Xenografting of gonadal tissues into mice as a possible method for conservation and utilization of porcine genetic resources
ABSTRACT
In vitro production of embryos, including in vitro maturation, fertilization of oocytes and their subsequent culture to the embryo stage, has become the most popular method of studying gametogenesis and embryogenesis in pigs. As well as their utility for basic studies, these procedures now enable us to generate viable embryos and offspring as a means of conserving genetic resources and rare animal breeds. Recently, more advanced technologies such as xenografting of gonadal (testicular and ovarian) tissues into immunodeficient experimental animals have been developed. In combination with in vitro embryo production techniques, this approach may provide many benefits. We have been carrying out studies to acquire basic information about the application of this method to porcine species, and to improve the existing techniques. Recently, we obtained oocytes from ovarian tissue xenografted and grown in nude mice that had the capacity to be fertilized and the ability to develop into early-stage embryos. We also obtained spermatozoa from the xenografted testicular tissues and injected them intracytoplasmically into in vitro-matured oocytes to produce piglets. Here we discuss the further possibilities of conservation and utilization of porcine gonadal tissue by xenografting into immunodeficient mice.
XENOGRAFTING OF OVARIAN TISSUE INTO MICE
Primordial follicles act as stores of ovarian follicles and can be potential sources of oocytes for medical, agricultural and zoological purposes. Ovarian grafting is a possible method of growing or maturing such oocytes in the primordial follicles (primordial oocytes) of large mammals. In humans and primates, grafting of ovarian tissues to another site in the body (autografting) has been used successfully to produce viable offspring (Donnez et al. 2004; Lee et al. 2004). Cross-species ovarian grafting (xenografting) seems to be more advantageous for the conservation and multiplication of domestic or endangered animals. In such cases, commercially available immunodeficent animals such as nude mice, severe combined immunodeficient (SCID) mice or nude rats are used as host animals. In 1994, Gosden et al. showed in a pioneering study that follicles were able to grow in cat and sheep ovarian tissues xenografted into SCID mice. In 2002, a further study demonstrated that mouse oocytes growing within ovarian tissue xenografted into nude rats acquired the ability to generate pups (Snow et al. 2002). To date, a number of reports have described the preparation of ovarian tissues from species phylogenetically distant from mice, including humans (Oktay et al. 1998; Weissman et al. 1999; Kim et al. 2002; Gook et al. 2003), dogs (Metcalfe et al. 2001), monkeys (Candy et al. 1995), sheep (Gosden et al. 1994), cattle (Senbon et al. 2003), pigs (Kaneko et al. 2003; Kagawa et al. 2005), tammar wallabies (Mattiske et al. 2002) and common wombats (Cleary et al. 2003, 2004), and their xenografting into immunodeficient mice. However, except for our study (Kaneko et al. 2003), none of these previous investigations succeeded in acquiring any oocytes that had been grown from primordial follicles, and which had matured or showed fertilization ability. Our study using porcine species was the first to demonstrate clearly that such oocytes were competent in these abilities. Furthermore, in our series of studies (Kaneko et al. 2006; Kikuchi et al. 2006), we have generated viable embryos from porcine primordial oocytes xenografted into nude mice. In this section, we summarize the recent data and discuss the possibility of further improvement of ovarian xenografting technology.
Maturation and fertilization in vitro of porcine oocytes grown in host mice
Ovarian tissues from 20-day-old piglets contain follicles at the primordial stage (Fig. 1). We transplanted pieces of these tissues under the capsules of the bilateral kidneys of ovariectomized nude mice (Kaneko et al. 2003). Histological evaluation showed that most of the follicles in the tissue were primordial. Forty-five to 70 days after grafting, the host mice in all groups showed vaginal opening with the appearance of cornified vaginal epithelial cells. This observation suggested that antral follicles were growing in the grafted ovarian tissues. The mice were then treated with 5 IU of equine chorionic gonadotropin (eCG) 10 days (eCG-10), 30 days (eCG-30), or 60 days (eCG-60) after the detection of cornified epithelial cells in their vaginal smears. Ovarian grafts and blood samples were collected 48 h after eCG treatment. Small antral follicles formed in the grafts in all of the mouse groups. After micro-dissection of the follicles with a surgical blade, we recovered a larger number of full-sized (> 115 µm in diameter) oocytes in comparison with the other groups. It has been reported that oocytes reaching 115 µm in diameter acquire the abilities to mature, to be fertilized and to develop (Motlik et al. 1984; Hirao et al. 1995). Treatment of host mice with eCG with optimal timing is important for obtaining good-quality oocytes, and the peripheral levels of total inhibin were highest in the eCG-60 group. This result also reflects the fact that enhanced growth of antral follicles occurred in this group. When 573 oocytes obtained from the eCG-60 group were matured in vitro, 98 (17.1%) of them were found to be at the metaphase-II stage. Moreover, 11 (55.0%) of 20 in vitro matured (IVM) oocytes with a first polar body were fertilized in vitro, resulting in both male and female pronucleus formation. These results clearly demonstrate that oocytes grown from porcine primordial follicles can become mature and fertilizable by a combination of xenografting into mice and in vitro culture (IVC).
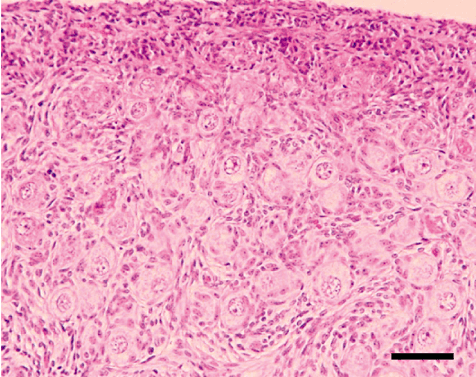
Histological appearance of porcine ovarian tissue before grafting into mice. Cortex area of neonatal (20 days old) donor porcine ovary before grafting. Scale bar indicates 100 µm.
In vitro and in vivo developmental ability of oocytes collected from host mice
For the next step, we evaluated the developmental ability to embryos derived from in vitro-fertilized oocytes after IVC or transfer to recipients (Kikuchi et al. 2006). The maturation rates, as determined from the presence of the first polar body, of oocytes collected from the host mice 48 h after treatment with eCG ranged from 25.1% to 42.5%. The matured oocytes were fertilized in vitro and then some of them were further cultured in vitro for 6 days, or transferred into estrus-synchronized recipients and recovered after 6 days. After IVC for 6 days, 15.4% (14/91) of the cultured oocytes had cleaved to the 2- to 8-cell stage; however, no morula or blastocyst was collected. In embryo transfer and recovery experiments (total of 4 trials), 59.9% (181/302) were recovered but all of them were found to have degenerated. These results suggest that the developmental ability of oocytes obtained from xenografts is insufficient, even after in vitro maturation.
Improvement of the developmental ability of oocytes grown in xenografts
Previous studies have demonstrated that the oocytes obtained from xenografts have no developmental ability when subjected to conventional maturation and fertilization procedures. To improve their developmental ability, we consider that the following two procedures may be applicable: (i) treatment of the host mice with gonadtropins, or (ii) manipulation of oocytes to confer developmental ability.
1 Treatment of host mice with gonadotropins
When we used ovaries from gilts obtained at a slaughterhouse, immature oocytes were obtained from follicles 3–5 mm in diameter, and these finally produced blastocysts at a rate of 25% (Kikuchi et al. 2002). In the case of ovarian xenografts, small numbers of follicles grew to the antral stage, but their diameters were generally less than 2 mm. The oocyte diameters were also smaller (< 115 µm) even after eCG treatment of the host mice (Kaneko et al. 2003). To improve the developmental ability of primordial oocytes xenografted into nude mice, we administered exogenous gonadotrophins to host mice with the aim of accelerating follicular growth (Kaneko et al. 2006). This approach was based on the idea that oocytes of appropriate size and good quality can be obtained only from larger follicles. Gonadotropin treatments were started around 60 days after vaginal cornification. Ovarian grafts were obtained 2 or 3 days after treatment with eCG (eCG-2 and eCG-3 groups, respectively), after porcine follicle-stimulating hormone (pFSH) infusion from an osmotic pump for 7 or 14 days, or after infusion of pFSH for 14 days with a single injection of antiserum against estradiol (pFSH-7, pFSH-14 and pFSH-14EA groups, respectively). This antiserum was expected to inhibit luteinization of follicles by suppressing the surge of mouse LH secrection, resulting in suppression of hemorrhage into the follicle antrum from the wall. Gonadotropin treatments accelerated follicular growth within the xenografts relative to that in control mice given no gonadotropins (Fig. 2a,b, respectively), consistent with the markedly higher circulating inhibin levels in the gonadotropin-treated mice. In contrast, circulating mouse FSH levels were depressed. These results indicated clearly that follicular growth can be supported by administration of exogenous gonadotropins, and not with endogenous mouse FSH. In this experiment, 56.4% (22/39) of IVM oocytes with a first polar body were fertilized in vitro in the pFSH-14EA group. After in vitro fertilization (IVF) and subsequent IVC for 7 days, one blastocyst (0.6%–0.9%) was obtained from each of the eCG-3, pFSH-7 and pFSH-14EA groups, whereas no blastocysts appeared in the other groups. These results suggested that exogenous gonadotropins, and not mouse FSH, stimulated the growing follicles that had grown from the primordial follicles in the xenografts: the effects were incomplete, but the meiotic and developmental abilities of the oocytes were improved to some extent.
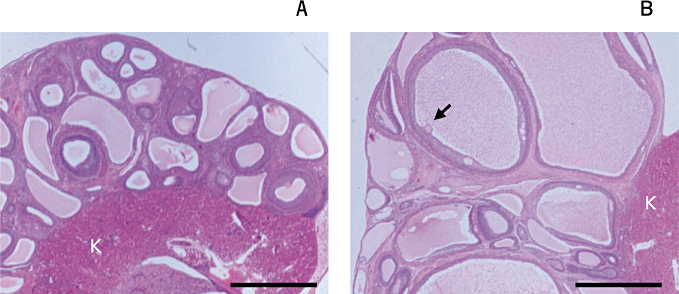
Histological appearance of porcine ovarian tissue after grafting into mice. Grafted tissues from a mouse from the control (a: no gonadotropin treatment, graft obtained 10 days after vaginal cornification) and follicle-stimulating hormone (FSH)-14EA groups (b: graft obtained after infusion of porcine FSH for 14 days followed by a single injection of antiserum against estradiol 7 days after the start of FSH infusion). An arrow indicates the oocyte enclosed in the cumulus of a well-grown follicle. K: kidney of a host mouse. Scale bars indicate 3 mm. Images are modified from Kaneko et al. (2006).
2 Fusion of cytoplasmic fragments prepared from oocytes with good developmental ability
Our previous studies suggested that, in pigs, oocytes derived from primordial follicles, even after in vivo growth with gonadotropin and IVM, appeared to have difficulty achieving full cytoplasmic maturation. We hypothesized that nucleus (cytoplasm) transfer or replacement might be capable of conferring developmental ability on such oocytes. This method is carried out by injecting or fusing a nucleus (and a small volume of the cytoplasm surrounding it) with the cytoplasm of a recipient oocyte having sufficient cytoplasmic maturity. Usually, the recipient cytoplasm can be generated by enucleation from oocytes matured from slaughterhouse ovaries. This method guarantees that the resulting reconstructed oocytes containing the necessary genetic information have an adequate degree of embryonic development. For this nuclear transfer, two major procedures have been reported, depending on the maturational stage of the oocyte: (i) germinal vesicle (GV) transfer (mice (Liu et al. 1999, 2003a; Moffa et al. 2002), rabbits (Li et al. 2001), cattle (Bao et al. 2003) and humans (Zhang et al. 1999)); and (ii) metaphase-II (M-II) chromosome transfer in mice (Wang et al. 2001; Liu et al. 2003b; Bai et al. 2006). With each procedure, successful embryo or offspring production has been reported in mice (Liu et al. 1999; Wang et al. 2001). However, in porcine species, nuclear replacement of oocytes either by GV transfer or M-II chromosome transfer has not yet been reported. These procedures can only be completed through micromanipulation by an experienced operator. Alternatively, we have recently reported the ‘centri-fusion’ method of nuclear transfer (Fahrudin et al. 2007; Nagai et al. 2007). This method can be applied for somatic cell nuclear transfer without utilization of special micromanipulators or injectors. Originally, cytoplasmic fragments without a metaphase plate (cytoplasts), which had been obtained after a combination of serial centrifugations of matured oocytes and zona pellucida removal, were attached with a somatic cell. They were then reconstructed by fusion with an electrical pulse. This method can be applied to generate mature oocytes by fusion of several cytoplasts and a cytoplasmic fragment with chromosomes (karyoplast). Theoretically, the resulting oocyte should be the same as a M-II-transferred oocyte (Maedomari et al. 2010). Furthermore, cytoplasm(s) can also be fused with an intact zona-free oocyte without centrifugation. This method is now expected to facilitate modification of cytoplasmic maturational ability, even in oocytes from xenografted ovaries. In fact, by this method, we were able to generate embryos of good quality (Fig. 3); the blastocyst rate was 14.8% and the cell number was 29.7 on average, after IVF and IVC (Kaneko et al. 2010). These results strongly suggest that fusion of cytoplasts prepared from oocytes with good developmental ability has promising potential for obtaining embryos of reasonable quality.
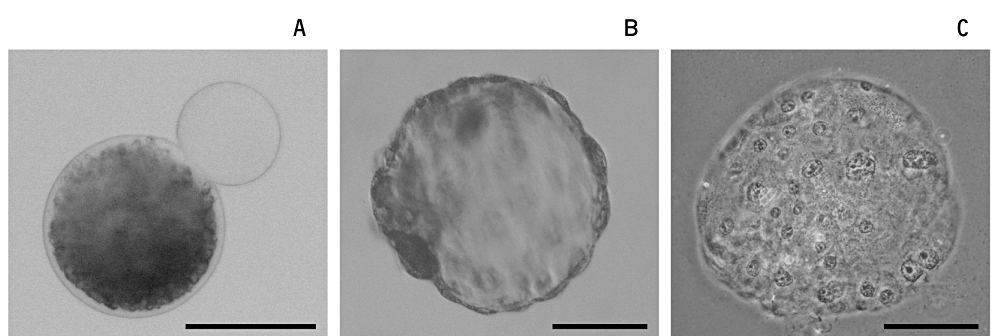
Development of embryos cultured from oocytes of xenografted tissue after the fusion of cytoplast(s). (a) Oocytes obtained from xenografts were each fused with a cytoplast prepared by the ‘centri-fusion’ method. The cytoplasts were free of lipid droplets and showed increased transparency compared with an intact oocyte. By this method, one or several cytoplasts could be fused simultaneously. (b and c) Embryos developed from an oocyte fused with three cytoplasms. Images before (b) and after (c) fixation and staining. This embryo had 120 cells. Scale bars indicate 100 µm.
Future studies of ovarian xenografting
We can now obtain oocytes grown from primordial follicles xenografted into mice. The oocytes can be fertilized and develop to blastocysts in vitro when they are fused with cytoplasts prepared by ‘centri-fusion’ from oocytes with good developmental ability. For the next step, we would like to confirm whether offspring can be obtained from those embryos. Evaluation of mitochondrial heterogeneity in oocytes fused with cytoplasm is also necessary; if possible, we would like to generate oocytes with full developmental ability without application of this fusion method. From the viewpoint of conservation of genetic resources, cryopreservation of ovarian tissues before xenografting may be further improved, and the possibilities of this approach for some species have been reported (Candy et al. 1995; Cleary et al. 2003; Kikuchi et al. 2010).
XENOGRAFTING OF TESTICULAR TISSUE INTO MICE
The possibility of spermatogenesis after transplantation of germ cells directly into the testis of a host animal has been suggested for mice (Brinster & Avarbock 1994; Brinster & Zimmermann 1994) and pigs (Honaramooz et al. 2002a). In these cases, germ cells, including spermatogonial stem cells that had been isolated by enzymatic treatment, were injected directly into the seminiferous tubules. It was expected that spermatozoa from the introduced cells would be produced in the ejaculate. However, this technique requires special skills for injection. Furthermore, sperm production is limited to small numbers, and accurate separation procedures are essential for selecting spermatozoa that have been derived from the introduced cells. Although germ cell transplantation into mice has allowed complete donor-derived spermatogenesis in rodents, this is not the case for large domesticated animals such as cattle, pigs and horses (Dobrinski et al. 2000).
Other approaches for inducing spermatogenesis include grafting of testicular tissues entopically (into the same site in the body) or ectopically (into another site in the body). Sperm have already been obtained from testicular tissues following entopic allografting (grafting into the same species) in mice (Shinohara et al. 2002), entopic xenografting (grafting between species) in rabbits (Shinohara et al. 2002), ectopic allografting in mice (Honaramooz et al. 2002b; Schlatt et al. 2003), and ectopic xenografting in hamsters (Schlatt et al. 2002), goats (Honaramooz et al. 2002b), rhesus macaques (Honaramooz et al. 2004), sheep (Zeng et al. 2006), cats (Snedaker et al. 2004) and pigs (Honaramooz et al. 2002b; Zeng et al. 2006, 2007; Kaneko et al. 2008; Nakai et al. 2009). In other domestic animals, only the spermatid, and not mature spermatozoa, can be obtained by ectopic xenografting in cattle (Oatley et al. 2004, 2005; Schmidt et al. 2006) and in horses (Rathi et al. 2006). Among these procedures, xenografting into immunodeficient experimental animals is the most attractive because host animals such as nude mice or SCID mice are available commercially. Ectopic xenografting of testicular tissues under the skin of the back can be performed much more easily and is thus advantageous (Honaramooz et al. 2002b). This approach has facilitated the production of caprine and porcine sperm grown in nude mice, showing also that spermatozoa from xenografts can fertilize mouse oocytes after intra-cytoplasmic injection (ICSI) to form the male pronucleus.
Recently, successful embryo production by using these sperm cells from xenografted testicular tissues has been reported, but limited to rhesus monkeys (Honaramooz et al. 2004) and pigs (Honaramooz et al. 2008; Nakai et al. 2009). In our previous study (Nakai et al. 2009), blastocysts with normal ploidy were produced after ICSI. Recently, we have also reported the successful production of live piglets when embryos just after ICSI using xenogeneic sperm were transferred to recipients (Nakai et al. 2010). Although the efficacy of piglet production remains low, the results clearly suggest that oocytes injected with a sperm that has differentiated from gonocytes within the xenografts have the ability to develop to viable offspring in a large mammal. In this section, we present recent data and discuss the possibility of further improvement of testicular xenografting technology.
Growing of porcine testicular tissues in host mice and spermatogenesis
In this series of experiments, we collected testicular tissues from newborn piglets (5–14 days after birth) and xenografted them immediately. As shown in Figure 4, testicular tissues before grafting contain seminiferous cords consisting of only gonocytes/spermatogonia and Sertoli cells. We first examined the growth status of the xenografted tissues and the possibility of sperm recovery (Kaneko et al. 2008). Ninety days after xenografting, formation of lumina was observed in the seminiferous cords. Spermatocytes and spermatids were detected in the seminiferous tubules at around 60 and 90 days, respectively, and spermatozoa were observed after 120 days. It was notable that the proportion of tubule cross-sections containing elongated spermatids and/or spermatozoa was lower in the grafted tissues at around 210 days than that of control tissues from normal boars (Fig. 5). Interestingly, part of the recovered graft contained seminiferous tubules in which spermatogenesis was confirmed, whereas other parts did not show completion of spermatogenesis (Fig. 6a). Spermatozoa were obtained after mincing of the tissues after 120 days (Fig. 6b); almost all the sperm had a cytoplasmic droplet and were morphologically similar to epididymal sperm. Recovery rates (no. mice producing sperm in the xenografts/total no. mice × 100) increased as the duration was prolonged to 210 days (30.7% for 120 days to 88.9% for 210 days).
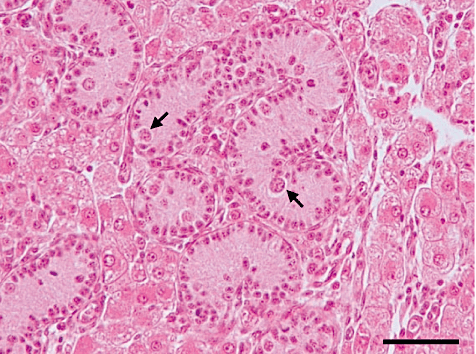
Histological micrograph of donor testis tissue from a 14-day-old piglet. The seminiferous cords contain only gonocytes and spermatogonia (arrows). Scale bar indicates 50 µm.
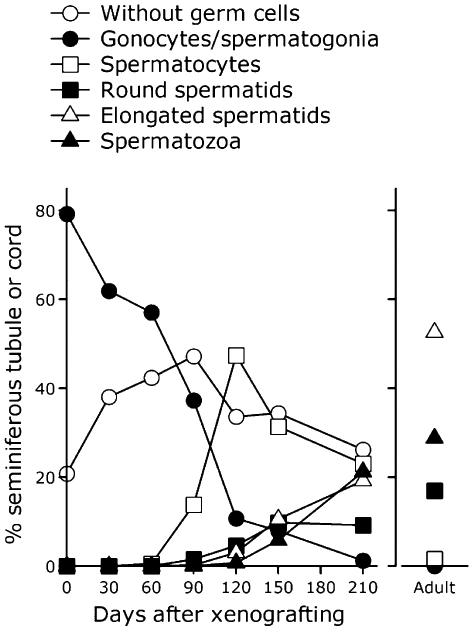
Percentage of seminiferous cord or tubule cross-section after xenografting. Histological sections of testicular tissues grafted into mice were prepared 0–210 days after xenografting. Those from adult boars were also prepared. The percentages were calculated after classification by the most advanced type of germ cell present. Averages from three mice in each group are presented. Data are represented from Kaneko et al. (2008).
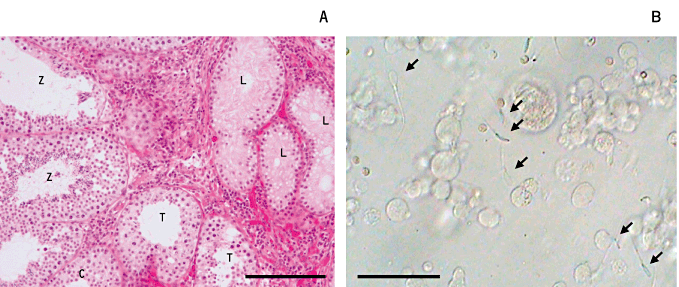
Spermatogenesis and recovery of spermatozoa from xenografted testicular tissues. (a) A histological section of porcine-grafted testicular tissue recovered 223 days after grafting. The recovered graft contained seminiferous tubules in which spermatogenesis was confirmed (Z), or with only spermatocytes (c) and with Sertoli cells (L). Scale bar indicates 100 µm. (b) Spermatozoa (arrows) collected from minced tissues 150 days after xenografting. They showed faint motility just after release into the medium; however, they were not considered applicable for artificial insemination or in vitro fertilization. Scale bar indicates 10 µm.
In vitro development of zygotes fertilized by ICSI of sperm obtained from xenografted tissues
In this study (Nakai et al. 2009), we obtained and used spermatozoa between 125 and 192 days after xenografting from 41 of 65 host mice (63.1%). As a positive control, we also collect testicular spermatozoa from adult boars. A single spermatozoon was injected into an in vitro-matured porcine oocyte, and the oocytes were electro-stimulated and cultured (graft-ICSI and testis-ICSI, respectively). Blastocyst rates in both ICSI groups (24.9% and 37.4%, respectively) were higher than those without the injection procedure (parthenogenetic; 12.7%) and after injection of a small amount of injection buffer (sham; 13.0%). Rates of diploid blastocysts in both the graft-ICSI and testis-ICSI groups (48.9% and 60.6%) were higher than those in the parthenogenetic and sham groups (13.5% and 28.0%). The qualities of the blastocysts did not differ among the experimental groups (Fig. 7). Therefore, we demonstrated that porcine oocytes injected with xenogeneic sperm have the ability to develop to the blastocyst stage in vitro.
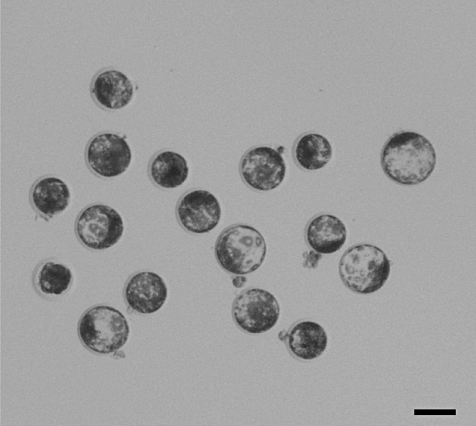
Porcine blastocysts developed from oocytes fertilized by injection of sperm obtained from xenografted testicular tissue and subsequent in vitro culture for 6 days. Scale bar represents 100 µm.
Development to piglets after transfer of ICSI-oocytes into recipient females
Development of embryos to term has been confirmed only in rabbits when sperm were obtained after entopic xenografting into mouse testes (Shinohara et al. 2002). We succeeded for the first time in obtaining embryo development to term in a large animal after ectopic xenografting (Nakai et al. 2010). Testicular tissues prepared from neonatal piglets were transplanted under the back skin of castrated nude mice. Between 133 and 280 days after xenografting, we collected morphologically normal spermatozoa. After ICSI of a single spermatozoon into an in vitro-matured porcine oocyte, the oocytes were electro-stimulated and transferred into estrus-synchronized recipients. Two out of 23 recipient gilts gave birth to one female and five male piglets (Fig. 8). The piglets have grown normally and both sexes have shown normal reproduction ability (H. Kaneko, K. Kikuchi, M. Nakai, N. Kashiwazaki, 2011, unpublished data).

A 50-day-old male piglet obtained after transfer of an oocyte injected with sperm from xenografted tissue. All male and the female piglet (total 6 piglets) grew normally to adulthood.
Future studies of testicular xenografting
As is the case for ovarian xenografting, cryopreservation of testicular tissues before xenografting should be examined. Although the possibilities of cryopreservation of testicular tissues have been reported in several species (Honaramooz et al. 2002b; Schlatt et al. 2002; Shinohara et al. 2002), the efficacy needs to be improved. The fertilization and developmental abilities of the sperm produced, and the normality of the offspring, will be assessed.
Conclusions and perspectives
We have demonstrated the feasibility of xenografting porcine gonadal tissues into nude mice. This approach can be used not only for the conservation of genetic resources but can also be applied to pigs and other large animals, allowing rescue of genetically modified animals that sometimes die before sexual maturity; the immature gonadal tissues can be preserved in experimental animals and used for the next generation. Furthermore, gametes or embryos can be obtained by these technologies within shorter periods when immature gonadal tissues are xenografted from juveniles or fetuses, compared with those from adult animals. For example, in cattle, we can usually obtain spermatozoa from adult bulls around 2 years of age; however, by using this procedure, it may be possible to shorten this period to a couple of months, being of considerable benefit for breeding programs. To meet these objectives, further basic research will be necessary.
ACKNOWLEDGMENTS
This series of studies was supported in part by Grants-in-Aid for Scientific Research (22380153 to K. K., 21380715 to H. K and 22658085 to N. K.) from the Japanese Society for the Promotion of Science. This research was also supported partially by a research project grant awarded by the Azabu University Research Services Division to N. K. The authors thank Drs J. Noguchi, J. Ito, T. Somfai, M. Ozawa, N. Maedomari, M. Hosoe, K. Ohnuma and T. Akita for the completion of experiments.




