MAGNETIC RESONANCE IMAGING FINDINGS OF PRESUMED CEREBELLAR CEREBROVASCULAR ACCIDENT IN TWELVE DOGS
Abstract
The magnetic resonance imaging (MRI) findings of presumed cerebrovascular accident in 12 dogs are described. Fourteen lesions were seen, commonly (11 of 14) within the gray matter of the cerebellar hemispheres or vermis. Thirteen lesions were hyperintense on T2-weighted images (in 11 dogs) and one was hypointense. Eleven of 14 lesions were within the region supplied by the rostral cerebellar artery or one of its main branches and there was no, or minimal, mass effect. Contrast enhancement was only seen in six lesions and was mild in all. Gradient-echo images provided additional information in two dogs. The appearance of infarction in dogs with diffusion-weighted images (DWI) is similar to that in humans, and provided supportive evidence for the diagnosis of infarction in five dogs. The use of gradient-echo and DWI is recommended for the evaluation of suspected cerebrovascular accidents in dogs. Six of the 12 affected animals were spaniels or spaniel crosses, suggesting a possible breed predisposition.
Introduction
Cerebrovascular disease is an abnormality of the brain attributable to a disturbance in blood supply.1 Cerebrovascular accident (stroke) is a sudden onset of focal brain signs secondary to infarction or hemorrhage resulting from cerebrovascular disease. Cerebrovascular accidents are broadly divided into two groups—ischemic infarction and hemorrhagic. Ischemic infarction may occur as a result of disturbance to the arterial or venous supply caused by a thrombus, embolus, or secondary to blood vessel abnormalities. Cerebrovascular accident is reported to be uncommon in small animals; however, the true incidence is unknown.1–3 Cerebrovascular accidents are increasingly being recognized in dogs since magnetic resonance imaging (MRI) has become more available.4 Until recently, most reports of cerebrovascular accident in dogs were based on postmortem results. This may affect the apparent incidence and prognosis, as it is likely that only the most severely affected dogs (or dogs where infarction is secondary to a disease with a poor prognosis, e.g. neoplasia) will die or be euthanized. As a result, the imaging findings, clinical findings, and prognosis of less severely affected animals may be different from that previously reported.
There are only 12 reported cerebrovascular accidents affecting the cerebellum in dogs5–8 and only three reports of the imaging features of cerebrovascular accident affecting the cerebellum/brainstem of dogs.7–9
The aim of this study is to describe the MRI features—distribution, appearance, pattern of contrast enhancement, and the use of gradient-echo and diffusion-weighted imaging (DWI) of dogs with presumed cerebrovascular accident affecting the cerebellum.
Methods
A retrospective study was performed of dogs referred to the neurology unit of the Animal Health Trust between January 2001 and March 2004 and undergoing MRI. One dog examined prior to the study period was included as it had postmortem confirmation of cerebellar infarction and allowed comparison of the pathologic and imaging findings. The diagnosis of presumed cerebrovascular accident as a cause for the clinical signs was based upon the following criteria:
- (i)
History—acute or peracute onset of signs and then arrest and nonprogression or improvement of neurological abnormalities (either as solitary or paroxysmal events).
- (ii)
Neurolocalization confined solely to the cerebellum or central vestibular system.
- (iii)
Cisternal cerebrospinal fluid (CSF) analysis—either normal or compatible with cerebrovascular accident (elevated protein, mild neutrophilic, or mononuclear pleocytosis (<30 cells/μl), xanthochromia, hemosiderosis, or normal).1,6,10,11 Dogs with other CSF abnormalities were excluded.
Dogs were excluded if neurolocalization suggested multifocal disease, peripheral vestibular disease, or a spinal cause for the ataxia. Dogs with MRI findings typical of neoplasia or a significant congenital malformation that could directly cause the neurological signs were excluded. Dogs with congenital anomalies or lesions considered incidental were not excluded. Congenital lesions that could theoretically predispose to infarction, such as Chiari malformation, but would not cause cerebellar–vestibular signs, were not excluded.
MRI was performed in 11 dogs using a 1.5-T MR unit* and one dog was imaged using a 0.5-T MR unit.† All dogs were anesthetized for MR examination and were imaged in dorsal recumbency using a human extremity coil. In all dogs a standard brain protocol was used including T2- and T1-weighted (pre- and postintravenous injection of 0.1 mmol/kg gadodiamide)‡ images. Images were obtained in transverse, sagittal, and dorsal planes. In addition, T2* gradient-echo (GRE) and T2-weighted fluid attenuated inversion recovery (FLAIR) images were obtained in 10 dogs. MR angiography (MRA) using a 3D time-of-flight technique (TOF) was performed in six dogs. Contrast-enhanced MRA of the carotid arteries was performed in one dog. DWI using an echo-planar technique with a b value of 1000 were obtained in six dogs. ADC maps were calculated from the DWI using a proprietary software package.§
Diagnostic tests to screen for potential underlying causes of cerebrovascular disease were performed as appropriate and included routine hematology, serum biochemistry, serology for Toxoplasma gondii, Neospora caninum, and canine distemper virus, adrenal function testing, thyroid screening, thoracic radiography, abdominal ultrasound, echocardiography, noninvasive blood pressure monitoring, coagulation profile, antithrombin 3 levels, and urinalysis.
Results
Twelve dogs fulfilled the inclusion criteria. The dogs ranged in age from 4.5 to 15 years with a median of 8.25 years and mean of 8.9 years. Seven dogs were male (four neutered) and five were neutered females. There were two mixed breed (one English Springer spaniel cross, one undetermined) and six pure breeds: four Cavalier King Charles spaniels (CKCS), two golden retrievers and one—English Cocker spaniel, Weimaraner, Border collie, and Greyhound.
All dogs had acute onset of neurological signs. In all dogs the neurolocalization was cerebellar or central vestibular (central vestibular localization in three, central vestibular with cerebellar involvement in eight, and cerebellar in one). In five, the dogs had shown brief (less than 10-min duration with rapid recovery to normal), episodic neurologic signs consistent with a vestibular neurolocalization prior to presentation (as reported by the owner or referring veterinarian).
Physical examination was normal in all dogs except for two, which had grade 3/6 systolic mitral heart murmurs. One dog had chronic exercise intolerance and clinical signs suggestive of a neuromuscular disorder since 18 months of age but was considered unrelated to the cerebellar infarct. Cisternal CSF analysis was normal in all but two dogs, one that had elevated protein levels, one of which also had phagocytosed erythrocytes on cytologic examination. Serology for T. gondii, N. caninum, and canine distemper virus was negative in all the dogs where it was performed.
The dogs were initially imaged between 1 and 10 days postonset of clinical signs (median 2 days, mean 3.3 days). One dog had another MR examination 45 days after the first and onset of clinical signs. MR images were abnormal in all cases. There were 14 cerebellar lesions; 10 dogs had single lesions, one dog had bilateral lesions, and one dog had two small separate lesions within the same cerebellar hemisphere. Eleven of 14 cerebellar lesions were within the tentorial side of the cerebellum compatible with the region supplied by the rostral cerebellar artery (Fig. 1). Two lesions were within the deep cerebellar gray matter and one was within the white matter of the cerebellar vermis. There was a bias toward left-sided lesions, with nine lesions in the left cerebellar hemisphere or paravermis, compared with only four in the right side, and one in the midline. The lesions were usually confined to gray matter but where white matter was involved the gray matter changes were more severe. Ten lesions were wedge or roughly rectangular in shape, three were pinpoint, and one was ovoid (in the region of the deep cerebellar nuclei). Twelve lesions were well defined and sharply demarcated from adjacent parenchyma and had a homogenous appearance. Two small (less than 2-mm diameter) lesions within the same cerebellar hemisphere were rather poorly marginated. No mass effect was seen in 12 lesions and mild parenchymal swelling was present in two lesions (imaged 30 h and 10 days postonset of clinical signs). One dog had MRI performed 30 h and 45 days postonset of clinical signs. On the initial examination there was subtle swelling of the affected parenchyma. On the second examination there was focal atrophy of the affected parenchyma (Fig. 2). Thirteen of the lesions were hyperintense on T2-weighted images (Fig. 3) and hypointense or isointense in T1-weighted images (Fig. 4). In one case a pinpoint lesion within the white matter of the vermis was hypointense on T2- and T1-weighted images (Fig. 5). On FLAIR images, the appearance of the lesions was similar to that on the T2-weighted images in all instances. In two dogs the lesions were slightly more conspicuous on FLAIR images but were still identified on the T2-weighted images. In the dogs where a gradient-echo sequence was used, the cerebellar lesions were either not visible or mildly hyperintense except for one in which a small hypointense focus was seen (Fig. 5). In addition to a cerebellar lesion, one dog had multiple small foci of low signal on GRE images, consistent with small hemorrhagic areas, within the cerebrum at the junction of gray and white matter, which were not visible on the T2-weighted images and T1-weighted images (Fig. 6). These were considered asymptomatic as neurolocalization was confined to a cerebellar–vestibular location. This dog also had symmetric increase in signal within the white matter of the parietal and occipital lobes, which were considered incidental findings as there were no neurologic signs that could be attributed to lesions in these locations. Contrast enhancement was seen in six of 14 lesions and was mild and peripheral in four (Fig. 7B and C). Two lesions had mild diffuse enhancement (Fig. 7A). Cerebral and carotid angiography was normal in the six dogs where it was performed. In five of the six dogs where DWI was performed (imaged between 1 and 5 days postonset of clinical signs) a clearly demarcated focal area, of uniform increased signal, was seen on the DW image, with a corresponding area of reduced diffusion (seen as low intensity) on the ADC map (Fig. 8). In one case where DWI was performed, a small area of low signal was seen within the white matter of the cerebellar vermis on both DW and ADC images. The lesions were more obvious on DWI than on conventional fast spin-echo images in all six dogs where DWI was performed. The lesions were between 30% and 100% larger on the DWI compared with the T2-weighted images in four dogs, and similar in size in two. Three of the four CKCS had Chiari malformation typical for the breed, with associated syringohydromyelia in one.12 No clinical signs were present that could be attributed to the Chiari malformation and given the high incidence in CKCS and poor correlation between MR findings and clinical signs, they were not considered significant.12
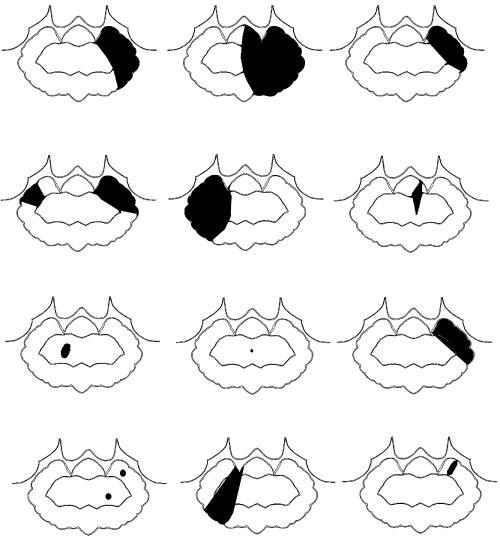
Distribution of cerebellar infarcts in 12 dogs on dorsal plane images. Rostral is to the top of the diagram; the right of the diagram represents the left side of the patient. The solid gray areas represent the location of the lesions drawn from the dorsal plane magnetic resonance image. Note the predominantly gray matter distribution and angular shape to the lesions.
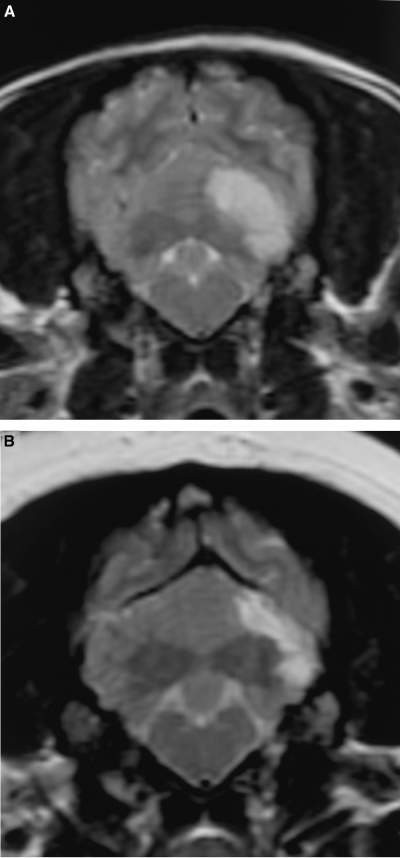
Transverse plane T2-weighted images of a 4-year-old Cavalier King Charles spaniel acquired 30 h (A) and 45 days (B) postonset of clinical signs. There is mild swelling of the gray matter of the folia of the left cerebellar hemisphere on the initial examination. Repeat magnetic resonance images show a reduction in the size of the lesion.

Transverse (A), dorsal (B), and left parasagittal (C) plane T2-weighted images of a 7-year-old neutered female Cavalier King Charles spaniel. Note the lack of mass effect, sharp demarcation between normal and abnormal parenchyma, and rostral distribution.
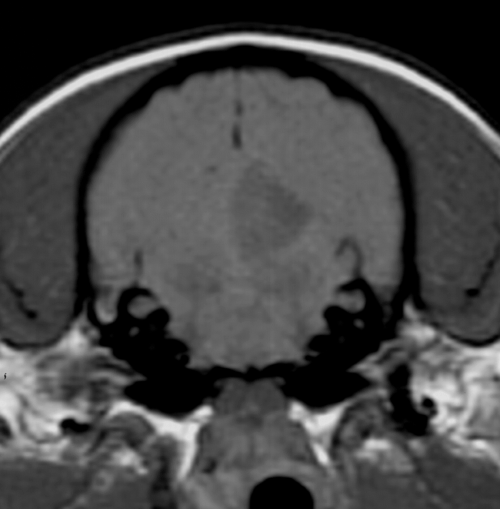
Transverse plane T1-weighted image of the same dog as in Fig. 3.
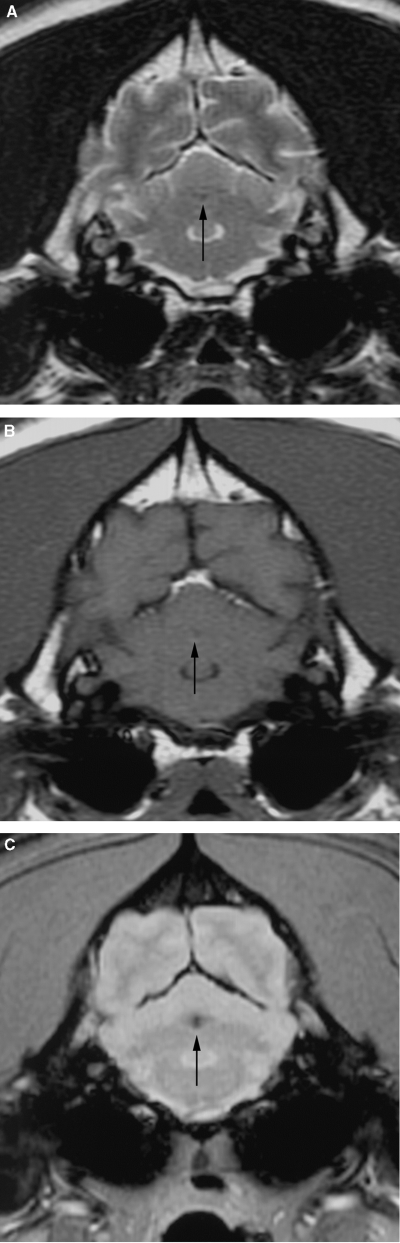
Transverse plane T2-weighted (A), T1-weighted (B), and T2* Gradient-echo (C) images of the cerebellum of a 9-year-old neutered male Golden retriever. (A) Small hypointense focus is present within the center of the cerebellum on all sequences (arrow) but is considerably more obvious on the Gradient-echo image.
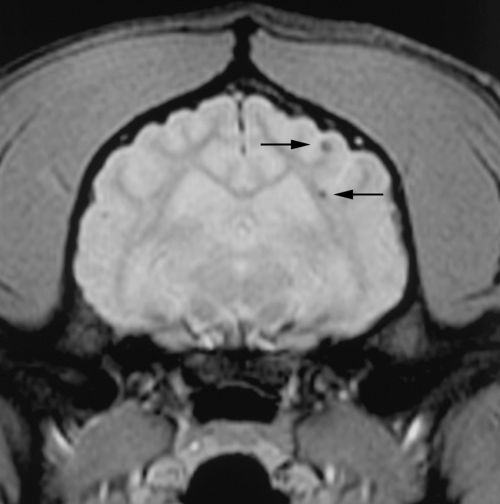
Transverse plane T2* Gradient-echo images of the cerebrum of a 15-year-old male English springer spaniel cross. Note the small hypointense foci (arrows) at the junction of the white matter and gray matter of the left parieto-occipital lobe.

Transverse plane T1-weighted images postcontrast of two different dogs (A, B). The diffuse enhancement seen in (A) was the most dramatic seen of all the cases. The faint peripheral enhancement in (B) was more common and was seen in four of the six cases that showed contrast enhancement. Figure 3 shows the precontrast T1-weighted image of the dog in (B).
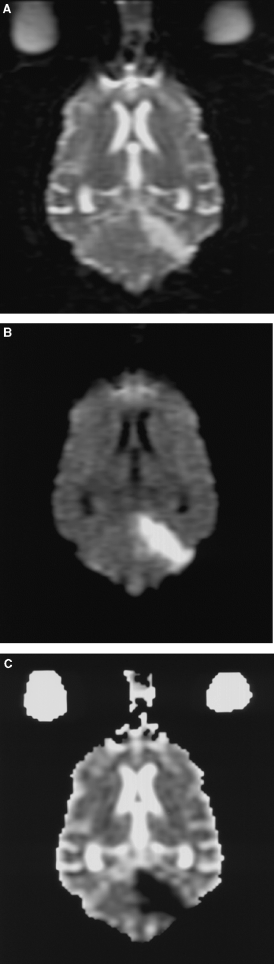
Dorsal oblique plane T2-weighted (A) diffusion-weighted image (DWI) (B), and apparent diffusion coefficient (ADC) (C) images of a 15-year-old male English Springer spaniel cross. The hyperintense lesion within the cerebellum on the DWI could be because of restricted diffusion or an artifact “T2 shine through.” By calculating the ADC map the lesions are confirmed as being due to restricted diffusion.
Discussion
Definitive diagnosis of a cerebrovascular accident is difficult when the animal recovers, as in 11 of the dogs reported here.13 The diagnosis of cerebrovascular accident was based upon inclusion criteria that were consistent with cerebrovascular accident and eliminated other potential causes for the signs. Acute onset, arrest, and then regression of neurologic signs in all except fatal strokes are the hallmark of cerebrovascular accident.1,14 Inflammatory and neoplastic disease is typically progressive but may wax and wane in severity with time. Acute decompensation of inflammatory disease or central nervous system neoplasia may result in apparent acute onset of clinical signs,1,15 but once present clinical signs do not typically regress without treatment as in cases of cerebrovascular accident. CSF analysis and MRI also helped to exclude inflammatory and neoplastic disease. In the majority of cerebrovascular accidents in humans, CSF analysis is normal, but a marked transient neutrophilic pleiocytosis is common in the first week following the event.11,16 Idiopathic vestibular disease may also be characterized initially by acute neurologic signs. However, although there is some debate, it is generally considered that idiopathic vestibular disease does not cause central or cerebellar signs.17 The MR appearance of cerebellar infarcts with no or minimal mass effect and predominantly gray matter distribution differs from neoplasia. A mass effect may be seen with cerebellar infarction in humans but was not seen in any of the patients reported here. Where a mass effect occurs it usually develops 3–5 days postinfarction because of vasogenic edema.18 One of the characteristic findings of infarction is that the mass effect resolves before the development of contrast enhancement. Trauma is usually readily differentiated by history and clinical examination.1
Five dogs had brief (less than 10-min duration) episodes of neurologic signs prior to admission similar to that described for transient ischemic attacks (TIA) in man.19,20 In several, these were reported as seizure activity by the referring veterinarian but on close examination of the history were more likely to have been acute vestibular episodes. Similar paroxysmal events have previously been reported in dogs with histologically proven infarction.21 In humans, TIAs are brief episodes of focal loss of brain function attributable to ischemia involving one of the vascular systems and lasting less than 24 h.22 TIAs because of vertebral–basilar disease may cause dizziness, nystagmus, ataxia, and weakness.19 In humans, TIAs are usually related to vascular stenosis and ulceration because of atherosclerosis and thrombus formation or cardioembolism and commonly precede cerebellar infarction.20,22
The age distribution in this series is similar to that previously reported, with infarcts typically occurring in middle-aged to older dogs. Including this study, 28 of 48 (58%) dogs with cerebrovascular disease were male and only 20 of 48 (42%) were females, possibly suggesting an increased risk for males, as also seen in humans.3,5–8,13,21,23–28 No breed predisposition has been reported for cerebrovascular accident in dogs. In this series, spaniels and spaniel crosses (50%) appear over-represented in comparison with the number of spaniels presented to the neurology service (11%) and the hospital population (29%). CKCS also appeared over-represented (27%) compared with CKCS presented to the neurology service (3.5%) and the hospital population (3%). There are several reasons why CKCS may be predisposed to cerebrovascular accidents. This breed has a high incidence of cardiac disease and also platelet abnormalities,29 which could potentially predispose to cardioembolic infarction. CKCS have a high incidence of Chiari-type malformation,12 which potentially could have some effect on vertebral–basilar blood flow. Further studies are required to investigate breed predisposition and underlying causes further.
In the dog, blood supply to the brain is from two main sources: the internal carotid artery and the basilar artery.1 There are two (left and right) rostral cerebellar arteries that have been described as arising as branches of the caudal cerebral arteries8 or alternately arising as branches of the caudal communicating/basilar arteries.30 Each rostral cerebellar artery gives off three terminal (lateral, intermediate, and medial) branches that supply the rostral part of the cerebellar hemisphere and vermis although this is variable.30,31 Extensive arterial anastamoses are present on the surface of the canine cerebellum that provide collateral circulation in the event of thrombosis of the cerebellar arteries.30,32 The blood supply to the cerebellum in dogs is similar to that in humans, with the rostral cerebellar artery in dogs corresponding to the superior cerebellar artery in humans.30 Most cerebellar infarcts in humans are territorial infarcts of the superior cerebellar artery.20 Infarcts within this artery in humans are commonly associated with cardioembolism or defects in the basilar or vertebral arteries secondary to atherosclerosis.20 Embolic causes of infarction are often bilateral and commonly result in hemorrhagic infarction.18 It has been suggested that most infarction in dogs is because of embolic disease but there is little evidence to support this hypothesis.33 Only in one patient of the present series was embolic disease likely, a dog with bilateral cerebellar infarcts that had clinical evidence of cardiac disease. This dog was borderline hypothyroid, which may cause atherosclerosis in dogs.34,23 It has been shown experimentally that the blood supply to the cerebellum is almost entirely via the vertebral and basilar arteries.35 Emboli to the cerebellum would have to enter the basilar–vertebral circulation rather than via the internal carotid arteries. Eleven of the 14 lesions seen within the cerebellum were within the region supplied by the rostral cerebellar arteries and resembled territorial infarcts seen in humans. There was an apparent bias toward lesions occurring within the left side of the cerebellum but the reason for this is unknown. Three infarcts were found in the deep cerebellar gray matter and it was not clear from which artery they arose. They resembled infarcts described as nonterritorial infarcts in humans, which resemble lacunar and watershed infarcts, but the classification of small deep cerebellar infarcts in humans is the subject of debate.36,20
In 10 dogs the MRI appearance of the lesions was similar to that reported for cerebellar infarcts and infarcts elsewhere in the brains of dogs.8,21 Infarcts are most commonly clearly demarcated lesions, which are hyperintense on T2-weighted images and often wedge shaped with minimal mass effect and often preferential gray matter involvement. The increased signal on T2-weighted images and reduced signal on T1-weighted images are consistent with an increase in water because of cytotoxic and vasogenic edema. Two dogs had lesions without the classic appearance of cerebellar infarction. One dog had a solitary hypointense lesion in the cerebellar white matter, and one dog had two rather ill-defined hyperintensities on T2-weighted images.
Contrast enhancement was seen in six of 14 lesions and was faint in all instances, peripheral in location in four, and diffuse in two. A similar pattern of mild peripheral enhancement was seen in two previously reported cerebellar infarcts.8 In two lesions contrast enhancement was only clearly visible on digital subtraction images where the precontrast data are subtracted from postcontrast data. Patients where enhancement was seen were imaged between 1 and 45 days postonset of neurologic signs (median of 3 days, mean of 13 days). The lesions that had contrast enhancement were considered to be nonhemorrhagic, which differs from another study of infarction in seven dogs where nonhemorrhagic infarcts did not have contrast enhancement.21 In humans, progressive contrast enhancement of arteries is seen within the first 5–7 days postinfarction followed by dense cortical enhancement, which begins at day 5–7 and persists for 6–8 weeks.18 The contrast enhancement is reported to be because of leakage of contrast medium through the damaged blood-brain barrier.18
The infarcts were considered to be nonhemorrhagic in 11 of the 12 dogs where a lesion was seen. This was based on the absence of hypointensity on GRE images (performed in 10 dogs) and uniform hyperintensity on T2-weighted images and absence of hyperintensity on T1-weighted images. One dog had a small focal hypointense lesion within the cerebellum on T2-weighted and GRE images, which was thought to be consistent with a small hemorrhagic area. In one study in dogs, hemorrhagic infarcts were mixed intensity on T1- and T2-weighted images21 but GRE images were not obtained. The nonhemorrhagic infarcts were hyperintense on T2-weighted images and hypointense on T1-weighted images as in this study. GRE sequences are the most sensitive for demonstrating hemorrhage within the brain, which appears hypointense at all stages.37 Low signal on T2-weighted images and GRE images is, however, not specific for hemorrhage but may also be seen with mineralization, gas, fibrous tissue, or iron deposits.38 In humans, hemorrhage associated with infarction usually occurs after the onset of infarction and is more common in cardioembolic stroke than atherosclerotic stroke.18 The mechanism by which nonhemorrhagic infarcts undergo hemorrhagic transformation is unknown but may be because of reperfusion.18 In two patients GRE provided additional information over the standard fast spin-echo sequences. In one a small lesion within the white matter of the cerebellar vermis was initially overlooked prior to performing the GRE sequence (Fig. 5). In another dog multifocal pinpoint foci of low signal on T2* GRE images were seen within the forebrain on the GRE images but were not visible on the fast spin-echo images (Fig. 6). In humans, small hypointensities are commonly seen on GRE images of the brain of older people. These are most commonly because of small lacunar infarcts (cerebral microbleeds) and are because of deposits of hemosiderin in most instances.39 A consistent correlation has been reported between cerebral microbleeds and ischemic cerebral lesions. Cerebral microbleeds are often secondary to cerebral amyloid angiopathy and hypertension in humans but are often asymptomatic.18,39
In the one dog that had repeat MR examination 6 weeks after initial imaging there was peripheral hyperintensity on T1-weighted images, which could be consistent with hemorrhage. High-intensity lesions on T1-weighted images have been seen with cortical laminal necrosis associated with brain infarction in people, possibly because of increased protein content.7 Hyperintensity on precontrast T1-weighted scans has been reported in an infarct in a dog secondary to intravascular lymphoma although the cause for the hyperintensity was uncertain.7
DWI were obtained in six dogs. Five had focal areas of reduced diffusion consistent with acute infarction. DWI is increasingly being used in the investigation of suspect stroke in humans as it has been more sensitive than conventional spin-echo sequences for demonstrating areas of ischemia.40 DWI are usually obtained using an echo planar spin-echo technique and map proton contrast reflecting the microvascular water environment and detect the effect of random molecular motion of water in the presence of a magnetic field gradient.41 A T2-weighted sequence is usually used with a pair of gradient pulses placed symmetrically around the 180° refocusing pulse of the spin-echo sequence. Static protons dephase and rephase completely and appear as they would if the symmetrical gradient pulses were not applied. Molecular diffusion as a result of Brownian motion causes protons to acquire a phase change resulting in signal loss on the DWI. Molecules diffuse in all three dimensions by Brownian motion. The diffusibility of the molecules is described by the diffusion coefficient. The average path length (L) that diffusing water protons travel through the microenvironment of the brain is related to the duration of the observation (t) and the diffusion coefficient (D) and can be expressed by the formula L=√2tD. In the brain, water movement is restricted because of tissue cellular elements and membranes. Tissue boundaries with different degrees of permeability hinder free diffusion decreasing the diffusion coefficient. In the brain, normal white matter tracts restrict movement of water perpendicular to the tract, which results in anisotropy. To overcome anisotropy a trace DWI is usually acquired with three independent sensitizing gradients applied in the x, y, and z planes and the mean (trace) DWI is applied. The trace DWI is relatively insensitive to the direction of the sensitizing gradient. Acute infarction results in water trapping within cells and causes reduced diffusion, which is seen as increased signal on the DWI. Because the DWI has some T2 weighting, lesions that are hyperintense on T2-weighted images may also appear hyperintense on the DWI (known as T2 shine through). To differentiate T2 shine through from true restricted diffusion the ADC can be calculated. ADC quantifies diffusion and is a function of the ratio of signal intensity ratio between two images of different diffusion weighting and a constant derived from the properties of the diffusion gradient called the b-value. b-value quantifies the diffusion sensitivity and is affected by the duration, amplitude, and interval of the applied diffusion gradient. Areas with restricted diffusion will have low ADC values and appear as low intensity on most ADC maps. The reduced diffusion is thought to be related to movement of water from the extracellular to the intracellular space. It is theorized that intracellular water has more restricted diffusion compared with extracellular water. Other theories for the restricted diffusion seen with infarction include increased water viscosity and extracellular tortuosity, changes in cytoplasmic circulation, decreased cellular membrane permeability, and water streaming.41 Abnormalities on the DWI may be seen within 1 h of onset of ischemia with reduction in ADC seen within 2.5 min. The classic appearance of acute infarction is hyperintensity on the DWI and reduced ADC. This pattern persists for the first 4 days after which the ADC values may have pseudonormalization after 4–10 days. ADC values typically change from reduced to high (dark to bright) after 7–10 days. The DWI images may remain hyperintense for up to 72 days postinfarction, which makes them unhelpful for dating the age of the infarct. High signal on DWI and low ADC despite having high sensitivity (up to 100%) and specificity (up to 100%) for the diagnosis of acute infarction has been shown in some patients to have false positives and negatives.42 A similar pattern has been seen with hemorrhage, abcesses, lymphoma, and Creutzfeld Jacob disease. These conditions can be differentiated from acute infarction on the basis of history and appearance on T2-weighted and postcontrast T1-weighted images.41,42 DWI in five dogs was suggestive of acute infarction and provided additional support for the diagnosis of cerebellar infarction. In two dogs the lesions were considerably more obvious on the DWI than the T2-weighted images.
Magnetic resonance angiography (MRA) was interpreted as normal in six dogs although the cerebellar arteries were inconsistently seen. A 3D TOF sequence was used, which is a noncontrast medium-based technique. A long train of short TR pulses is used to saturate spins in stationary tissue.43 The spins of inflowing blood are fully relaxed and appear hyperintense to the surrounding tissue. The contrast depends upon the flow velocity and path of the vessel flowing through the imaged volume.43 Artifacts may be seen as tissue with a short relaxation time, e.g., fat and gadolinium may also appear hyperintense.43,44 Ideally, the imaged slice should be perpendicular to blood flow as flow within the slice may become saturated resulting in loss of contrast. The image volumes were placed perpendicular to the basilar artery, which results in the cerebellar arteries partly running in plane, which may have decreased visualization of the vessels. The cerebellar arteries in dogs are small, and in the authors' experience may not be visible in normal dogs. Maximum intensity projection (MIP) algorithms were used postprocessing in addition to assessing the raw images. MIP images are reported to result in loss of information and may prevent visualization of small or faint vascular structures.44 It is possible using a contrast media-based MRA technique that the cerebellar arteries may have been more clearly visualized.
Conclusion
The MRI appearance of canine cerebellar infarcts appears characteristic and similar to that reported in humans and elsewhere in the brain in dogs. They occur most commonly within the region supplied by the rostral cerebellar artery and are possibly more common in the left cerebellar hemisphere and in spaniels. Gradient-echo images are recommended for visualization of hemorrhage and DWI may be useful to provide support for the diagnosis.




