Low-Dose IL-2 for In Vivo Expansion of CD4+ and CD8+ Regulatory T Cells in Nonhuman Primates
Abstract
IL-2 is a known potent T cell growth factor that amplifies lymphocyte responses in vivo. This capacity has led to the use of high-dose IL-2 to enhance T cell immunity in patients with AIDS or cancer. However, more recent studies have indicated that IL-2 is also critical for the development and peripheral expansion of regulatory T cells (Tregs). In the current study, low-dose IL-2 (1 million IU/m2 BSA/day) was administered to expand Tregs in vivo in naïve nonhuman primates. Our study demonstrated that low-dose IL-2 therapy significantly expanded peripheral blood CD4+ and CD8+ Tregs in vivo with limited expansion of non-Treg cells. These expanded Tregs are mainly CD45RA− Foxp3 high activated Tregs and demonstrated potent immunosuppressive function in vitro. The results of this preclinical study can serve as a basis to develop Treg immunotherapy, which has significant therapeutic potential in organ/cellular transplantation.
Abbreviations:
-
- IL-2
-
- interleukin-2
-
- Tregs
-
- regulatory T cells
Introduction
Regulatory T cells (Tregs) play a critical role in a variety of human physiologic and pathologic processes including autoimmunity (1), cancer (2) and solid organ transplantation (3). Treg immunotherapy has been of great interest in the treatment of autoimmune disease or organ/cellular transplant recipients. Significant progress has been made in methods to expand Treg ex vivo (4), which has been applied to reduce the incidence or severity of graft versus host disease (GVHD) in postbone-marrow transplant recipients (5). In vivo expansion of Tregs is an alternative Treg immunotherapy, and methods to expand Tregs in vivo have also been studied. Among various cytokines, IL-2 has been identified as an essential cytokine for development and expansion of Tregs (6), which was also found to expand functional Tregs with moderate to lower dose (7–10). Saadoun et al. recently reported successful treatment of hepatitis C virus-induced vasculitis by expanding Tregs by low-dose IL-2 treatment (11). Most recent studies by Koreth et al. demonstrated that low-dose IL-2 treatment induced preferential and sustained Treg expansion in bone-marrow transplant recipients with clinical improvements of GVHD (12). However, to date, the in vivo effects of low-dose IL-2 on Tregs have only been shown in rodent models or severely immunocompromised patients. In this study, aiming at possible application for organ/cellular transplantation, we evaluated low-dose IL-2 administration for in vivo expansion of Tregs in naïve cynomolgus monkeys. Our study demonstrated that low-dose IL-2 therapy significantly expanded both CD4+ and CD8+ functional Tregs in vivo with limited expansion of non-Treg cells in nonhuman primates (NPH).
Material and Methods
Animals
Naïve male cynomolgus monkeys that weighed 3 to 7 kg were used (Charles River Primates, Wilmington, MA). All surgical procedures and care of animals were performed in accordance with National Institute of Health guidelines for the care and use of primates and were approved by the Massachusetts General Hospital Subcommittee on Animal Research.
IL-2 treatment: Cynomolgus monkeys were treated with daily subcutaneous injections of IL-2 (Aldeleukin, human recombinant IL-2, 1 000 000 IU/m2 BSA/day; Prometheus San Diego, CA) for 28 days.
Flow cytometric analyses
Peripheral blood mononuclear cells (PBMC) were labeled with a combination of the following mAbs: CD3 (SP34), CD4 (L200), CD8 (Sk 1), CD16 (3G8), CD20 (2H7), CD45RA (5H9), CD56 (B159), CD62L (SK11), CD95 (DX-2), NKG2A (Z199) and isotype-matched irrelevant control mAbs (BD Pharmingen, San Jose, CA). To assess intracellular protein expression, Foxp3 (PCH101), Ki67 (B56) and CD152 (BNI3), cells were permeabilized using fixation/permeabilization solution (eBioscience, San Diego, CA). PBMCs were then analyzed using FACS Calibur and FACS Scan flow cytometers and Cell Quest Software (BD Pharmingen), or FlowJo software (TreeStar Inc., Ashland, OR).
Suppression assay
20×103 of PBMCs were incubated in medium containing RPMI 1640 (Mediatech, Inc., Manassas, VA) and 10% monkey plasma, with stimulation of plate-bound CD3-Ab (SP34, 10 μg/mL) and soluble CD28-Ab (28.2, 1 μg/mL) in the presence of different ratios (1:1, 1:1/4 and 1:1/16) of CD4+CD25high cells sorted by a FACS Aria (BD Biosciences, Bedford, MA). CD4+CD25dim cells were used as a control. Cells were incubated for 110 hours, and during the last 24 hours, plates were pulsed and proliferation was evaluated by incorporation of (3H) thymidine as previously reported (13). In some experiments, CD3+CD8+CD25high cells and CD3+CD8−CD25dim cells were sorted as CD8Treg. (Figure 3B).
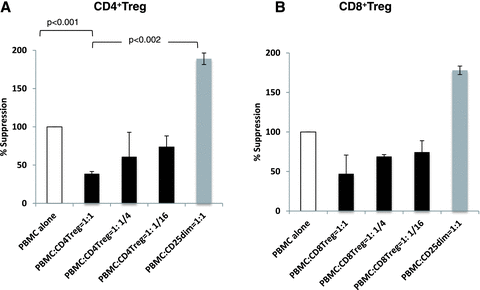
Suppression assays of expanded CD4+ and CD8+ Tregs. A: Each column represents mean +SD of fold suppression, comparing with PBMC alone from three separate experiments. Addition of CD4+CD25high to PBMCs (20 × 103 cells) stimulated with abs anti-CD3/CD28 resulted in dose-dependent suppression of T cell activation (black columns). In contrast, addition of CD25dim cells instead of CD4+CD25high resulted in 1.5- to 2-fold higher responses (dark gray columns) than PBMC alone, which was significantly higher (p < 0.002) than PBMC responses with CD4+CD25high. B: Similar suppressive function was observed with CD3+CD8+ CD25high cells. The results were mean +SD of fold suppression, comparing with PBMC alone from two separate experiments.
Statistical analysis
We used a repeated measures analysis of variance to test whether the abundance of each cell was greater during the treatment period (days 6–27) than during the baseline (day 6). Data were log-transformed before analysis. In addition, we calculated the log-fold change for each cell and compared them in pairs using a paired t-test.
Results
Low-dose IL-2 injection expanded CD4+and CD8+T regulatory cells in vivo
In the peripheral blood, normal adult (age 3–6) cynomolgus monkeys (n = 7) were found to have 4.4 ± 2.0% of CD4+CD25+ Foxp3+ cells among CD4+ cells (Figure 1A). These cells were further divided by the expression of CD45RA, according to the recent studies in human Tregs (14). Similar to human Tregs, resting Treg (rTreg, CD45RA+Foxp3low), activated Treg (aTreg, CD45RA−Foxp3high) and nonsuppressive T cells comprise 40 ± 3%, 19 ± 8% and 42 ± 16% among CD4+Foxp3+ cells, respectively (Figure 1B).

Treg expansion after low-dose IL-2 in cynomolgus monkeys. (A) In the peripheral blood, CD4+CD25+ Foxp3+ cells comprise 4.4 ± 2.0% among CD4+ cells in adult cynomolgus monkeys (n = 7). (B) CD4+ cells were further divided by staining with CD45RA and Foxp3. Resting Treg (R: rTreg, CD45RA+Foxp3low), activated Treg (A: aTreg, CD45RA−Foxp3high) and Foxp3+ nonsuppressive T cells (N, CD45RA−Foxp3low) comprise 40 ± 3%, 19 ± 8% and 42 ± 16% among CD4+Foxp3+ cells, respectively. (C) A representative data of flow cytometric analysis (CD4+ gated) before and after daily injection of IL-2 (1 million unit/m2/day). Before IL-2 injection, percentage of CD25+Foxp3+ cells was 3.4%, but it was increased to 17.3% on day 13. (D) Daily treatment with low-dose IL-2 significantly expanded absolute counts of Tregs. In contrast, such expansion of Tregs was not observed with lower dose of IL-2 [0.1 (n = 2) and 0.6 million IU/m2 BSA(n = 1)]. (E) The majority of expanding Tregs during IL-2 injection were aTregs, which were Foxp3highCD45RA−CD62L−CTLA4high and KI-67+. (F) Flow cytometric analysis of a representative monkey. CD8+Foxp3+ cells were 0.05% before IL-2 injection but increased to 0.77% on day 13. (G) Absolute counts of Foxp3+ cells among CD8+ cells significantly increased after low-dose IL-2.
During the course of daily subcutaneous injections of IL-2 (1 million IU/m2 BSA) into five naïve monkeys for four weeks, there was significant expansion of absolute numbers of Tregs in the peripheral blood (Figure 1C and D). During IL-2 treatment, no adverse effects, including capillary leak syndrome, were observed. High Treg counts were maintained as long as IL-2 was administered but quickly returned to the pretreatment level after discontinuation of IL-2. In contrast, such expansion of Tregs was not observed with lower dose of IL-2 (0.1 and 0.6 million IU/m2 BSA; Figure 1D). The majority of these proliferating cells were aTreg, CD45RA−Foxp3highCTLA4+ CD62Llow and KI-67 positive (Figure 1E), which have been reported to be most immunosuppressive (14). IL-2 treatment also significantly expanded CD8+ Tregs in vivo (Figure 1F). In normal cynomolgus monkeys, CD8+Foxp3+ cells are of a minor population, typically comprising less than 0.5% of CD8+ cells (absolute count 2–4 cells/μl), but they were increased to as high as 30/mm3 during low-dose IL-2 treatment (Figure 1G).
Effects of low-dose IL-2 on non-Treg cells
Since normal values of lymphocyte subsets vary for each monkey, we analyzed relative increase of each cell population to the pretreatment level to compare the effect of low-dose IL-2 on Tregs and non-Treg cell populations in five monkeys (Figure 2). There was no significant expansion observed in CD8+ memory T cells and NK cells. The most significant expansion was observed in CD4+CD25+Foxp3+ Tregs (p < 0.0001), which were maintained 14–17 fold higher than pretreatment levels during IL-2 treatment. Although less significant, CD4+CD25+Foxp3− (p < 0.0001) and CD8+ Tregs (p < 0.0001) also showed 5.8 ± 1.8 and 9.3 ± 3.0 fold expansion, respectively. Expansion of CD4+CD25+Foxp3+ was rapid, within 1 week after IL-2 treatment but CD4+CD25+Foxp3− and CD8+ Tregs tended to increase after 2 weeks of IL-2 treatment. Significant, but less than threefold expansion of CD4+ memory T cells (p = 0.0002), CD4 (p = 0.017) and CD8 (p = 0.009) naïve cells were also observed (Figure 2).
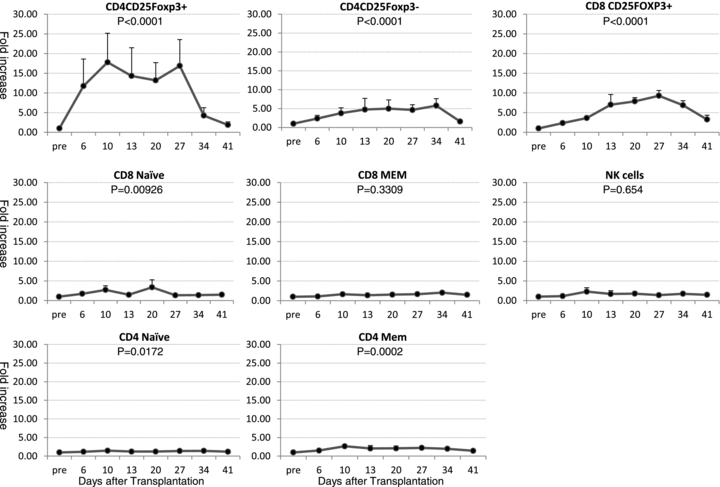
The effect of low-dose IL-2 on Tregs and non-Treg cell population. To compare the effect of low-dose IL-2 on Tregs and non-Treg cell population, relative increase of each cell population to the pretreatment level was analyzed (n = 5) (Figure 2). There was no significant expansion observed in CD8+ memory T cells and NK cells. Most significant expansion was observed in CD4+CD25+Foxp3+ Treg (p < 0.0001), followed by CD4+CD25+Foxp3− (p < 0.0001) and CD8+ Tregs (p < 0.0001). Significant expansion of CD4+ memory T cells (p = 0.0002), CD4 (p = 0.017) and CD8 (p = 0.009) naïve cells was also observed but their expansion was much less significant than those observed in CD4+ Tregs.
Expanded CD4+ and CD8+Tregs are suppressive
The suppressive function of expanded Tregs was evaluated by measuring 3H thymidine incorporation after PBMC activation by plate-bound CD3/soluble CD28 mAbs with or without CD4+CD25 high cells. By adding sorted CD4+CD25high cells to PBMC (20 × 103 cells) in different ratios (1:1, 1:1/4, 1:1/16), T cell proliferation was significantly suppressed (Figure 3A, black columns), comparing with T cell proliferation without Tregs. Adding CD25dim cells instead of Tregs in 1:1 ratio resulted in approximately twofold higher response (PBMC: CD25dim= 1:1) than PBMC alone. PBMC responses with CD25dim cells were significantly higher (p < 0.002) than PBMC responses with CD4+Tregs (Figure 3A). Similar suppressive function was observed in CD8+Tregs (Figure 3B).
Discussion
IL-2 was originally identified as a potent growth factor for antigen-activated T cells and NK cells, thereby enhancing innate and adaptive immunity. These properties have led to the use of high-dose IL-2 for treatment of metastatic renal cell carcinoma and malignant melanoma. However, IL-2 often induced immunosuppressive effects rather than enhancing immune responses especially with low-dose treatment (15,16). In clinical settings, low-dose IL-2 treatment had been applied to patients with HIV infection in an attempt to improve CD4+ cell counts and to expand NK cell expansion and augment innate and adaptive immune responses. Unfortunately, IL-2 treated patients did not experience a better clinical outcome (7) and further studies revealed significant expansion of functional Tregs in these patients (8, 9). In pediatric patients with sarcoma, low-dose IL-2 was administered with autologous lymphocyte infusions after cyclophosphamide-based chemotherapy, which also resulted in significant expansion of functional Tregs (10). Most recently, Koreth et al. applied low-dose IL-2 treatment in the treatment of chronic GVHD in bone-marrow transplant recipients and observed preferential and sustained Treg expansion with clinical improvements of GVHD (12). Since Tregs constitutively express all three subunits of the high affinity IL-2R (α, β and γ), they can presumably respond to physiologically low doses of IL-2. Low-dose IL-2 may also induce JAK/STAT signaling rather than PI3K signaling, which preferentially expand Treg rather than conventional effector T cells (17,18). Therefore, depending on the dose, IL-2 treatment may potentially be useful for immunotherapy by expanding Tregs.
In the current study, using naïve cynomolgus monkeys, we studied low-dose IL-2 treatment for in vivo expansion of Tregs. The dose of IL-2 was determined based on a clinical study with low-dose IL-2 treatment (12,18), which was approximately 1/100–1/200 of the dose that has been used for treatment of malignancies (600 000–720 000 IU/kg every 8 hours) (19). Our preliminary studies also showed no effects in lymphocytes by 0.1 million IU/m2 and limited effects with 0.6 million IU/m2 of IL-2 (Fig. 1D). Therefore, we estimated that the minimal dose of IL-2 that can expand Tregs would be 0.6–1.0 million IU/m2.
We found that daily low-dose IL-2 injections markedly expanded absolute counts of both CD4+ and CD8+Tregs with limited expansion of other T cells and NK cells. The majority of these expanded Tregs were CD45RA−FOXP3highCTLA4+ Ki67+, which is considered the most immunosuppressive subpopulation among Tregs (14). From the observations that these Tregs were highly proliferative and their expansion was less significant in the lymph nodes or bone marrow (data not shown), we speculate that these expanded Tregs originated from either preexisting resting Tregs or nonregulatory CD4+ T cells in the peripheral blood. However, we cannot conclude how much Tregs were originated from naturally occurring Tregs in this study. Since these expanded Tregs have significant suppressive function, in vivo expansion of Tregs by low-dose IL-2 may be useful for induction therapy or even for tolerance induction in organ/cellular transplantation. However, it may be difficult to apply for treatment of rejection, since low-dose IL-2 did expand non-Tregs (CD4+CD25+Foxp3-). Further studies of concomitant immunosuppressive medications are necessary to ensure exclusive expansion of Tregs without the activation of conventional effector T cells.
Acknowledgement
This study was supported in part by 5U19DK080652–02 NHL-BI, POI-HL18646, NIH-NIAID, ROIA137692 and NIH/NIAID 5R01 AI50987–03 and NIH R00000000008108.
We thank Ms. Susan Shea for technical assistance and Ms. Felicia Libby for editorial assistance.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. This manuscript was also not prepared or funded by any commercial organization.




