CALVARIAL hYPEROSTOSIS SYNDROME IN TWO bULLMASTIFFS
Abstract
Two bullmastiffs with calvarial hyperostosis syndrome are described and are the first documented examples in females. The clinical and radiologic features were similar to those previously reported in males. Magnetic resonance (MR) imaging findings have not previously been reported. One dog underwent MR imaging and abnormalities included thickening of the frontal bones with loss of normal fat signal and changes in the overlying soft tissues. In one of the dogs, long bone changes were seen in the femora and resembled those seen with craniomandibular osteopathy.
Introduction
Calvarial hyperostosis syndrome of bullmastiffs is a recently described benign bone disease affecting the skull.1,2 It is similar in clinical and histopathologic characteristics to craniomandibular osteopathy and human infantile cortical hyperostosis. To date it has only been reported in young male bullmastiffs and has been speculated to be sex linked.1,2 This report describes the clinical and imaging features of calvarial hyperostosis syndrome in two young female bullmastiffs. To the authors' knowledge these are the first documented cases in females. The radiographic features of this syndrome have been reported but there are no published descriptions of the magnetic resonance (MR) imaging changes seen with this disease.1,2
Dog 1
A 7-month-old intact female bullmastiff presented to the referring veterinarian with an acute onset of focal swelling of the head of 4 days duration. The dog resented its head being touched, had reduced appetite and appeared subdued. Jaw pain or difficulty opening the mouth could not be detected. Radiographically there was bony proliferation along the dorsal aspect of the skull. Incisional surgical biopsies of the bony swelling were obtained. Histologically the abnormal bone had benign reactive changes with well-differentiated woven to lamellar bone and remodelling changes concentrated on the periosteal aspect. Mild periosteal fibroplasia with edematous widening of the intertrabecular spaces was present. The remodelling bone spicules were consistently rimmed by a single layer of well-differentiated osteoblasts. Occasional intertrabecular spaces contained small numbers of perivascular neutrophils, lymphocytes, and scattered plasma cells. The dog was treated with carprophen.* Nine days after initial presentation the dog became lame on its left pelvic limb with a nonpainful swelling below the tarsus. The dog was referred to the Animal Health Trust for further evaluation. An asymmetrical, domed appearance to the head was present with a more prominent bulge on the left side of the calvarium, caudal to the orbit, and medial to the left ear. The swelling measured approximately 6.5 cm × 3.5 cm × 1.5 cm and was nonpainful on palpation. The dog had a marked 9/10 left hindlimb lameness and resented stifle and tarsal manipulation. No effusions, crepitus or instability were detected. A routine biochemistry profile and complete blood count (CBC) were normal. Under general anesthesia radiographs of the skull, stifles, and tarsal joints were obtained. Radiographs of the joints were normal but there was subtle increase in opacity within the medullary cavity of both distal femoral diaphyses. The skull was abnormal with severe, diffuse thickening and increased opacity of the parietal and occipital bones with loss of visualization of the underlying trabecular pattern (Fig. 1). Dorsally, the bone appeared dense and sclerotic with a gradual transition to normal bone ventrally. The transition zone between the abnormal and normal bone was poorly defined. Along the external sagittal crest there was smooth periosteal new bone with a separate band of smooth, dense, paraperiosteal new bone adjacent to the occipital protuberance. The remainder of the skull was normal. Based on the radiographic and histologic findings, a diagnosis of calvarial hyperostosis syndrome in bullmastiffs was made. Treatment with carprophen was continued. One month after discharge the dog became lame again with swelling over the distal left femur. The clinical signs were unresponsive to medical treatment with carpophen. Radiographs were made at the referring practice, showing extensive endosteal and periosteal new bone formation along the length of the left femoral diaphysis (Fig. 2). The changes were most severe distally but spared the metaphysis and epiphysis. The periosteal and cortical thickening was smooth and benign in appearance but there was a faint collar of paraperiosteal new bone within the soft tissues adjacent to the femur. Within the right femur there was mild patchy increased opacity within the medullary cavity. The changes resembled those seen in craniomandibular osteopathy with limb involvement and it was thought they were probably related to the calvarial hyperostosis syndrome. In view of the poor response to nonsteroidal antiinflammatories and as calvarial hyperostosis syndrome has been purported to be associated with bacterial osteomyelitis,1 the dog was treated with antibiotics. Following treatment with metronidazole† and cephalexin,‡ there was a rapid improvement in clinical signs with resolution of the swelling and lameness. No further relapses have occurred in the following 3 years.
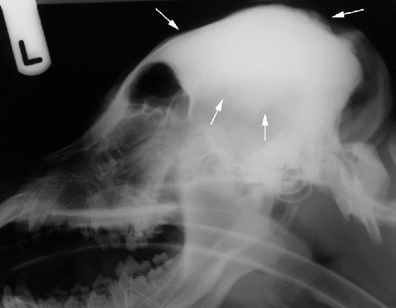
Lateral radiograph of the skull of dog 1. Note the focal thickening of the dorsal calvarium centered along the external sagittal crest (arrows).
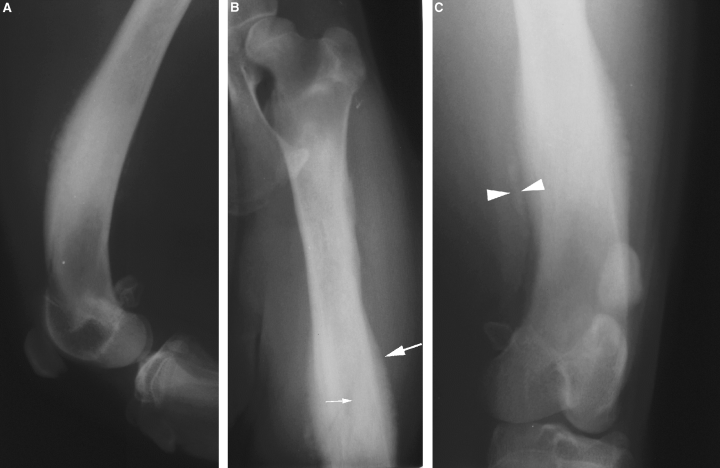
(A) Mediolateral and (B, C) caudocranial radiographs of the left femur of dog 1. There is extensive endosteal (small arrow) and periosteal (large arrow) new bone formation extending the length of the diaphysis. A faint collar of mineralization is present within the soft tissues adjacent to the distal diaphysis (arrowheads).
Dog 2
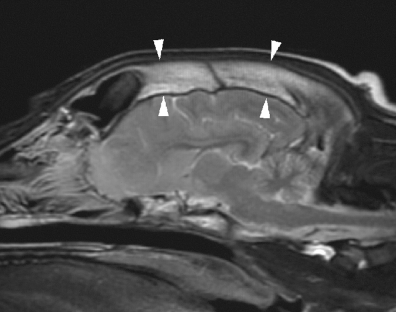
A 6-month-old intact female bullmastiff presented to the referring veterinarian with head pain. The dog was treated symptomatically with carprophen and prednisolone,§ which resulted in partial improvement in demeanour but the head pain persisted and the dog developed swelling over the right eye 1–2 weeks later. Routine hematology was unremarkable. As the dog was still symptomatic, treatment with clavulanic acid potentiated amoxycillin¶ was initiated. The dog was referred for further evaluation of the head swelling 4 weeks after onset of clinical signs. At this time clinical examination of the dog was normal except for a small firm swelling over the right frontal bone. Magnetic resonance (MR) images of the head were obtained under general anesthesia using a 1.5-T MR unit∥ and a human extremity coil. Images were obtained in dorsal, sagittal, and transverse planes and included T2-weighted, T2* gradient-echo (GRE) and T1-weighted (pre and postintravenous injection of 0.1 mmol/kg gadobenate dimeglumine#) sequences. Radiography of the head was also performed. On the radiographs there was thickening and increased opacity of the frontal bone and dorsal aspects of the temporal and parietal bones extending caudally to the mid-external sagittal crest (Fig. 3A). The sclerotic bone gradually transitioned to normal bone caudally. The changes were most severe over the frontal sinuses. The changes were asymmetrical with the right frontal bone being more severely affected and thicker than the left. A faint, smooth periosteal reaction was present superficial to the affected bone. On MR imaging there was similar asymmetry with thickening of the frontal bones, most severe on the right. The abnormal bone was reduced in signal on T1- and T2- weighted images because of loss of fat signal (3-5). Within the overlying soft tissues there was ill-defined increased signal on T2-weighted images. A thin band of increased signal on T1- and T2-weighted images was present adjacent to the surface of the affected bones and possibly represented periosteal changes. No alteration in signal within the skull bones could be detected on GRE images. Following administration of contrast medium there was ill-defined, diffuse enhancement of the frontal bones and overlying soft tissues (Fig. 4). The abnormal bone extended from the level of the rostral aspect of the frontal sinuses caudally for 6 cm. The remainder of the head was within normal limits. Based on the clinical findings, history and diagnostic imaging results a diagnosis of calvarial hyperostosis syndrome was made. No treatment was prescribed and prednisolone therapy was tapered and withdrawn. The dog continued to improve and 2 months later was asymptomatic. One year following initial evaluation there was subtle swelling over the right frontal sinus but the dog was otherwise normal.
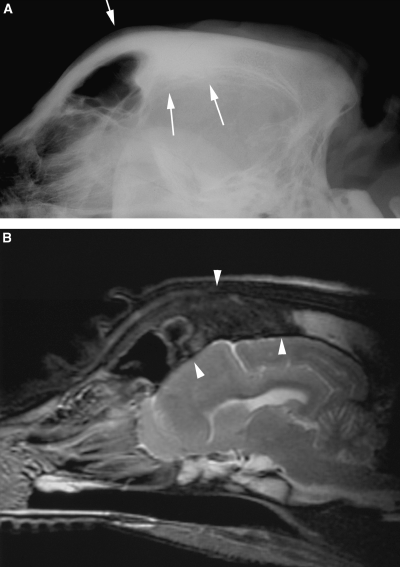
(A) Lateral radiograph and (B) sagittal T2-weighted magnetic resonance (MR) image of the head of dog 2. Sclerosis with loss of visualization of the trabecular pattern of the dorsal frontal bones is seen radiographically (arrows). On the MR image the changes are seen as reduction in signal of the abnormal bone (arrowheads) compared with the normal high-signal intensity of the occipital bone.
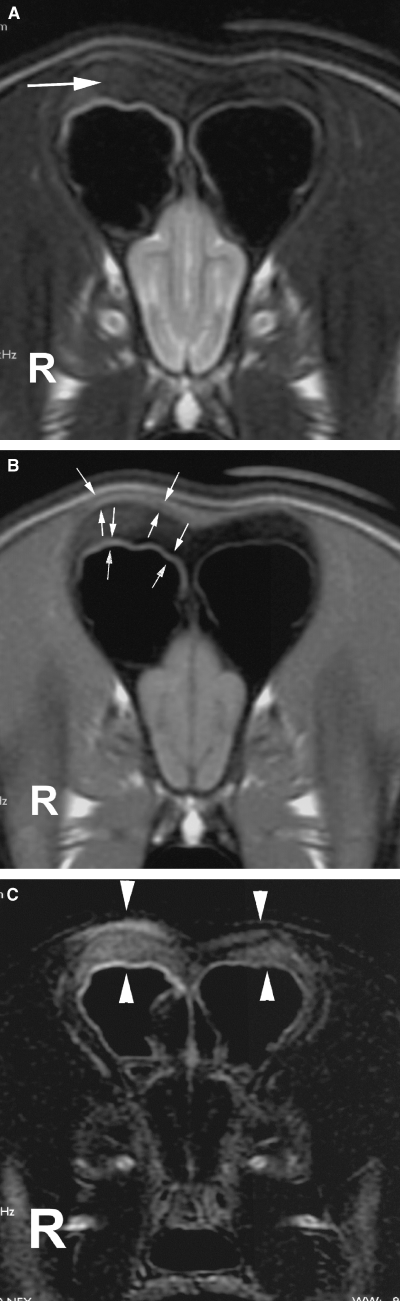
Transverse (A) T2-weighted, (B) T1-weighted, and (C) digital subtraction image of the frontal region. Note the asymmetry and thickening of the frontal bones with loss of signal from the bone marrow (large arrow). There is increase in signal and thickening of the soft tissues on the right (possibly representing periosteal changes) (small arrows). On the digital subtraction image (C), where the precontrast T1-weighted image is subtracted from the postcontrast T1-weighted image, there is abnormal diffuse contrast enhancement of the frontal bones and adjacent soft tissue (arrowheads).
Sagittal plane T2-weighted magnetic resonance image of the head of a normal 4-month-old Labrador Retriever. Note the hyperintense fat within the frontal and parietal bones (arrowheads) in comparison to the changes seen in dog 2 (Fig. 3).
Discussion
Calvarium hyperostosis syndrome in bullmastiffs is a poorly described condition and shares some similar features with craniomandibular osteopathy and infantile cortical hyperostosis in man. As the name suggests, the principle change seen in calvarial hyperostosis syndrome is bony enlargement of the calvarial bones. Clinical signs reported with the condition include painful bony swelling of the skull, lymphadenopathy, eosinophilia, and pyrexia.1,2 Seizures, hydrocephalus, lameness and suppurative osteomyelitis have also been reported.1 The clinical findings described here are similar to those previously reported and it appears that most dogs initially present for painful skull lesions although occasional limb involvement is also seen. The lesion localization is characteristic of the disease and the absence of involvement of the mandibles is the main differentiating feature from craniomandibular osteopathy.
Craniomandibular osteopathy involving only the calvarium, with sparing of the mandibles and petrous temporal bones, has been reported in breeds other than bullmastiffs.3,4 Given the similar radiographic and clinical findings with calvarial hyperostosis syndrome, it is possible that calvarial hyperostosis syndrome is simply a variant of craniomandibular osteopathy. There is, however, confusion regarding the pathological findings, with craniomandibular osteopathy reported to be both similar to and different from infantile cortical hyperostosis.1,2,5–7 Infantile cortical hyperostosis and calvarial hyperostosis syndrome are reported as being predominately inflammatory subperiosteal or periosteal diseases.1,2,7 Craniomandibular osteopathy by comparison is reported to differ from infantile cortical hyperostosis by predominately resulting in irregular deposition and resorption of bone resulting in a mosaic of poorly mineralized tissue.6 The inflammatory component of craniomandibular osteopathy is variable. The confusion may simply be because of the fact that bone has a limited number of responses to injury and different diseases result in similar radiographic changes. It is possible that craniomandibular osteopathy and calvarial hyperostosis syndrome are because of a variety of underlying causes (e.g., genetic, infectious), as infantile cortical hyperostosis is in man. This may explain some of the differences in presentation seen in the reported cases of calvarial hyperostosis syndrome.
The radiographic features of calvarial hyperostosis syndrome are well described and appear as periosteal proliferation along the dorsal aspect of the calvarium involving the frontal, parietal, and occipital bones.1,2 The long bone signs in dog one are similar to those reported in infantile cortical hyperostosis and some patients with craniomandibular osteopathy.5,8,9 It is possible the long bone changes were because of a concurrent disease such as panosteitis or hypertrophic osteodystrophy. The lack of metaphyseal changes is however atypical for hypertrophic osteodystrophy and paraperiosteal soft-tissue mineralization is not a typical feature of panosteitis. The cause of the long bone changes in dog one is unknown but appeared antibiotic responsive. Long bone involvement has also been seen in one case of calvarial hyperostosis syndrome, which at postmortem had evidence of suppurative osteomyelitis.1 Osteomyelitis of long bones has also been reported in a case of infantile cortical hyperostosis in man, where the similar clinical and radiographic features of infantile cortical hyperostosis and osteomyelitis lead to confusion of the diagnosis.10 The radiologic differential diagnoses of infantile cortical hyperostosis includes osteomyelitis, trauma, scurvy, osteosarcoma, congenital syphilis, vitamin A intoxication, long-term prostaglandin therapy for congenital cyanotic heart disease, and battered child syndrome.8,10
In man survey radiography is considered adequate for diagnosis in most patients with infantile cortical hyperostosis but MR imaging may be helpful in the early stages of the disease.8,11 The appearance of infantile cortical hyperostosis on MR images in humans is described as soft tissue edema and inflammation. The lesions typically involving the diaphyses of the long bones, clavicles, and mandibles.12 On MR images the lesions are characterized by increased signal on T2-weighted images, surrounding the affected bones.8,11
In dog 2, the MR images were characterized by the soft tissue changes and nondestructive bony changes similar to the radiographic findings. The hypointense signal on T1- and T2-weighted images in the affected bones in dog 2 was thought to be because of loss of fat from the bone marrow. Reduction in signal within the bone marrow on T1- and T2-weighted images is because of reduction in amount of fat and may occur in a variety of conditions such as fibrosis, sclerosis, hemosiderosis, or marrow infiltration/replacement and is a relatively nonspecific change. It has been shown in mature dogs that high signal within bone marrow on T1- and T2-weighted images is because of high-fat content in yellow marrow.13 The appearance of normal bone marrow in immature dogs has not been reported but may be expected to have less fat content than in adult dogs because of a greater percentage of red marrow. In the authors' experience, by 6 months of age the appearance of the bone marrow in the calvarium resembles that seen in adult dogs. Alteration in the shape and architecture of the bone, distribution of marrow changes and soft tissue involvement are also important in narrowing the differential diagnoses.
A standard head MR protocol was used for the MR imaging of dog two, with the addition of a T2* GRE sequence. On T2* GRE images bone appears hypointense and bony changes are often easier to detect compared to T1- and T2-weighted spin–echo images. Fat in bone marrow results in high signal on fast-spin–echo T1- and T2-weighted images and it may be difficult to see contrast enhancement within the marrow. In retrospect it may have been preferable to obtain the postcontrast T1-weighted images with the use of an additional spectral fat saturation pulse. This would have suppressed the high signal in the bone marrow and allowed visualization of the enhancement without the need for digital subtraction. The digital subtraction images were however useful in demonstrating the abnormal enhancement within the bone. The use of a short tau inversion recovery (STIR) sequence, to give a fat suppressed T2-weighted image, would have been useful to allow evaluation of any bone marrow edema. In dogs, MR imaging is probably not routinely required for the diagnosis of calvarial hyperostosis syndrome, which can be achieved by survey radiography alone.
Craniomandibular osteopathy is hereditary in West Highland White Terriers and an inherited form of infantile cortical hyperostosis also exists.12,14 Genetic studies in humans suggest that the familial form of infantile cortical hyperostosis may be associated with a COL1A1 missense mutation.12,15 It is possible that calvarial hyperostosis syndrome in bullmastiffs also has a partial genetic basis. To date all the published reports of calvarial hyperostosis syndrome (seven dogs), have been in male dogs (five) or the gender was unspecified (two), which has lead to speculation that the condition may be sex linked. As these two new patients show, this is not true and the apparent absence of female dogs is probably related to the small number of described patients.
It has been speculated that nongenetic factors may also be involved in craniomandibular osteopathy.4 Irish setters with canine leuckocyte adhesion deficiency are reported to develop similar long bone and skull changes to craniomandibular osteopathy, which may be secondary to underlying infection.16 Sporadic nonfamilial cases of infantile cortical hyperostosis have been associated with the administration of prostaglandin E1 and prostaglandin E2, and elevated prostaglandin E levels have been found in some children with infantile cortical hyperostosis. Whether the elevated prostaglandin E levels is a cause or effect of infantile cortical hyperostosis is unknown. Recent studies in humans of the familial form of infantile cortical hyperostosis indicate that the disease may be because of a collagen-related disorder.12,15 It is unknown if dogs with calvarial hyperostosis syndrome or craniomandibular osteopathy have collagen abnormalities or alterations in prostaglandin levels but this warrants further study.
These two dogs suggest that calvarial hyperostosis syndrome is not a sex-linked syndrome as previously thought, however, further evaluation of a larger population is still needed. The underlying cause(s) is still not known and the role of infectious disease or collagen abnormalities in calvarial hyperostosis syndrome requires further study.




