 3 times the risk of developing a uremic crisis or dying of renal failure than dogs with urine protein:creatinine ratio <1.
For a case–control study, it is necessary to select subjects that have the relevant outcome and to select a control group from the same population. The proportions of affected subjects and control subjects that have the variable under study are then compared. For example, we performed a case–control study of the radiographic signs associated with acute angiostrongylosis in dogs.5 This was done by collecting a sample of 16 sets of thoracic radiographs of dogs with proven angiostrongylosis and comparing them with thoracic radiographs from a control group of 49 dogs that had similar acute presentation and clinical signs (i.e., cough, dyspnea, and/or collapse) but were negative for angiostrongylosis. Interpretation of the radiographs was done by a single observer without knowledge of the dogs' histories or diagnoses, using a standardized reporting form to help ensure that the radiographs were assessed consistently. Alveolar pattern and, to a lesser extent, bronchial thickening were observed in a greater proportion of radiographs of dogs with acute angiostrongylosis.5 In this study, 13/16 (81%) dogs with angiostrongylosis had an alveolar pattern compared with 9/49 (18%) dogs with other conditions. Odds ratio (OR)=(13/3)/(9/40)=19.3 (95% confidence interval 4.5–82.0). Hence, the odds of observing an alveolar pattern in the lung of a dog with acute angiostrongylosis were 19.3 times the odds of this finding in a dog without angiostrongylosis, which suggests that this sign may be useful as a diagnostic aid.
Another recent case–control study described the association between computed tomography (CT) signs and histologic diagnosis in cats with sinonasal disease.6 From a population of cats with suspected sinonasal disease, cats were selected that had CT and endoscopic nasal biopsies within 2 weeks, and within this sample, the cats with neoplasia were compared with the cats with rhinitis. A control group of cats without nasal disease was not used. Various CT signs were found to be significantly associated with nasal neoplasia, including unilateral lysis of ethmoturbinates (OR 11.0), and soft tissue/fluid within the sphenoidal sinus (OR 15.3).6
A cross-sectional study is one in which a group of subjects that share common variables are identified, and the prevalence of variables is compared in different subgroups of subjects. Data are collected that represent one point in time for each of the subjects of the study. For example, a recent cross-sectional study examined the relationship between age of cats and the presence of a dilated pancreatic duct.7 From a population of 1434 cats of known age that had abdominal ultrasonography, 21/776 (3%) cats aged 10 years or less had a dilated pancreatic duct (>1.3 mm) compared with 83/658 (13%) cats aged 11 years or more. OR=(83/575)/(21/755)=5.1 (95% confidence interval 3.2–8.5). Hence, the odds of finding a dilated pancreatic duct in a cat aged 11 years or more were 5.1 times the odds of finding one in a cat aged 10 years or less.
When reading a paper that does not explicitly state the study design, the design may be determined by answering a series of questions about its key features (Fig. 1). The study design determines what outcome should be measured. The outcome of cohort studies is the relative risk (the risk ratio). Risk is the probability that the event will occur during a specified period of time; therefore, risk calculations are meaningful only for designs such as cohort studies that encompass a specified period of time. Because there is no need to measure risk in order to calculate the odds, ORs can be used to describe the results of studies that do not depend on observing subjects over a period of time, for example cross-sectional and case–control studies.
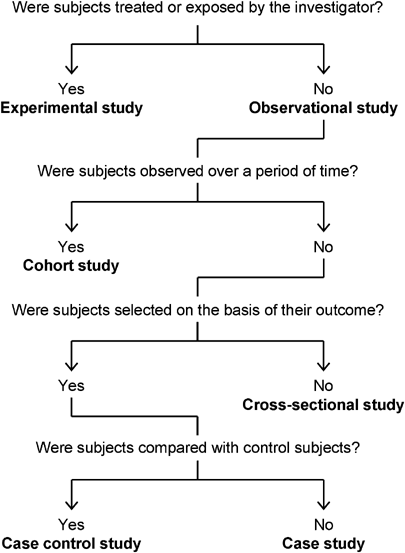
Classification of study designs.
Each of the observational study designs has advantages and disadvantages (Table 2). All observational study designs share the disadvantage of being susceptible to confounding variables, which have similar, but inseparable effects to the variables under study and therefore confuse interpretation of the results.8
| Design | Advantages | Disadvantages |
|---|---|---|
| Cohort | Suitable when a clinical trial or experimental study is unethicalCan establish sequence of events, which helps establish cause and effectCan be used to study multiple outcomesMeasures of effect include incidence and relative risk (risk ratio); enables calculation of number needed to treat | May require large sample sizeInefficient for rare outcomesProspective study can be time-consuming, expensiveNot immune from bias or confounding |
| Case-control | Can be used to study multiple exposuresUseful for studying rare conditionsShort duration, inexpensiveMay generate useful data from relatively few subjects | Limited to one outcome variableMeasures of effect include odds ratio, which is less useful than relative riskNot immune from bias or confounding |
| Cross-sectional | Can be used to study multiple outcomesShort duration, inexpensiveUseful for providing background information about a population, e.g. as a pilot study | Measures of effect include prevalence, which is less useful than incidenceUnable to distinguish cause and effectNot immune from bias or confounding |
Observational studies may be used during all phases of research into diagnostic tests (Table 3), although questions about the effect on patient outcomes of using a new test (or new treatment) are best answered using randomized clinical trials.
| Phase | Diagnostic Testing | Therapeutic Trials |
|---|---|---|
| 1 | Does test produce different results in affected patients versus normal individuals? Negative result avoids need to proceed to phases 2–4 | Does the drug produce any adverse reactions in healthy volunteers? |
| 2 | Are patients with a certain test result more likely to have target disorder than patients with other results? | Does the drug appear to be safe and effective in selected patients? |
| 3 | Does test result distinguish patients with target disorder from patients with other conditions that could produce similar clinical signs? | Does the drug convey a therapeutic benefit in randomized clinical trials? |
| 4 | Do patients that undergo this test have lower morbidity and/or lower mortality than similar patients who are not tested? | Do patients given this drug have lower morbidity and/or lower mortality than similar patients who are not treated? |




