Calf production from vitrified bovine sexed embryos following in-straw dilution
ABSTRACT
The objective of this study was to develop an in-straw dilution method suitable for direct transfer of vitrified bovine sexed embryos. Embryo sexing was performed by molecular diagnosis. Several sexed and vitrified-warmed embryos were transferred after evaluation of morphologically embryonic survival at warming and in-straw dilution (Evaluation group). The other embryos were immediately directly transferred to recipients without first being expelled from the straws after in-straw dilution (Non-evaluation group). The pregnancy rates of vitrified sexed embryos were 38.7% and 34.8% in the Evaluation group and Non-evaluation group, respectively, which were not significantly different. The viability of lower quality embryos before vitrification tended to be lower (P = 0.087) than that of the higher quality embryos regardless of evaluating embryos after warming and in-straw dilution. The abortion rates were similar, and there was no difference between the two groups (13.9% and 12.5%, respectively). These results demonstrate that vitrified bovine sexed embryos can be vitrified and diluted by the in-straw method and that the vitrified and warmed sexed embryos can develop to term.
INTRODUCTION
Embryo transfer of bovine sexed embryos has a major impact on both dairy and beef industries. For commercial application of the technology, it is extremely important to cryopreserve bovine sexed embryos. Bovine sexed embryos after cryopreservation resulted in reduced viability and pregnancy rates after embryo transfer (Thibier & Nibart 1995; Hasler et al. 2002). In general, it is known that during vitrification of mammalian embryos there must be no intracellular ice formation, which is a major cause of cell injury during freezing (Kasai 1997). Although many vitrification methods have been developed for cattle embryos (Massip et al. 1987; Douchi et al. 1990; Ishimori et al. 1993; Kasai 1997), most of these protocols require several steps for removal of the cryoprotectant before embryo transfer (Massip et al. 1987; Douchi et al. 1990; Ishimori et al. 1993). Moreover, to apply this technology extensively on farms it would be required that vitrified bovine sexed embryos can be warmed, diluted and transferred non-surgically into recipients under farm conditions very similar to those used for artificial insemination.
On the other hand, Leibo (1984) and Suzuki et al. (1984) reported on a one-step straw method in which bovine embryos were frozen with glycerol, which subsequently could be diluted in straws with sucrose solution before being transferred into the recipients. In addition, Massip et al. (1987), Voelkel and Hu (1992) and Dochi et al. (1998) reported on a direct transfer method in which frozen bovine embryos could be transferred directly to recipients after thawing without diluting the cryoprotectant.
The objective of the present study was to develop an in-straw dilution method suitable for direct transfer of vitrified bovine sexed embryos. We also examined gestation duration and sex of born calves derived from the sexed embryos.
MATERIALS AND METHODS
Embryo collection
Embryos were collected from superovulated cows of the Japanese Black and Holstein strains by a non-surgical flushing technique 7–8 days after the onset of estrus. The embryos were examined microscopically to determine their morphological qualities and developmental stages as previously described (Robertson & Nelson 1998). Embryos developed into the compacted morula to expanded blastocyst stages with code 1 (morphologically excellent or good embryos) and code 2 (fair embryos) according to a previous report (Robertson & Nelson 1998) were used in the present study.
Biopsy and sexing of embryos
Embryos were placed into microdrops of phosphate-buffered saline (PBS) supplemented with 0.2 mol/L sucrose under an inverted microscope equipped with a micromanipulator (Narishige Co Ltd, Tokyo, Japan). A small piece of each embryo was removed from the edge of the compact morulae or from the trophoblast of blastocysts by cutting with a microrazor blade (Bio-cut blade, Feather Safety Razor Co Ltd, Osaka, Japan) attached to the micromanipulator.
The biopsied embryos were cultured for 3–5 h in TCM199 (Gibco, Invitrogen, Grandland, NY, USA) supplemented with 20% fetal calf serum (FCS) and 0.1 mmol/L β-mercaptoethanol (Sigma-Aldrich Chemicals, St. Louis, MO, USA) (ME199) at 38.5°C under 5% CO2 in humidified air.
The small pieces of embryos were sexed with a Loop-mediated Isothermal Amplification (LAMP) Kit (Eiken Chemical, Tokyo, Japan), XY selector (Itoham Foods Inc, Hyogo, Japan) or YCD primer (Unicoop Japan, Tokyo, Japan). The sex of biopsied embryos as the counterparts was predicted by the sex of the small pieces.
Vitrification of biopsied embryos
The vitrification procedure is based on the method originally designed for intact bovine embryos by Ishimori et al. (1993), with slight modifications. The sexed embryos were exposed to 200 µL of equilibrated solution (VS50, 50% vitrification solution) consisting of 12.5% ethylene glycol (EG, Nacalai Tesque Inc., Kyoto, Japan), 12.5% dimethylsulfoxide (DMSO, Nacalai Tesque) dissolved in PBS supplemented with 0.3% bovine serum albumin (BSA, Sigma-Aldrich) at room temperature for 60 s. The embryos were then exposed to vitrification solution (VSED) consisting of 25% EG, 25% DMSO dissolved in PBS supplemented with 0.3% BSA and kept for 30 s. As shown Fig. 1, the sexed embryos were loaded into 0.25-mL plastic straws (IMV, L'Aigle, France) containing dilution solution 5% EG, 0.15 mol/L sucrose (Nacalai Tesque) in PBS supplemented with 20% FCS, then with two 11-µL droplets of VSED. The straws were immediately heat-sealed, placed horizontally in liquid nitrogen vapor at −180°C for 2 min and then immersed into liquid nitrogen for storage. The sexed embryos were stored for at least 5 days.
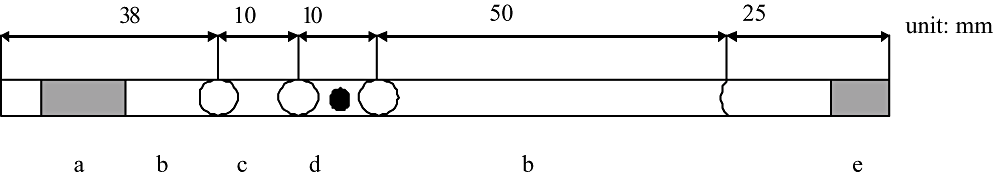
The loading constructions of the straws for in-straw dilution. (a) Cotton plug. (b) Dilution solution (5% ethylene glycol and 0.15 mol/L sucrose solution). (c) Vitrification solution (25% ethylene glycol 25% dimethylsulfoxide solution). (d) Embryo included in vitrification solution. (e) Heat seal.
The procedure for the in-straw dilution is shown in Figure 2. Straws containing embryos were exposed in air for 5 s and then plunged into 20°C water to warm the diluents for 30 s. Straws were then removed from the water and shaken to mix the columns of the straws. Straws were then held vertically in the same water bath for 3 min. Several warmed embryos were expelled into ME199 and held for 3 min at room temperature. The embryos were then washed and cultured for 3–5 h in ME199 at 38.5°C under 5% CO2 in humidified air to evaluate the embryonic morphology.
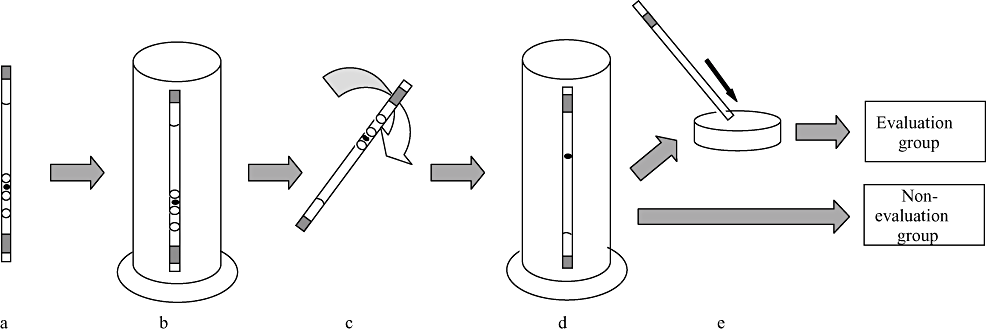
In-straw dilution procedure of vitrified bovine sexed embryos. (a) The straw was exposed to air for 5 s. (b) The straw was plunged vertically into a 20°C water bath for warming until the dilution solution melted. (c) The straw, closed with the heat seal, was shaken to mix the columns of liquid. (d) After mixing, the straw was held vertically in the same water bath for 3 min to dilute the cryoprotectants. (e) The sexed embryos were transferred to recipients after evaluation for survival (Evaluation group). The sexed embryos were directly transferred to recipients without being expelled from the straws (Non evaluation group).
Embryo transfer
The recipients used in the present study were cows and heifers. Most recipients were Holsteins, although other breeds, including Japanese Black and cross-breeds, were employed. Embryo transfers were performed on day 7 or 8 (day 0 = onset of estrus) by trained technicians. Pregnancy was diagnosed by palpation via the rectum or ultra-sonography after day 35 of embryo transfer.
The post-warmed surviving embryos (Evaluation group) were re-loaded into 0.25-mL plastic straws and non-surgically transferred into the uterine horn ipsilateral to the ovary bearing corpus luteum (one embryo per recipient). The other vitrified-warmed embryos (Non-evaluation group) were non-surgically transferred to the recipient after in-straw dilution without being expelled from the straws.
Statistical analyses
The significance of differences between means (rate of viability, rate of pregnancy) was compared by Pearson's χ2 test or Fisher's exact test using the SPSS program (SPSS 11.5J; SPSS Inc., Tokyo, Japan). Differences with probability values P < 0.05 were considered significant.
RESULTS
The viability of vitrified bovine sexed embryos after in-straw dilution is shown in Table 1. There were no significant differences in survival rates and pregnancies related to the quality of embryos. However, the viability of the code 2 (lower quality) embryos tended to be lower (P = 0.087) than that of the code 1 (higher quality) embryos regardless of evaluating embryos after warming and in-straw dilution. There were also no significant differences in rates of abortion between the Evaluation group (13.9%, 5/36) and the Non-evaluation group (12.5%, 4/32).
| Groups† | Embryo quality before vitrification‡ | No. of vitrified/ warmed embryos | No. of surviving embryos (%) | No. of recipients | No. of pregnancies (%)§ | No. of abortions (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Evaluation group | Code 1 | 71 | 67 | (94.4) | 67 | 29 | (40.8) | 2 | (6.9) |
| Code 2 | 22 | 18 | (81.8) | 18 | 7 | (31.8) | 3 | (42.9) | |
| Total | 93 | 85 | (91.4) | 85 | 36 | (38.7) | 5 | (13.9) | |
| Non-evaluation group | Code 1 | 77 | 77 | 28 | (36.4) | 3 | (10.7) | ||
| Code 2 | 15 | 15 | 4 | (26.7) | 1 | (25.0) | |||
| Total | 92 | 92 | 32 | (34.8) | 4 | (12.5) | |||
- † Vitrified-warmed embryos in the Evaluation group were transferred to recipients after evaluation of embryo survival. Vitrified-warmed embryos in the Non-evaluation group were transferred to recipients without being expelled from the straws.
- ‡ Code 1: morphologically excellent or good embryos, Code 2: fair embryos (Robertson & Nelson 1998).
- § §Diagnosis by palpation via the rectum on day 35 after embryo transfer.
The viability of vitrified bovine sexed embryos and embryonic stages before vitrification on Evaluation and Non-evaluation groups is shown in Table 2. Survival rates and pregnancy rates in various developmental stages of embryos were not significantly different.
| Groups† | Embryonic stages | No. of warmed embryos | No. of surviving embryos (%) | No. of recipients | No. of pregnancies (%)‡ | ||
|---|---|---|---|---|---|---|---|
| Evaluation group | CM | 13 | 11 | (84.6) | 11 | 5 | (38.5) |
| EB | 33 | 28 | (84.8) | 28 | 14 | (42.4) | |
| BL | 40 | 39 | (97.5) | 39 | 15 | (37.5) | |
| EXB | 7 | 7 | (100.0) | 7 | 2 | (28.6) | |
| Non-evaluation group | CM | 23 | 23 | 6 | (26.1) | ||
| EB | 25 | 25 | 7 | (28.0) | |||
| BL | 39 | 39 | 18 | (46.2) | |||
| EXB | 5 | 5 | 1 | (20.0) | |||
- † Vitrified-warmed embryos in the Evaluation group were transferred to recipients after evaluation of embryo survival. Vitrified-warmed embryos in the Non-evaluation group were transferred to recipients without being expelled from the straws.
- ‡ ‡Diagnosis by palpation via the rectum on day 35 after embryo transfer. BL, blastocyst; CM, compacted morula; EB, early blastocyst; EXB, expanded blastocyst.
As for time required for embryo transfer, there were no significant differences in pregnancy rates of vitrified sexed embryos regardless of evaluating embryos (Table 3).
| Groups† | Time required for embryo transfer‡ | No. of recipients | No. of pregnancies (%)§ | |
|---|---|---|---|---|
| Evaluation group | Within 5 min | 55 | 22 | (40.0) |
| More than 6 min | 8 | 3 | (37.5) | |
| Non-evaluation group | Within 5 min | 65 | 26 | (40.0) |
| More than 6 min | 25 | 6 | (24.0) | |
- † Vitrified-warmed embryos in the Evaluation group were transferred to recipients after evaluation of embryo survival. Vitrified-warmed embryos in the Non-evaluation group were transferred to recipients without being expelled from the straws.
- ‡ ‡The time required from the placement of the straws in the transferring gun until embryo transfer.
- § §Diagnosis by palpation via the rectum on day 35 after embryo transfer.
The gestation period of Holstein calves (n = 17) was 279.2 ± 8.1 days, and that of Japanese Black calves (n = 41) was 285.7 ± 4.3 days. The sex accuracy of the sexed embryos was assessed by the sex of calves at birth. A total of 16 male and 3 female calves were derived from embryos identified as male, and 2 male and 28 female calves were derived from embryos identified as female. The accuracy rate of vitrified bovine sex- embryos was 89.8% (44/49).
DISCUSSION
The objective of the present study was to develop an in-straw dilution method which is suitable for direct transfer of vitrified bovine sexed embryos. In the present study, vitrified bovine sexed embryos were transferred to recipients after in-straw dilution, thus allowing direct transfer of vitrified and warmed embryos without the need to recover embryos from the devices for containing embryos. Therefore, the procedure is useful for application in cattle farms.
Vajta et al. (1995) reported that in vitro-fertilized (IVF)-derived blastocysts vitrified and diluted in straws showed high rates of re-expanding and hatching. Saha et al. (1996) reported that five vitrified-warmed bovine embryos, using 40% EG + 11.3% trehalose + 20% polyvinylpyrrolidone, were directly transferred to five recipients, and three recipients gave birth to three normal calves. Van wagtendonk-de leeuw et al. (1997) also reported that vitrification of bovine embryos using 6.5 mol/L glycerol and in-straw dilution with 1.0 mol/L sucrose, yielded a pregnancy rate comparable to that obtained using controlled slow freezing and three-step dilution. Survival rates of vitrified bovine sexed embryos after in-straw dilution were found to be the same as those reported previously for intact (not sexed) bovine embryos (Vajta et al. 1995; Saha et al. 1996). There were no significant differences in pregnancy rates regardless of evaluating embryos after warming and in-straw dilution in the present study. The results demonstrate that bovine sexed embryos vitrified with VSED could be warmed by in-straw dilution and transferred directly to recipients. This in-straw dilution and direct transfer method reduces the requirements for equipment and technical skill, which are obstacles to the application of embryo transfer technology in cattle farms where appropriate laboratory conditions are not available.
Hasler et al. (2002) reported that the biopsy of bovine embryos judged as fair, whether they are fresh or frozen, resulted in significantly lower pregnancy rates compared to those of code 1 embryos judged as higher quality. Ushijima et al. (1995) also reported on a comparable result. In the present study, the survival rate of the embryos morphologically judged as fair (code 2) tended to be lower (P = 0.087) than that of the embryos morphologically judged as excellent or good (code 1) in the Evaluation group. The pregnancy rate of the embryos morphologically judged as fair (code 2) was also lower than that of the embryos morphologically judged as excellent or good (code 1), about 10% in each group, but the difference was not significant. These results indicate that it may improve pregnancy rates by selecting the embryos according to morphological quality before vitrification.
In the present study, there were no significant differences in survival rates and pregnancies between the developmental stages (compacted morula-expanded blastocyst stages) of embryos in both groups. Massip et al. (1987) and Douchi et al. (1990) reported that the pregnancy rates of bovine morulae after vitrification and warming were similar to those obtained after conventional slow freezing, but the pregnancy rates were not higher than those obtained using blastocysts. On the other hand, Ishimori et al. (1993) and Campos-chillón et al. (2005) demonstrated that there was no difference in the hatching rate or pregnancy rates between the vitrified morulae and blastocysts.
In the present study, the time required from the placement of the straws in the transferring gun until embryo transfer was recorded for each embryo transfer in both groups, and this ranged from 3 to 10 min, with an average time of 4.9 min. In both groups, a lower pregnancy rate was obtained when more than 6 min were required to complete embryo transfer than if the transfer was completed within 5 min, but the difference was not significant. Dochi et al. (1998) reported that in a conventional direct transfer after slow freezing, a lower pregnancy rate was obtained when more than 11 min were required to complete an embryo transfer than if the transfer could be completed within 10 min. Maintaining embryos in the freezing medium for longer periods may be detrimental even after thawing, thus lowering the pregnancy rate after transfer. Also, Matoba et al. (2003) reported that the post-thaw viability of embryos frozen with 1.5 mol/L EG is influenced by the holding period in the freezing medium after thawing. In the present study, maintaining embryos in diluted vitrification solution for longer periods may cause the lower pregnancy rate after transfer, similar to the effect seen in conventional direct transfer. In addition, Kasai (1997) has pointed out that if handling of the embryos is treated inappropriately during the vitrification process, the viability of embryos is reduced by chemical toxicity, osmotic injury and de-vitrification. We suggest that the viability of vitrified embryos after in-straw dilution should be examined in relation to maintenance temperature and the hold time to promote the field use of the in-straw dilution and direct transfer method.
In the present study, the abortion rates and the gestation period of calves derived from vitrified sexed embryos is in agreement with those of other studies (King et al. 1985a,b; Agca et al. 1998; Dochi et al. 1998; Numabe et al. 2001). King et al. (1985a) reported recipient abortion rates of approximately 5% at 2–7 months of gestation when fresh embryos had been transferred. Dochi et al. (1998) reported recipient abortion rates of 9.6–13.3% when frozen embryos had been transferred and demonstrated that the abortion rate was not significantly related to cryoprotectants or dilution methods. The abortion rates of vitrified bovine sexed embryos in our study were similar to those noted above, and we found no difference between the two groups (Evaluation group, 13.9%; Non-evaluation group, 12.5%).
Agca et al. (1998) demonstrated that the mean gestation period of artificial insemination calves were no different from those of calves derived from vitrified sexed embryos. Comparable results were obtained in the present study.
Hirayama et al. (2004) reported that the pregnancy rate of sexed embryos was 57.4% and all calves born were of the predicted sex (12 male and 21 female). Similarly, Hasler et al. (2002) also reported that the accuracy of sex determination was 98.7% for male and 94.4% for female embryos. Thibier and Nibart (1995) demonstrated that the accuracy of sex determination was 98%. In the present study, the accuracy of sex determination was 89.8% (44/49); the sex of five newborn calves did not agree with the pretransfer determination. In particular, the three female calves were derived from three male embryos, so the variance seems to be a result of contaminations with bovine DNA different from that of the samples. Herr and Reed (1991) reported that contaminations can arise from FCS or BSA used in the culture medium, from spermatozoa or cells adhering to the zona products of previous assays. To apply the sexing of embryos, it is necessary to be aware of these sources of error.
In conclusion, the results of the present study demonstrate vitrified bovine sexed embryos can be vitrified with VSED and diluted by the in-straw method and that the vitrified and warmed sexed embryos can develop to term. However, further studies are required to improve the viability of vitrified bovine sexed embryos after in-straw dilution.
ACKNOWLEDGMENT
This work was supported in part by the Japanese Ministry of Agriculture, Forestry and Fisheries.




