Inheritance and expression of stripe rust resistance in common wheat (Triticum aestivum) transferred from Aegilops tauschii and its utilization
Abstract
Stripe rust is one of the most destructive diseases for wheat crops in China. Two stripe rust physiological strains, i.e. CYR30 (intern. name: 175 E 191) and CYR31 (intern. name: 293 E 175) have been the dominant and epidemic physiological strains since 1994. One Aegilops tauschii accession (SQ-214) from CIMMYT was found immune from or highly resistant to Chinese new stripe rust races CYR30 and CYR31 at adult stage. SQ-214 was crossed with a highly susceptible Ae. tauschii accession As-80. Analysis of data from F1-F2 populations of SQ-214/As-80 revealed that the resistance was controlled by a single dominant gene. To exploit the resistance for wheat breeding, SQ-214 was crossed with Chinese Spring (CS) and backcrossed by two Chinese commercial wheat varieties MY26 and SW3243. The resistance from SQ-214 was suppressed in the F1 hybrids (CS/SQ-214) and the F2 population of CS/SQ-214//MY26. However, the resistance of SQ-214 was expressed in several F2 individuals of CS/SQ-214//SW3243. Eleven advanced lines with high level of resistance to the Chinese stripe rust CYR30 and CYR31 have been developed. This result suggested that SW3243 does not suppress the expression of the Chinese stripe rust and should be used as wheat germplasm for exploiting resistance of Ae. tauschii in wheat breeding. The gliadin electrophoretic pattern of the eleven advanced lines with high stripe rust resistances was compared with their parents SQ-214, CS and SW3243 by acid polyacrylamide gel electrophoresis. The omega-gliadin bands of Gli-Dt1 in Ae. tauschii SQ-214 were transferred to some advanced lines and freely expressed in common wheat genetic background. One of advanced lines possesses a null Gli-D1 allele, where the omega-gliadin bands encoding by the Gli-D1 allele were absent. The potential utilization of this advanced line for wheat quality and stripe rust resistance breeding is also discussed in this paper.
The stripe rust (yellow rust) of the hexaploid wheat (Triticum aestivum L.) caused by Puccinia striiformis f. sp. tritici is considered one of the most serious diseases in many wheat growing regions over the world, especially in areas under cool and wet environmental conditions (Roelfs et al. 1992). It is one of the most destructive wheat diseases in China. There has been five boom and bust cycles of the stripe rust epidemics in China since the late 1950s. The latest epidemics happened in 1990, caused by the Chinese stripe rust physiological strain 28 (CYR28, intern. code: 175 E 142) and 29 (CYR29, intern. code: 175 E 158). Nearly 6.7×106 ha of wheat fields in Shaanxi, Henan, Hubei and Gansu provinces were attacked, which caused 2.65×109 kg loss in wheat production (Wang et al. 1995). Thus, breeding of wheat varieties resistant to stripe rust is one of the most important breeding strategies in China.
Two stripe rust physiological strains, i.e. CYR30 (intern. name: 175 E 191) and CYR31 (intern. name: 239 E 175) were found in 1991 and 1993, respectively (Wang et al. 1996). The CYR30 and CYR31 have become epidemic physiological strains in China since 1994. Noticeably, in the spring of 2001, the epidemics occurred in most wheat production areas in Sichuan and Gansu provinces, simply because almost all of the released wheat varieties growing in these areas were highly susceptible to the CYR30 and CYR31 strains. To date, very few advanced lines in the provincial and national yield testing nurseries have displayed resistance to the CYR30 and CYR31. For example, among the 36 tested advanced lines in the Sichuan yield testing nurseries in 2001, only one line derived from the shuttle breeding program of Sichuan/CIMMYT (The International Maize and Wheat Improving Center) showed resistance to the CYR30 and CYR31. This situation indicated that urgent action needs to be taken to exploit new stripe rust resistant germplasm for wheat breeding, which is the most important task in western China, where the stripe rust diseases are the most serious problems for wheat production.
Aegilops tauschii Coss. (DD, 2n=14), the donor of D genome to common wheat (AABBDD, 2n=42), is a valuable source of genes with diversified disease and pest resistance, apart from its great variation in seed storage proteins (Kerber and Dyck 1979; Gill and Raupp 1987; Lagudah and Halloran 1988; William et al. 1993; Yang et al. 1998, 1999, 2000a). Benefit from its complete homology with the wheat D-genome, Ae. tauschii is probably one of the most suitable species for direct introgression of useful traits to wheat (Gill and Raupp 1987; Cox et al. 1991). Alonso and Kimber (1984) have in fact determined that one backcross with their F1 hybrids re-stalled 92 % of the genotype of the recurrent parent. Therefore, direct transfer should be considered as a suitable and efficient technique for exploiting Ae. tauschii in wheat breeding (Mujeeb-Kazi and Hettel 1995). Several genes of resistance to leaf rust and Hessian fly have been transferred to common wheat by this approach (Kerber and Dyck 1979; Gill and Raupp 1987; Gill et al. 1991; Cox et al. 1991). However, it was reported that the resistant genes from an alien species were occasionally suppressed after they were transferred from a lower ploidy species to a higher ploidy crop (Kerber and Green 1980; Kerber 1983; Bai and Knott 1992; Ma et al. 1995; Gert et al. 1995). The suppression of resistant genes in the host crop hindered free made application of the resistance in many cases (Bai and Knott 1992). Previous authors reported that suppressor(s) existing in some of T. turgidum wheat would restrain the expression of resistant genes from Ae. tauschii in a synthetic hexaploid background (Gert et al. 1995; Ma et al. 1995; Assefa and Feharmann 2000; Yang et al. 2001). Therefore, a better understanding the genetic expression of Ae. tauschii genes for stripe rust resistance under the common wheat background by direct hybridization will facilitate the effective utilization of this important resource for stripe rust resistance.
Gliadins are alcohol-soluble seed storage proteins that show great level of intervarietal polymorphism when studied by a standard method of acid polyacrylamide gel electrophoresis (A-PAGE) (Zillman and Bushuk 1979). Gliadins of Ae. tauschii are mainly controlled by Gli-Dt1 and Gli Dt2 loci (Lagudah and Halloran 1988; William et al. 1993). William et al. (1993) reported that the omega-gliadin bands of Ae. tauschii were always expressed in the synthetic hexaploids. This technology can be used as a probe to detect the successful transferring of the alien genes encoded by Gli-Dt alleles.
During 1996–1997, 48 accessions of Ae. tauschii were evaluated for resistance to physiological strains CYR30 and CYR31 of wheat stripe rust in China (Yang et al. 1998). Out of 48, 28 accessions displayed seedling and adult resistance. One of the highly resistant Ae. tauschii accessions (SQ-214) was used as resistant germplasm to cross with Sichuan commercial wheat varieties for exploiting its resistance in our research program. The objectives of this study were to (i) analyze the inheritance of stripe rust resistance in the Ae. tauschii accession SQ-214, (ii) analyze genetic expression and suppression of the resistant genes in the hybrid progenies derived from Ae. tauschii SQ-214 under the common wheat background, and (iii) develop high resistance advanced lines by transferring the resistance from Ae. tauschii SQ-214 into Sichuan commercial wheat varieties and compare the gliadins of advanced lines with their parents.
MATERIAL AND METHODS
Plant material
Three accessions of common wheat, Chinese Spring (CS), MY26, and SW3243 that were highly susceptible to physiological strains CYR30 and CYR31 of wheat stripe rust in China, and two accessions of Aegilops tauschii SQ-214 and As-80 were included in this study. Ae. tauschii SQ-214 was highly resistant to the two physiological strains, but Ae. tauschii As-80 was highly susceptible. MY26 and SW3243 are major commercial wheat varieties that are widely cultivated in Sichuan and adjacent provinces. Mingxian 169, a widely adopted susceptible line for stripe rust in China, was used as susceptible check and stripe rust inoculating spreader in the present study.
Resistance evaluation
Two Chinese stripe rust physiological strains, CYR30 and CYR31 isolated from the mixture of urediospores from infected wheat plants in Sichuan and Gansu provinces by Wang et al. (1996), were used in the present study. The two strains were virulent to the cultivar Hybrid46. The virulent genes of the CYR30 and CYR31 are identified as 1, 2, 3a, 3b, 4a, 4b, 6, 7, 8, 9, SD and C5; and 1, 2, 3a, 3b, 4a, 4b, 6, 7, 9, 15, SD, Su and C5, respectively (Wang et al. 1996).
In China the stripe rust usually infects winter wheat and the spring-habited winter wheat at their adult stage in early spring. Noticeably, the infection of adult plants causes much more serious yield losses than the infection of seedlings due to the formation of shriveled grains at the adult stage. The present experiment therefore only focused on the evaluation of adult-plant resistance.
The host materials were evaluated in the field at the experimental station of the Research Institute of Plant Protection, Sichuan Academy of Agricultural Sciences, which had an ideal artificial inoculating condition and favorable environment for stripe rust development. A randomized complete block design with two replicates was adopted in the experiment. Each block contained 50 plots, and each plot (0.2×0.5 m) contained two rows. Seeds of common wheat and the segregated offspring of CS/SQ214//SW3243 were directly planted to plots, while the embryo rescued hybrid plants of CS/SQ214 were transplanted from tissue culture laboratory to plots. To overcome the strong winter-growth habit of Ae. tauschii, seedlings of this species were vernalized for 7 weeks under low temperature (3–4°C) and long day (14–15h light/9–10h dark) conditions. After vernalization, the seedlings of Ae. tauschii were transplanted to the plots designed as above. The susceptible variety Mingxian169 was used as susceptible check and was sown at normal seeding time, located at every five plots and both sides of a block. The stripe rust epidemic was initiated by inoculating spreader rows of cultivar Mingxian169 with the mixed urediospores of the CYR30 and CYR31. At the same time, the experimental rows were also inoculated. The stripe rust infection type was recorded three times at 10-day intervals. The first disease notes were taken when the susceptible check had reached 100 % severity (Ma et al. 1995). The infection types (ITs) were scored as I-HR (immune-high resistant), R (resistant), MR (middle resistant), MS (middle susceptible), S (susceptible), HS (high susceptible) following to the concept of basic and expanded scales described by McNeal et al. (1971) and Line and Qayoum (1991).
Gliadin electrophoresis
Gliadin proteins were extracted with a solution of 70 % ethanol and 0.01 % methyl green, and fractionated by a standard acid polyacrylamide gel electrophoresis at pH 3.1, following the procedure of Cooke (1987). The gliadins of the eleven advanced lines were compared with that of their Ae. tauschii parent SQ-214, and the two common wheat parents CS and SW3243 (Fig. 2).
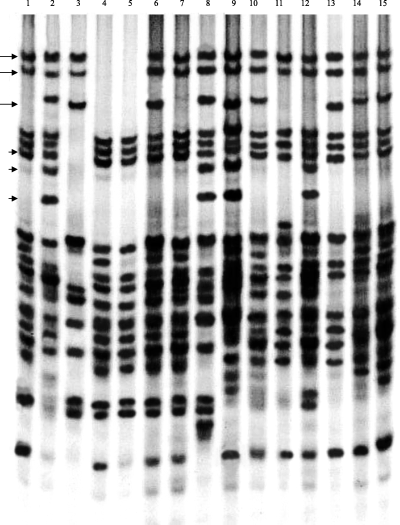
Gliadin electrophoretic patterns of Triticum aestivum (lane 1, CS; lane 2, SW3243), Aegilops tauschii (lane 3, SQ-214), and their advanced lines developed from the interspecific hybrids (lane 4–5, ADL-1; lane 6, ADL-2; lane 7, ADL-3; lane 8, ADL-4; lane 9, ADL-5; lane 10, ADL-6; lane 11, ADL-7; lane 12, ADL-8; lane 13, ADL-9; lane 14, ADL-10; and lane 15, ADL-11). Arrows indicated the omega-gliadin bands encoding by Gli-Dt1 derived from Ae. tauschii SQ-214, arrowheads indicated the gliadin marker Gli1B3 of 1BS (1BL/1RS translocation) derived from one of the common wheat parents, SW3243.
RESULTS
Inheritance of stripe rust resistance in Ae. tauschii
As presented in Table 1, the F1 hybrids of SQ-214 crossed with susceptible accession As-80 were highly resistant as its resistant parent SQ-214. This indicated that the resistance of SQ-214 is controlled by a dominant gene(s).
| Combination or parent | Display of infection types | Year of data collection | ||||||
|---|---|---|---|---|---|---|---|---|
| I-HR | R | MR | MS | S | HS | Totals | ||
| SQ-214 | 15 | 15 | 1995–1996 | |||||
| As-80 | 15 | 15 | 1995–1996 | |||||
| F1 individuals of SQ-214×As-80 | 14 | 14 | 1996–1997 | |||||
| F2 individuals of SQ-214×As-80 | 132 | 17 | 34 | 183 | 1997–1998 | |||
The F2 population, derived from the F1 hybrids between the resistant SQ-214 and susceptible As-80 segregated resistant (I-HR) and susceptible (S-HS) types (Table 1). The chi-square test confirmed that frequency of resistant versus susceptible individuals was in accordance with a 3:1 ratio (X132:512=0.8033, P value 0.250–0.500) (Table 1). This indicated the resistance of SQ-214 being controlled by one dominant gene.
Genetic expression of stripe rust resistance of Ae. tauschii
The interspecific hybrids between CS and Ae. tauschii accession SQ-214 were medium susceptible (MS) as its common wheat parent CS (Table 2). This indicated that the resistant gene of Ae. tauschii accession SQ-214 was inhibited by a suppressor(s) of CS in the interspecific hybrids.
| Combination or parent | Display of infection types | Year of data collection | ||||||
|---|---|---|---|---|---|---|---|---|
| I-HR | R | MR | MS | S | HS | Totals | ||
| SQ-214 | 15 | 15 | 1995–1996 | |||||
| CS | 30 | 30 | 1995–1996 | |||||
| SW3243 | 30 | 30 | 1995–1996 | |||||
| F1 individuals of CS×SQ-214 | 19 | 19 | 1996–1997 | |||||
| BC1F2 individuals of CS/SQ-214//SW3243 | 11 | 2 | 12 | 31 | 107 | 163 | 1998–1999 | |
| BC1F2 individuals of CS/SQ-214//MY26 | 20 | 57 | 119 | 196 | 1998–1999 | |||
The interspecific hybrids were backcrossed with Sichuan commercial varieties SW3243 and MY26. Since the number of obtained backcross seeds was limited, therefore they were planted directly in Kunming, Yunan Province, for producing sufficient F2 (BC1F2) population. The backcross F1 (BC1F1) plants were not used for stripe rust evaluation. The individual resistance response in the BC1F2 population of CS/SQ-214//SW3243 was different from that of CS/SQ-214//MY26. Of 163 individuals in the BC1F2 population of CS/SQ-214//SW3243, eleven displayed immune or highly resistant (I-HR) as their resistant parent Ae. tauschii SQ-214, fourteen individuals displayed infection types (ITs) between the resistant parent Ae. tauschii SQ-214 and the susceptible common wheat parents CS and SW3243, the others displayed ITs as the two susceptible common wheat parents (Table 2). However, there was no individual that displayed high level of resistance as its resistant parent Ae. tauschii SQ-214 in the BC1F2 population of CS/SQ-214//MY26 (Table 2). Of 196 individuals, only 20 displayed MR, and the others displayed MS to HS as the two susceptible common wheat parents. This indicated that the expression of resistance from SQ-214 that was suppressed in the interspecific hybrid (CS/SQ-214) was expressed in some individuals of the BC1F2 population of CS/SQ-214//SW3243, but was still suppressed in the BC1F2 population of CS/SQ-214//MY26.
Development and gliadin analyses
The procedure of transferring stripe rust resistances from Ae. tauschii into common wheat for developing advanced lines was shown in Fig. 1. The eleven highly resistant individuals from the BC1F2 population of CS/SQ-214//SW3243 were propagated every year both at Chengdu, Sichuan Province and Kunming, Yunnan Province for shortening the breeding period. The propagated materials were evaluated for stripe rust resistance in F4 and F6 generations at Chengdu. Ten plants with the best agronomic characters and high resistance were selected from each of the eleven highly resistant individuals derived from the F2 population of CS/SQ-214//SW3243. One of the ten plants was randomly selected for gliadin analyses to detect the genetic material derived from Ae. tauschii SQ-214 in advanced lines by A-PAGE in 2001.
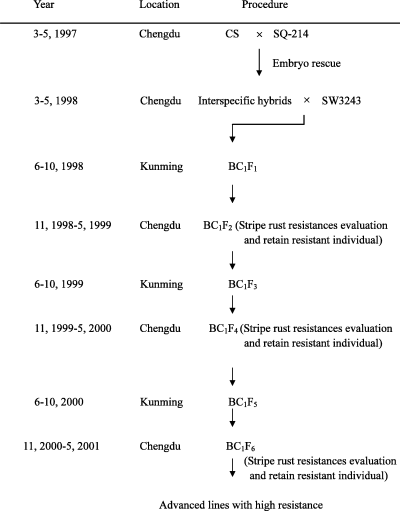
The procedure of transferring stripe rust resistances into common wheat from Ae. tauschii
As shown in Fig. 2, the eleven advanced lines possess recombinant bands of gliadins derived from Ae. tauschii SQ-214, CS and SW3243 and have completely different gliadin electrophoretic patterns from each other. The advanced line-1 (ADL-1) possesses a null Gli-D1 allele without the omega-gliadin bands encoding by the Gli-D1 allele (Fig. 2, lane 4–5). ADL-2, ADL-5 and ADL-9 possess the Gli-Dt1 allele encoding omega-gliadin bands derived from SQ-214 (Fig. 2, lane 6, 9 and 13). ADL-3, ADL-7 and ADL-8 possess the Gli-D1 allele encoding omega-gliadin bands derived from CS (Fig. 2, lane 7, 11 and 12). ADL-4, ADL-6, ADL-10 and ADL-11 possess the Gli-D1 allele encoding omega-gliadin bands derived from SW3243 (Fig. 2, lane 8, 10, 14 and 15). The results indicated that the omega-gliadin bands of Gli-Dt1 in Ae. tauschii SQ-214 were transferred to some advanced lines and freely expressed in common wheat genetic background.
Sozinov et al. (1987) had identified a block of gliadin bands (Gld1B3) as a marker for the presence of 1RS chromosome segment from rye in wheat. Using Gld1B3 as marker, it was found that three advanced lines (ADL-4, ADL-5 and ADL-8) carried the 1RS chromosome segment (Fig. 2, lane 8, 9 and 12), which derived from the Sichuan commercial variety SW3243. The results indicated that SW3243 possessed 1B/1R translocation.
DISCUSSION
Wheat is one of the most important food crops and it will become even more significant as the global population increases. However, the frequent use of limited parental genotypes in the modern wheat breeding practices and the monotonous plantation of only a few improved wheat varieties with narrow genetic background in large scales cause serious problems of genetic erosion in cultivated wheat (Porceddu et al. 1988). As a consequence, the genetic erosion not only reduces genetic diversity of wheat varieties and constrains further improvement of wheat, but also makes wheat become increasingly vulnerable to the biotic and abiotic stresses (Nevo 1995). The introduction of alien genes from wild relative species will increase the genetic diversity of cultivated wheats, therefore, the distant hybridization becomes an essential and important approach to exploit desirable genes of wild species in the tribe Triticeae (Jiang et al. 1994).
Wild relatives in the genus Aegilops have been extensively utilized in wheat breeding. One accession of Ae. tauschii (SQ-214), with high resistance to Chinese stripe rust physiological strains CYR30 and CRY31 was used in this study (Yang et al. 1998). Genetic analyses indicated that the resistance of Ae. tauschii SQ-214 was controlled by a single dominant gene. Our data showed that the resistance of SQ-214 could be easily transferred into common wheat though sexual hybridization. However, the problems have been experienced by many wheat breeders that the resistance gene was occasionally suppressed after transferring into bread wheat or synthetic hexaploid wheat, which significantly hindered the effective utilization of the valuable germplasm. Kerber and Green (1980) reported that the long arm of chromosome 7D carried a gene that suppresses the expression of a gene or genes for steam rust resistance in the A or B genome of bread wheat. Kerber (1983) further demonstrated that the function of leaf rust resistance from a durum wheat (T. turgidum var. durum) was inhibited by the suppressors on the D genomes of bread wheat and Ae. tauschii in their hybrids (durum wheat/bread wheat) and the synthetics (durum wheat/Ae. tauschii), respectively. The suppressors of the stripe rust were occasionally observed both in the A or B and D genomes of T. turgidum and Ae. tauschii, respectively, by other authors (Gert et al. 1995; Ma et al. 1995). The suppression of stripe rust resistance seemed to be donor specific to T. turgidum or Ae. tauschii in synthetic hexaploids (Gert et al. 1995; Ma et al. 1995; Yang et al. 2001).
In this study, the resistance of Ae. tauschii SQ-214 was also suppressed by certain common wheat parents. It was clearly shown that the resistance of Ae. tauschii SQ-214 was suppressed by CS and MY26 in their interspecific hybrid plants (CS/SQ-214) and the BC1F2 population (CS/SQ-214//MY26). However, the suppressed resistance from Ae. tauschii SQ-214 in the CS/SQ-214 hybrid was expressed in some individuals of the BC1F2 population (CS/SQ-214//SW3243) when wheat variety SW3243 was used as a backcrossing parent. The results of this experiment suggested that the SW3243 did not possess a suppressing allele(s), but CS and MY26 obviously possess a suppressor(s) inhibiting the expression of stripe rust resistance of Ae. tauschii SQ-214. Our other study showed that the powdery mildew resistance of another Ae. tauschii accession (SQ-309) was freely expressed in hybrid between SW3243 and SQ-309 (Yang et al. 2000b). The SW3243 was released in 1998 and later named as Chuanmai30. It is a high-yielding variety derived from the shuttle-breeding program of Sichuan/CIMMYT and was widely cultivated in Sichuan and adjacent provinces. Because it does not have a suppressor for the disease resistance (Bai and Knott 1992), we suggested to use the SW3243 as a receiving common wheat for exploiting the resistances of stripe rust and powdery mildew in Ae. tauschii accessions through sexual hybridization. Our previous results indicated that the SW3243 possessed the dominant loci of Kr genes (Yang et al. 2000) resulting a low crossability with rye and Ae. tauschii, when it was used as a male parent in hybridization. However, the same combination resulted relatively high crossability when Ae. tauschii was used as female to cross with common wheat (Yang et al. 2000a). We therefore suggested that Ae. tauschii should be used as female to cross with SW3243 to achieve an efficient frequency of transferring the stripe rust and powdery mildew resistant gene(s) from Ae. tauschii into common wheat.
Eleven advanced lines with high resistance to Chinese stripe rust physiological strains CYR30 and CYR31 derived from the Ae. tauschii SQ-214 were developed in the present study. Since 1994, the CYR30 and CYR31 have been epidemic physiological strains in China. However, only two major resistant resources Nanrong9851 and Guirong21, with resistances derived from T. durum wheat (AABB, 2n=28) were used in wheat breeding in recent years in China. Therefore, these materials with resistance derived from Ae. tauschii should be used as new resistant germplasm for wheat breeding in China.
In addition, one of the advanced lines, ADL-1 was found to possess a null Gli-D1 allele without the omega-gliadin bands encoding by the Gli-D1 allele. Branlard and Dardevet (1994) found that a French wheat cultivar Darius has excellent bread-making quality, even though it possesses the high molecular weight glutenin subunit combination 2, 7 and 12, which are usually associated with poor quality, and a null allele at the Gli-D1 locus. It was found that the null allele of Darius, characterized by the absence of the Gli-D1 encoded omega-gliadins, was associated significantly with higher dough tenacity P, and strength W (Branlard and Dardevet 1994). Redaelli et al. (1997) further determined that the impact of the null allele at Gli-D1 on gluten strength was highly positive in NILs possessing HMW-GS 2+12, and negligible or negative in NILs containing HMW-GS 5+10. It was suggested that breeders could improve dough quality by using this allele in combination with HMW-GS 2+12 (Branlard and Dardevet 1994; Redaelli et al. 1997). In general, most Chinese wheat varieties possess the HMW-GS 2+12 and have poor bread-making quality for most varieties (He et al. 1995; Yen 1999). Therefore, the advanced line ADL-1, possessing both the null Gli-D1 allele and high resistance of stripe rust, could be used as elite genetic resources to improve the dough quality and stripe rust resistance of commercial wheat varieties with high yield potential and HMW-GS 2+12 in China.
Acknowledgements
This research was sponsored by National Nature Science Foundation of China (39470450 and 30070472), Sichuan Provincial Science Fund for Distinguished Young Scholars, and the Science and Technology Department of Sichuan Province. The authors are highly grateful to Mr. Liu Zhengde and Miss Yang Jiaxiau of Research Institute of Plant Protection, Sichuan Academy of Agricultural Sciences, China, for providing the isolates of the Chinese stripe rust physiological strain CYR30 and CYR31, and for their kind assistance in resistance evaluation. Thanks also extend to Prof. Yen Chi, Sichuan Agricultural University, China, and to Dr. Mujeeb-Kazi, A., CIMMYT, Mexico, for providing Ae. tauschii accessions As-80 and SQ-214.




