DNA methylation analysis on satellite I region in blastocysts obtained from somatic cell cloned cattle
ABSTRACT
Many observations have been made on cloned embryos and on adult clones by somatic cell nuclear transfer (SCNT), but it is still unclear whether the progeny of cloned animals is presenting normal epigenetic status. Here, in order to accumulate the information for evaluating the normality of cloned cattle, we analyzed the DNA methylation status on satellite I region in blastocysts obtained from cloned cattle. Embryos were produced by artificial insemination (AI) to non-cloned or cloned dams using semen from non-cloned or cloned sires. After 7 days of AI, embryos at blastocyst stage were collected by uterine flushing. The DNA methylation levels in embryos obtained by using semen and/or oocytes from cloned cattle were similar to those in in vivo embryos from non-cloned cattle. In contrast, the DNA methylation levels in SCNT embryos were significantly higher (P < 0.01) than those in in vivo embryos from non-cloned and cloned cattle, approximately similar to those in somatic cells used as donor cells. Thus, this study provides useful information that epigenetic status may be normal in the progeny of cloned cattle, suggesting the normality of germline cells in cloned cattle.
INTRODUCTION
Somatic cell nuclear transfer (SCNT) technique is useful to produce multiple copies of genetically elite animals for breeding and transgenic animals for pharmaceutical protein production or for experimental model of human diseases (Schmidt 2006). Especially in livestock animals, because the productive performance greatly depends on genetic background, SCNT technique has the potential to produce unlimited copies of animals with high performance or other desirable traits. In fact, a previous study suggested that the same breeding performance was expected from mating a cloned dam and sire as from mating the animals used as nuclear donors for clones (Kasai et al. 2007). Therefore, in order to copy the elite with high economic value, many studies concerning SCNT technique have been conducted and more than 500 cloned cattle have been produced only in Japan (Watanabe & Nagai 2008). In addition, Hoshino et al. (2009) recently succeeded in the resurrection of a bull by SCNT from unprotected frozen tissue of a dead sire. Thus, developing the SCNT technique could be beneficial for the stock-raising industry. However, cloning efficiency is still low and various abnormalities have been observed, namely, aberrant epigenetic status (Kang et al. 2001, 2005; Wee et al. 2006; Sawai et al. 2010), abnormal gene expression (Amarnath et al. 2007; Sawai 2009), placental dysfunction (Hashizume et al. 2002) and high incidences of pregnancy loss and neonatal death (Watanabe & Nagai 2009). In addition, global gene expression profiles by using microarray analysis demonstrated abnormal gene expression in cloned mice which even appeared healthy on delivery (Kohda et al. 2005). For these reasons, even if cloned cattle healthily develop to adulthood, social acceptance for use of their products has not been completely gained at present.
Under such present conditions, the European Food Safety Authority (EFSA) and Food and Drug Administration (FDA) of the USA recently assessed whether products from cloned animals and their offspring posed any risks to other animals by being consumed as a food. As a result, their reports concluded that food products originating from healthy clones that meet the existing requirements for meat and milk in commerce pose no increased consumption risk(s) relative to comparable products from sexually derived animals (EFSA 2008; FDA 2008). In addition, a nationwide survey in Japan also suggested the normality of cloned cattle and their progeny (Watanabe & Nagai 2008, 2009). In other words, previous studies have investigated the health status, growth, productive performance and fertility of cloned cattle and offspring (Miyashita et al. 2003; Shiga et al. 2005; Yonai et al. 2005; Kasai et al. 2007; Watanabe & Nagai 2008). Based on these findings obtained from these reports, there was no remarkable difference in health status and food products among conventionally bred cattle, SCNT cloned cattle developed to adulthood and their offspring. However, there are few studies reporting the epigenetic characteristics of embryos from cloned cattle, even though abnormal epigenetic reprogramming was consistently observed in bovine SCNT embryos (Kang et al. 2001, 2005; Wee et al. 2006; Sawai et al. 2010). It is important to gain social acceptance of cloned animals, their offspring and food products when the SCNT technique is used for animal breeding. Therefore, the objective of this study was to investigate whether embryos produced from cloned dams and sires exhibit normal status of DNA methylation.
MATERIALS AND METHODS
Animals were treated according to preapproved animal care and use guidelines established for the National Agricultural Research Center for Kyushu Okinawa Region, Japan.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA), unless otherwise stated.
Production of cloned cattle
Cloned female and male cattle derived from Japanese Black cattle were produced by somatic cell nuclear transfer as described by Goto et al. (1999). Cumulus cells derived from cumulus-oocyte complexes (COCs) which were collected by ovum pick-up technique were used as donor cells, while a clonal bovine intramuscular preadipocyte line isolated from intramuscular white adipose tissue (Aso et al. 1995) was used as donor cells for production of a male clone. SCNT embryos developed to the blastocyst stage were transferred into recipient cows (Holstein). Viable cloned offspring were housed and fed under standard conditions, and then four cloned sires and a cloned dam were used in this study. These cloned cattle have normal fertility. In addition, it was confirmed that the length of pregnancy and birth weight of calves derived from these cloned cattle were similar to those of calves obtained from non-cloned cattle, and these calves had normally grown.
Collection of in vivo derived embryos
Collection of in vivo derived embryos was performed as described by Hashiyada et al. (2005). Briefly, superovulation was performed by injection of 20 IU of follicle-stimulating hormone (FSH: ANTORIN® R-10; Kawasaki Pharmaceutical, Kawasaki, Japan) and 2 mL of prostaglandin F2α (RESIPRON®-C; ASUKA-pharmaceutical, Tokyo, Japan) followed by artificial insemination (AI). AI was performed both on the day of estrus (day 0 of gestation) and the next morning, using frozen semen collected from a Japanese black bull produced by conventional breeding or SCNT. On day 7 after AI, embryos were recovered by uterine flashing. Blastocyst-stage embryos were selected and used for experiments.
Production of in vitro produced embryos
In vitro maturation, fertilization and culture procedures were performed as described previously (Balboula et al. 2010). Briefly, bovine ovaries were obtained from a local abattoir. COCs were aspirated from ovarian follicles with a syringe connected to a 19-gauge needle. After, washing with tissue culture medium-199 (TCM-199: Gibco, Grand Island, NY, USA), 50 COCs were cultured in 500 µL TCM-199 containing FSH (0.02 AU/mL; Denka, Kawasaki, Japan), estradiol-17β (1 µg/ mL), gentamicin (10 µg/mL) and 5% (v/v) fetal calf serum (FCS: Cat. no. 12483-020, Lot no. 1404232, Gibco) for 22 h at 38.5°C in a humidified atmosphere of 5% CO2 in air.
Spermatozoa were washed by centrifugation (700 × g for 10 min) in 90% (v/v) Percoll solution (GE Healthcare Bio-Sciences AB, Buckinghamshire, UK). After removing the supernatant, the pellet was diluted with IVF100 solution (Research Institute for the Functional Peptides, Yamagata, Japan) and further centrifugation was applied at 600 × g for 5 min. The spermatozoa pellet was diluted with IVF100 to prepare the final sperm cell concentration at 5–10 × 106/mL. After maturation, COCs were washed three times with IVF100 and then transferred into drops of sperm cell suspension (20 oocytes/100 µL drop). Fertilization was carried out for 6 h at 38.5°C in a humidified atmosphere of 5% CO2 in air.
After insemination, the cumulus cells surrounding putative zygotes were removed by pipetting. Presumptive zygotes were cultured in 50 µL micro-drops (20–30 zygotes/drop) of CR1aa (Rosenkrans et al. 1993) containing 5% FCS at 38.5°C in a humidified atmosphere of 5% O2, 5% CO2, and 90% N2 for 7 days.
Somatic cell nuclear transfer
Bovine fibroblast cells were obtained from the ear of a 20-day-old Japanese Black male calf and used as donor cells. Preparation procedure was performed as described previously (Yamanaka et al. 2010). For long-term storage, cells were collected after trypsin treatment, were frozen in Dulbecco's Modified Eagle's Medium (DMEM, Gibco) supplemented with 20% FCS and 10% dimethyl sulfoxide, and stored in liquid nitrogen. When needed, the cells were thawed and cultured in DMEM plus 5% FCS and passages 5–10 (based on an estimate of two cell doublings per passage) were used for experiments. For use as donor nuclei, cells were cultured 4–5 days past confluence.
After cultivating the oocytes up to maturation as described above, expanded cumulus cells were removed by pipetting in TCM-199 containing 1 mg/mL of hyaluronidase. Matured oocytes showing with a first polar body were selected and used for production of SCNT embryos. A portion of the zona pellucida near the first polar body was cut with a cutting needle prior to enucleation in a 50 µL drop of TCM-199 supplemented with 5% FCS. Then a small volume of cytoplasm surrounding the polar body was squeezed out through the slit made with the same cutting needle. The enucleated polar body and putative karyoplast were stained with 2 µg/mL Hoechst 33342 and observed by fluorescent microscopy (Nikon, Tokyo, Japan) to enable the successful selection of enucleated oocytes. Donor cells were trypsinized, washed by centrifugation, and resuspended in TCM199 supplemented with 0.5% bovine serum albumin (BSA). Donor cells were introduced into the perivitelline space of the recipient oocytes through the opening made during enucleation. The doublets were transferred in the Zimmerman's cell fusion medium and induced with two direct current electric pulses of 20 V/mm for 10 µs with a 0.1 s interval using an LF101 (TR Tech, Tokyo, Japan) equipped with a needle electrode. The fused oocytes were activated in CR1aa supplemented with 5% FCS, 10 µg/mL cycloheximide and 5 µg/mL cytochalasin D for 1 h and then in 10 µg/mL cycloheximide for 4 h. After washing with CR1aa supplemented with 5% FCS, we then cultured about 20 embryos in 50 µL drops of CR1aa supplemented with 5% FCS at 38.5°C in a humidified atmosphere of 5% O2, 5% CO2 and 90% N2 for 7 days.
DNA methylation analysis
Each blastocyst was separated into two parts, namely, ICM + TE which contains intact inner cell mass (ICM) with surrounding trophectderm (TE), and pure TE by micro-surgical blade (Bio-cut blade; Feather Safety Razor Co., Osaka, Japan). We used pure TE as the sample for DNA methylation analysis. In addition, in order to confirm the accuracy of results, we prepared bovine fibroblast cells and bovine fibroblast cells treated with DNA methyltransferase (DNMT) inhibitor 5-aza-2′-deoxycytidine (10 µmol/L for 1 week) for DNA methylation analysis as controls. Genomic DNA isolation and bisulfite treatment reaction were conducted using the EZ DNA Methylation-Direct™ Kit (Zymo Research, Orange, CA, USA) according to the manufacturer's instructions. After bisulfite treatment, satellite I region (which has 12 highly conserved CpG sites) was amplified by polymerase chain reaction (PCR). To amplify part of satellite I region, we used the PCR program consisting of 35 cycles (95°C for 30 s for denaturing, 46°C for 30 s for annealing, and 72°C for 20 s for extension) and primer set 5′-AATACCTCTAATTTCAAACT-3′ and 5′-TTTGTGAATGTAGTTAATA-3′ as described previously (Kang et al. 2001). The resulting PCR products contain two target sequences (CpG-4 and CpG-7) for the AciI restriction enzyme which only recognizes the methylated sequences, 5′-CCGC-3′ and 5′-GCGG-3′. For combined bisulfate restriction analysis (COBRA), 5 µL of the amplified PCR products were digested with 5 U of AciI restriction enzyme (New England Biolabs, Tokyo, Japan) for 1 h at 37°C, and then resolved on 7.5% polyacrylamide gel. After electrophoresis, the gel was stained with SYBR Gold nucleic acid gel stain (Molecular Probes, Carlsbad, CA, USA). Intensities of digested bands were calculated using image analyzer ChemiDoc and software Quantity One (Bio-Rad, Hercules, CA, USA). For DNA sequencing, the amplified PCR products were cloned into the pTAC-2 vector (BioDynamics Laboratory, Tokyo, Japan), transformed into competent cells, and selected colonies were amplified using the TempliPhi DNA Amplification Kit (GE Healthcare) according to the manufacturer's instructions. A DNA sequencing service (Greiner bio-one, Frickenhausen, Germany) was used, and DNA methylation analysis of the sequences obtained was performed using QUantification tool for Methylation Analysis (QUMA, http://quma.cdb.riken.jp/top/index.html) (Kumaki et al. 2008).
Statistical analysis
All data were analyzed using analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test. A P-value < 0.05 denoted a statistically significant difference.
RESULTS
In vivo embryos were prepared by AI in four experimental groups which were produced by different combinations of bulls (semen) and recipient (oocytes), namely, non-cloned recipient × non-cloned semen (nCL♀ × nCL♂), cloned recipient × non-cloned semen (CL♀ × nCL♂), non-cloned recipient × cloned semen (nCL♀ × CL♂) and cloned recipient × cloned semen (CL♀ × CL♂). Moreover, SCNT embryos were prepared as a control for showing aberrant DNA methylation pattern. DNA methylation patterns were analyzed by two methods, that is COBRA and DNA sequencing. For COBRA, band intensities were measured after electrophoresis of PCR products digested with restriction enzyme AciI, and the ratio of digested fragments was calculated as the level of DNA methylation on the satellite I region (Fig. 1). Different digestion percentages between control groups (i.e. bovine fibroblast cells treated with or without 5-aza-2′-deoxycytidine) were detected (81.9 vs. 60.7%). As shown in Figure 1B, COBRA showed that DNA methylation level on satellite I region in in vivo embryos was not different among experimental groups regardless of whether the origin of semen and/or oocytes was non-clone or cloned cattle (14.9–17.6%). In contrast, the DNA methylation level in SCNT embryos (61.4%) was significantly higher (P < 0.01) compared with those of any other experimental groups, including IVF embryos (21.9%).
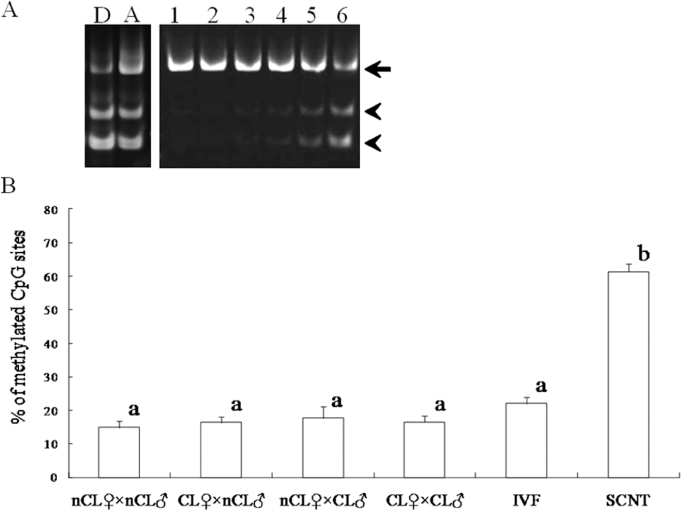
DNA methylation analysis of the satellite I region in blastocysts derived from cloned cattle by combined bisulfate restriction analysis. (A) Electrophoresis photograph shows different digestion patterns according to the level of DNA methylation. After digestion with AciI, polymerase chain reaction products of satellite I region were separated on 7.5% polyacrylamide gel. The percentage of DNA methylation was calculated from the intensity of digested fragments relative to the combined intensities of both digested (arrow head) and non-digested (arrows) fragments. D, donor cells (bovine fibroblast cells); A, donor cells treated with 5-aza-2′-deoxycytidine; 1–6, blastocysts produced by different embryo production techniques (1, nCL♀ × nCL♂; 2, CL♀ × nCL♂; 3, nCL♀ × CL♂; 4, CL♀ × CL♂; 5,IVF; 6, SCNT). (B) Histograms indicate the percentage of DNA methylation on the satellite I region. a,bindicate significant differences in the percentage of DNA methylation among groups (P < 0.01). Data are presented as the means ± SEM. nCL♀ × nCL♂, non-cloned recipient × non-cloned semen (n = 7); CL♀ × nCL♂, cloned recipient × non-cloned semen (n = 22); nCL♀ × CL♂, non-cloned recipient × cloned semen (n = 9); CL♀×CL♂, cloned recipient × cloned semen (n = 15); IVF, in vitro fertilized embryo (n = 13); SCNT, somatic cell nuclear transfer embryo (n = 17).
Next, we analyzed DNA methylation by using DNA sequencing. DNA methylation level in bovine fibroblast cells used as donor cells was also analyzed. The satellite I region in donor cells was highly methylated (80.0% and 82.5%, Fig. 2). As a control, the decrease in the level of DNA methylation level on the satellite I region was confirmed when these cells were treated with 5-aza-2′-deoxycytidine (63.9% and 64.5%, Fig. 2). As shown in 2, 3, DNA sequencing analysis showed the similarity in DNA methylation level among in vivo embryos originated from non-cloned and cloned cattle (10.8–20.2%), whereas satellite I region in SCNT embryos were highly methylated (68.7 ± 3.3%). These results were in agreement with those by COBRA (Fig. 1B).
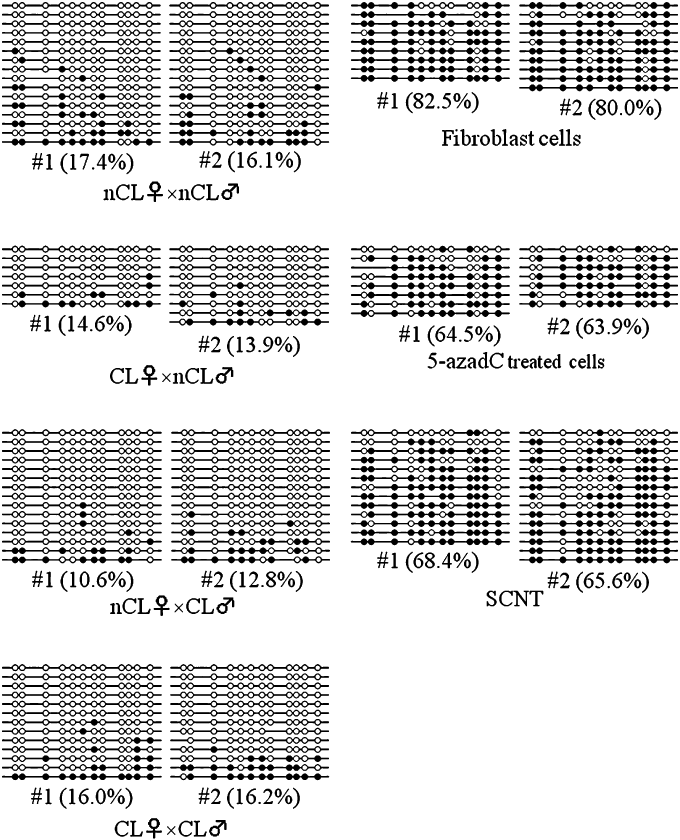
DNA methylation status of satellite I sequences in blastocysts derived from cloned cattle. Two representative data are shown from each experimental group. Methylation profiles of 12 CpG dinucleotides were analyzed, with methylation scores for each CpG obtained by sequencing polymerase chain reaction clones derived from bisulfite-treated genomic DNAs. Blank and filled circles represent unmethylated and methylated CpGs, respectively. Some CpG sites are absent from certain clones due to mutations present in their satellite sequence. Percent methylation is the proportion of methylated CpG sites relative to the total number of CpG sites. nCL♀ × nCL♂, non-cloned recipient × non-cloned semen; CL♀ × nCL♂, cloned recipient × non-cloned semen; nCL♀ × CL♂, non-cloned recipient × cloned semen; CL♀ × CL♂, cloned recipient × cloned semen; SCNT, somatic cell nuclear transfer embryo, fibroblast cells, bovine fibroblast cells used as donor cells; 5-azadC treated cells, bovine fibroblasts cells treated with 5-aza-2′-deoxycytidine.
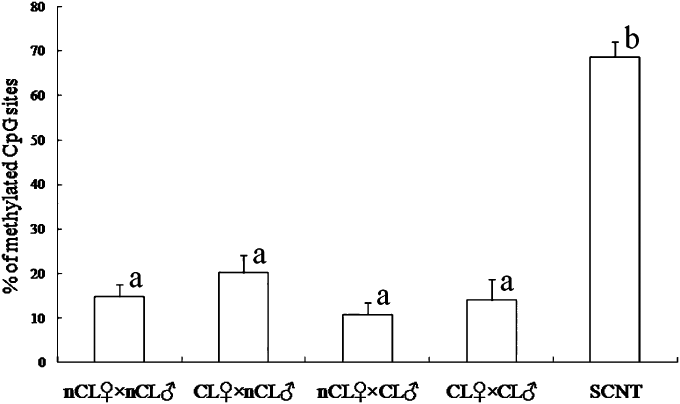
Summary results of DNA methylation analysis on satellite I region by bisulfite sequencing. nCL♀ × nCL♂, non-cloned recipient × non-cloned semen (n = 6); CL♀ × nCL♂, cloned recipient × non-cloned semen (n = 12); nCL♀ × CL♂, non-cloned recipient × cloned semen (n = 7); CL♀ × CL♂, cloned recipient × cloned semen (n=8); SCNT, somatic cell nuclear transfer (n = 11). a,bValues with different superscripts within each column are significantly different (P < 0.01). Data are presented as the means ± SEM.
DISCUSSION
In order to accumulate further information for evaluating the normality of cloned cattle and their progeny, we focused on DNA methylation status on satellite I region in bovine SCNT embryos. Because previous studies have reported that hypermethylation status of genomic DNA in TE cells directly influence developmental competence by altering gene expression (Kang et al. 2001; Beaujean et al. 2004), DNA methylation status of satellite I region in TE cells was analyzed in this study. As a result, we found that DNA methylation status on satellite I region in embryos derived from cloned cattle was similar to that in embryos derived from non-cloned ones.
DNA methylation at cytosine residues in CpG dinucleotides is known as one of the epigenetic modifications which regulates gene expression pattern (Jaenisch & Bird 2003). The DNA methylation status dramatically changes during embryonic development (Santos & Dean 2004). The genome during pre-implantation stages undergoes global demethylation, resulting in a low level of DNA methylation status (Santos et al. 2002; Armstrong et al. 2006). However, previous studies have demonstrated that the SCNT embryo does not fully undergo DNA demethylation (Dean et al. 2001; Kang et al. 2001; Wee et al. 2007). Thereby, SCNT embryos keep the DNA hypermethylation status originated from the donor cells. In addition, the difference in DNA methylation level on satellite I region between in vitro fertilized (IVF) and SCNT embryos was more remarkable in comparison with those of other satellite regions up to the blastocyst stage (Kang et al. 2005). In fact, in this study, the levels of DNA methylation on satellite I region in SCNT blastocysts were significantly higher than those of in vivo and IVF blasytocysts. As one of factors to affect DNA methylation status, Suzuki et al. (2009) demonstrated that in vitro culture led to abnormal expression of the maternally imprinted SNRPN gene in bovine embryos and fetuses, by altering DNA methylation level at this locus. However, although it is unclear whether the effect of environmental conditions on DNA methylation status is different among genes, there is no difference in DNA methylation levels on satellite I region between blastocysts produced by AI and IVF in this study. On the other hand, DNA methylation levels of SCNT blastocysts were significantly higher than those of AI and IVF groups (Fig. 1). This result is consistent with previous studies reporting that the level of DNA methylation on satellite I region in the SCNT blastocyst was significantly higher than those of normally fertilized blastocysts (Kang et al. 2001, 2005; Sawai et al. 2010). Taken together, these findings demonstrate that DNA hypermethylation on satellite I region in SCNT embryos was an essential matter which was caused by the SCNT technique, not in in vitro culture. For these reasons, abnormal DNA hypermethylation on satellite I region is one of the typical characteristics in SCNT embryos. Therefore, its pattern could be used as one of the indicators for evaluating the normality of SCNT embryos.
Fulka et al. (2004) reviewed that epigenetic reprogramming occurs at two steps in SCNT embryos. The first step of reprogramming is achieved after the donor nucleus is transferred into the enucleated oocyte and during the next few divisions. By the first step of reprogramming process, about 1–5% of SCNT embryos are capable of developing to viable offspring, even with some epigenetic errors. Next, the second step of reprogramming occurs only in developing fetal germ cells. By the second step of reprogramming, errors that were not corrected during the first step are repaired only in germ cells. For this reason, it is hypothesized that cloned animals have germ cells that are epigenetic error-free, but have somatic cells with some errors. In fact, although some cloned animals might possess genomic and physiological errors even if they developed to fertile adults, their offspring did not possess such errors (Ortegon et al. 2007). Our study shows that DNA methylation patterns on satellite I region in blastocysts produced by using semen and/or oocytes obtained from cloned cattle appeared normal compared with those produced by conventional methods such as artificial insemination and in vitro fertilization. This data indicated that there might be no epigenetic error in embryos obtained from cloned cattle, unlike SCNT embryos. Thus, this finding has supported the hypothesis that any errors in cloned cattle are not passed on to their progeny.
In regard to DNA methylation analysis, we assessed DNA methylation status by using two methods (i.e. DNA sequencing and COBRA) in this study. Although DNA sequencing can detect DNA methylation in all methylation sites, it is a complicated processes and takes a long time to obtain analytical results. On the other hand, although COBRA is an easy and rapid (approximately 8 h) method for DNA methylation analysis, it can only detect the sites containing the sequences which specific restriction enzymes can recognize. However, there are few reports showing the direct comparison of data obtained from each method. Here, we showed that there is no difference in results between DNA sequencing and COBRA in respect to DNA methylation analysis on satellite I region. This finding indicated that COBRA enables a rapid and simple DNA methylation analysis, which might be useful for analyzing biopsy samples before embryo transfer.
On the other hand, Sawai et al. (2010) recently reported that DNA methylation level on satellite I region in SCNT embryos became similar to that of normally fertilized embryos at the elongated stage even if the level was significantly higher at the blastocyst stage. Although it is unclear whether only demethylated SCNT embryos can develop to the elongated stage, various abnormalities are observed in SCNT fetuses beyond the elongated stage (Watanabe & Nagai 2009). These findings indicated that DNA methylation level on satellite I region in SCNT embryos should be corrected at an earlier stage. Although analyzing the fluctuation of DNA methylation according to developmental stages is required, our data clearly demonstrate that DNA methylation levels on satellite I region in embryos obtained from cloned cattle are normal at the blastocyst stage, unlike SCNT embryos.
In conclusion, this study is the first report showing that DNA methylation levels are similar between in vivo embryos derived from cloned and non-cloned cattle. Further studies which investigate spatial and temporal DNA methylation analysis are needed, not only for evaluating epigenetic normality of embryos, but for elucidating epigenetic reprogramming.
ACKNOWLEDGMENTS
We thank Dr Ahmed Zaky Balboula, Ms. Natsuko Matsumoto, Ms. Michie Teramoto and Ms. Etsuko Kawano for assistance with experiments. We also thank Kumamoto Meat Trading Center Ltd. and Kumamoto the Prefecture Meat Inspection Office for helping us to collect bovine ovaries.




