Epidemiology of Posttransplant Lymphoproliferative Disorders in Adult Kidney and Kidney Pancreas Recipients: Report of the French Registry and Analysis of Subgroups of Lymphomas
Abstract
A registry of posttransplant lymphoproliferative disorders (PTLD) was set up for the entire population of adult kidney transplant recipients in France. Cases of PTLD were prospectively enrolled between January 1, 1998, and December 31, 2007. Ten-year cumulative incidence was analyzed in patients transplanted after January 1, 1989. PTLD risk factors were analyzed in patients transplanted after January 1, 1998 by Cox analysis. Cumulative incidence was 1% after 5 years, 2.1% after 10 years. Multivariate analysis showed that PTLD was significantly associated with: older age of the recipient 47–60 years and >60 years (vs. 33–46 years, adjusted hazard ratio (AHR) = 1.87, CI = 1.22–2.86 and AHR = 2.80, CI = 1.73–4.55, respectively, p < 0.0001), simultaneous kidney–pancreas transplantation (AHR = 2.52, CI = 1.27–5.01 p = 0.008), year of transplant 1998–1999 and 2000–2001 (vs. 2006–2007, AHR = 3.36, CI = 1.64–6.87 and AHR = 3.08, CI = 1.55–6.15, respectively, p = 0.003), EBV mismatch (HR = 5.31, CI = 3.36–8.39, p < 0.001), 5 or 6 HLA mismatches (vs. 0–4, AHR = 1.54, CI = 1.12–2.12, p = 0.008), and induction therapy (AHR = 1.42, CI = 1–2.02, p = 0.05). Analyses of subgroups of PTLD provided new information about PTLD risk factors for early, late, EBV positive and negative, polymorphic, monomorphic, graft and cerebral lymphomas. This nationwide study highlights the increased risk of PTLD as long as 10 years after transplantation and the role of cofactors in modifying PTLD risk, particularly in specific PTLD subgroups.
Abbreviations:
-
- AHR
-
- adjusted hazard ratio
-
- ATG
-
- antilymphocyte globulin
-
- CI
-
- confidence interval
-
- CMV
-
- cytomegalovirus
-
- CNI
-
- calcineurin inhibitor
-
- CTS
-
- collaborative transplant study
-
- EBV
-
- Epstein–Barr virus
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- GIT
-
- gastro-intestinal tract
-
- LDH
-
- lactate dehydrogenase
-
- PRA
-
- panel reactive antibodies
-
- PTLD
-
- posttransplant lymphoproliferative disorders
-
- PY
-
- patient years
-
- SKP
-
- simultaneous kidney pancreas transplantation
-
- UNOS
-
- United Network for Organ Sharing
-
- USRDS
-
- United States Renal Data System
The term posttransplantation lymphoproliferative disorders (PTLD) was introduced in 1984 by Starzl (1) to describe lymphoproliferations, which, unlike lymphomas, are not always monoclonal and whose morphologic features often differ from those of lymphomas. PTLD are the second most frequent neoplasia in transplant patients after skin carcinomas, their risk being about 10–20 times higher than in the general population. PTLD incidence varies depending on the organ transplanted, and ranges from 0.5% in adult kidney or liver recipients to more than 10% in lung, intestinal recipients or pediatric transplant patients (2). Risk factors for PTLD are well known, i.e. Epstein-Barr seronegativity and immunosuppressive therapy (3–5). Recipients who are EBV seronegative have a higher risk of developing EBV-induced lymphomas (6), particularly pediatric patients (7). Rather than one particular immunosuppressive drug, it is generally accepted that the burden of immunosuppression explains the increased incidence of lymphomas in immunocompromised patients. Nevertheless, T-cell depleting agents are more prone to alter antitumoral cytotoxicity and thus to favor PTLD (8–10). Data on maintenance therapies are more conflicting. Calcineurin inhibitors increased the risk of lymphoma in some series, while new antimetabolites like mycophenolate mofetil are thought to be associated with a lower risk of PTLD (2,4,11). Little is known about the long-term risk of lymphoma development. Indeed, most large series only report a short follow-up, for example 3 years posttransplant in American databases (4,12,13). In addition, most existing registries do not contain precise information about PTLD presentation, histological or virological data, particularly EBV data (13).
Our purpose was to better describe the long-term incidence and risk factors of PTLD in a large series of kidney transplant patients. We prospectively collected data on all posttransplant lymphoproliferative disorders diagnosed during a 10-year period in a nationwide population of adult kidney recipients. This is the first extensive long-term nationwide registry in France that contains data on (i) a very large number of PTLD and subgroups of lymphomas, (ii) changes in PTLD incidence over time, (iii) risk factors of lymphoma and subgroups in the entire French adult kidney transplant population.
Patients and Methods
Patients
New cases of PTLD diagnosed in all adult kidney transplant centers (n = 35) in France between January 1, 1998 and December 31, 2007, were prospectively recorded. Inclusion criteria were recipient age >18 years at time of transplantation, kidney or simultaneous kidney–pancreas (SKP) transplantation, histological diagnosis of lymphoma (or strong clinical suspicion when biopsy was not possible), occurrence of lymphoma between 1 month after transplantation and 3 months after graft failure as it is known that after cessation of immunosuppression, the risk of neoplasia becomes the same as in the general population (14–17). The following histological forms were included: lymphoid hyperplasia, polymorphic lymphomas and monomorphic lymphomas, including Hodgkin's disease. Extensive data from donors and recipients were collected at the time of transplantation, at PTLD diagnosis, and subsequently once a year up to 10 years after diagnosis. Immunosuppressive drugs were recorded at discharge after transplantation and at the time of lymphoma diagnosis. Histological analyses were performed by the pathologist at each center. PTLD treatment was conducted in accordance with the general practice at each center.
PTLD incidence
A total of 327 cases of PTLD out of 150 088 patient years (PY) were included in this analysis. The incidence of PTLD was calculated for each year after transplantation, from the 1st to the 10th year posttransplantation. Incidence was calculated by dividing the number of cases occurring in the nth year of transplant by the number of patient years still at risk of lymphoma at the beginning of that year. The analysis was limited to patients transplanted between January 1, 1989 (considered for 10 years after transplantation) and December 31, 2006 (considered only during the first year posttransplantation). Incidence was calculated for each year of follow-up after transplantation, and cumulative incidence was the sum of annual incidences. Cumulative incidences as a function of the location of the lymphoma (graft, cerebral and gastro-intestinal PTLD) were computed in the same way. Cumulative incidence as a function of the period of transplantation (1998–2001 and 2002–2005) was computed using the Kaplan–Meier survival method, censoring all events that occurred more than 3 years post-transplantation to obtain the same follow-up time in the two groups. Statistical differences between the two periods were measured with the log-rank test.
PTLD risk factors
A total of 181 patients who were transplanted between January 1, 1998 and December 31, 2007 and who developed a PTLD were compared to all other kidney recipients (n = 21 170) transplanted in the same period who did not develop lymphoma despite undergoing immunosuppressive therapy (from the transplant date to the end of the study (December 31, 2008), or to the date of graft failure or death). Analysis was limited to patients transplanted after 1998 because many data from before 1998 were missing in the National French Transplant database (called Cristal) for patients without PTLD. Time to PTLD was defined as the time from most recent renal transplant to the date of diagnosis of PTLD, with patients censored at death, loss to follow-up, dialysis or the end of the study. Risk factors included donor and recipient age and gender, primary kidney disease, time since first dialysis, year of transplant, PRA, rank of transplantation, donor type, donor or recipient ABO group, cold ischemia time, number of HLA mismatches, donor and recipient EBV, CMV, HBV and HCV serostatus at time of transplantation and immunosuppression at discharge (induction and maintenance therapy). The cumulative percentage of lymphomas for risk factors was computed using the Kaplan–Meier survival method. Risk factors were analyzed by univariate analysis using a Cox proportional hazard model; variables with p < 0.20 in univariate analysis for a relationship with PTLD occurrence were used as covariates in a multivariate model. Risk factors for particular subtypes of lymphoma—early (<12 months posttransplant) and late onset PTLD (>12 months), EBV positive and negative PTLD, polymorphic and monomorphic PTLD, graft and cerebral PTLD—were studied using univariate analysis. Statistical analyses were performed using SAS software version 9.2.
Results
PTLD incidence
Cumulative incidence was 1% PY 5 years and 2.1% PY 10 years posttransplant (Figure 1A). Incidence was slightly higher in the 1st year, leveled off in the 2nd year and remained stable until the 7th year, and then increased in the 8th–10th year (Figure 1B). Analyzing PTLD incidence according to the period of transplantation (Figure 1C) revealed a decrease in lymphoma incidence during the first 3 years posttransplant in the more recent period (2002–2005 vs. 1998–2001, p < 0.001).
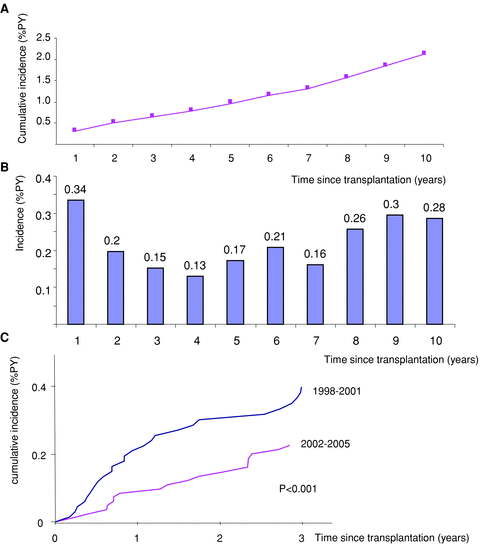
PTLD cumulative incidence per year since transplantation. (A) Cumulative incidence of PTLD over a period of 10 years. (B) Histogram of occurrence of PTLD as a function of time since transplant. (C) Three-year PTLD cumulative incidence as a function of the transplant period (1998–2001 vs. 2002–2005). PY = patient years.
Analyzing PTLD incidence according to the site of the lymphoma revealed differences in the time of the occurrence of graft, cerebral and intestinal PTLD (Figure 2). Graft PTLD occurred mainly in the first 2 years after transplant and their incidence became very low in the following 8 years. Cerebral PTLD occurred mainly between the second and the seventh year posttransplant. Incidence of gastro-intestinal tract PTLD was relatively low in the first 5 years and then increased dramatically from the sixth year posttransplant to exceed other locations after the seventh year.
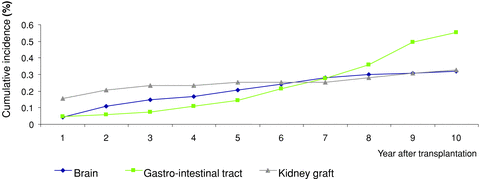
Ten-year cumulative incidence of PTLD as a function of the location of PTLD: graft, cerebral and digestive lymphomas.
Description of the cohort and analysis of PTLD risk factors
The baseline characteristics of the 181 patients with PTLD and their 21 170 controls are listed in Table 1. Table 2 lists the features of the lymphomas in the 181 recipients at the time of PTLD diagnosis. Treatment and outcome of patients with PTLD are detailed in Table 3. Thirty-five patients lost their graft (19%) leading to a graft survival of 88% at 1 year and 60% at 5 years (vs. respectively 91% and 80% in patients without lymphoma, p < 0.001). Overall survival of PTLD patients was 73% at 1 year, 60% at 5 years and 55% at 10 years posttransplant.
| PTLD n = 181 | Patients without PTLD N = 21 170 | |
|---|---|---|
| Recipient gender, n (%) | ||
| Female | 67 (37) | 8 103 (38) |
| Male | 112 (63) | 13 067 (62) |
| Missing | 2 | 0 |
| Age at transplantation, n (%) | ||
| 18–32 | 23 (13) | 3 482 (16) |
| 33–46 | 41 (23) | 6 352 (30) |
| 47–60 | 71 (42) | 7 952 (38) |
| >60 | 48 (21) | 3 384 (16) |
| Missing | 2 | 0 |
| Primary kidney disease, n (%) | ||
| APKD | 21 (12) | 2 939 (14) |
| Diabetic | 17 (9) | 1 513 (7) |
| GN | 63 (35) | 6 542 (31) |
| TICN | 20 (11) | 2 695 (13) |
| Vascular | 9 (5) | 1 325 (6) |
| Other | 14 (8) | 2 347 (11) |
| Unknown | 36 (20) | 3 809 (18) |
| Deceased donor | 176 (98) | 19 878 (94) |
| Living donor | 3 (2) | 1 292 (6) |
| SKP, n (%) | 10 (6) | 636 (3) |
| First transplantation | 157 (87) | 18 087 (85) |
| Second transplantation | 24 (13) | 3 262 (15) |
| Serological status, n (%) | ||
| Recipient | ||
| CMV + | 104 (60) | 12 990 (62) |
| Missing | 9 | 332 |
| EBV− | 26 (16) | 697 (3) |
| Missing | 5 | 1 079 |
| HCV + | 3 (2) | 1 267 (7) |
| Missing | 7 | 2 585 |
| HBV + | 19 (11) | 2 228 (11) |
| Missing | 4 | 412 |
| Donor | ||
| CMV + | 98 (56) | 10 609 (51) |
| Missing | 5 | 549 |
| EBV + | 156 (94) | 18 966 (94) |
| Missing | 11 | 893 |
| MM CMV | 31 (18) | 3 583 (18) |
| Missing | 12 | 860 |
| MM EBV | 23 (15) | 581 (3) |
| Missing | 24 | 1 913 |
| Immunosuppressive therapy at discharge, n (%) | ||
| Induction | 138 (79) | 14 522 (71) |
| ATG or OKT3 | 120 (66) | 11 523 (54) |
| AntiIL2R | 14 (18) | 3 017 (14) |
| CNI | 166 (92) | 19 827 (96) |
| Cyclosporine | 104 (59) | 10 552 (51) |
| Tacrolimus | 62 (36) | 9 276 (45) |
| Azathioprin | 36 (21) | 2 238 (11) |
| MMF | 137 (79) | 17 373 (85) |
| Steroids | 170 (97) | 20 127 (98) |
| Missing | 6 | 677 |
- APKD = autosomal polycystic kidney disease; GN = glomerulonephritis; TICN = tubulo-interstitial chronic nephropathy; SKP = simultaneous kidney-pancreas transplantation; MM = mismatch; ATG = antithymocyte globulins; antiRIL2 = interleukin 2 receptor antibodies; CNI = calcineurin inhibitor.
| Immunosuppressive therapy | n (%) |
|---|---|
| At diagnosis, n (%) | |
| CNI | 160 (88) |
| Cyclosporine | 88 (49) |
| Tacrolimus | 72 (40) |
| Anti metabolites | 142 (78) |
| Azathioprin | 22 (12) |
| MMF | 120 (66) |
| PSI | 16 (9) |
| Steroids | 129 (71) |
| Missing | 5 (3) |
| Time to PTLD | |
| Early onset PTLD | 87 (48) |
| Late onset PTLD | 94 (52) |
| Localization of PTLD | |
| Graft | 43 (24) |
| Brain | 37 (20) |
| GIT | 34 (19) |
| LN only | 17 (9) |
| ORL | 12 (7) |
| Skin and mucosa | 10 (5) |
| Lung | 8 (4) |
| Hematopoietic organs | 7 (4) |
| Liver | 6 (3) |
| Other | 6 (3) |
| Missing | 1 |
| Single site | 116 (64) |
| Disseminated disease | 64 (35) |
| Biological data | |
| Dysglobulinemia | 16 (15) |
| Missing | 72 |
| LDH>480 UI/L | 53 (45) |
| Missing | 64 |
| SCreat>130 μmol/L | 99 (55) |
| Missing | 2 |
| Positive EBV viremia | 74 (67) |
| Missing | 70 |
| Histological data | |
| B-cell lymphoma | 162 (94) |
| T-cell lymphoma | 8 (5) |
| Non-T non-B-cell lymphoma | 2 (1.1) |
| Missing | 9 |
| Polymorphic lymphoma | 42 (28) |
| Monomorphic lymphoma | 108 (72) |
| Hodgkin lymphoma | 6 (4) |
| Myeloma | 5 (3) |
| Missing | 31 |
| EBV positive PTLD | 111 (75) |
| Missing | 33 |
| Polyclonal PTLD | 18 (22) |
| Monoclonal PTLD | 52 (88) |
| Missing | 111 |
- CNI = calcineurin inhibitors; PSI = proliferation signal inhibitors; GIT = gastro-intestinal tract; LN = lymph nodes; LDH = lactate dehydrogenase; Screat = serum creatinine; EBV = Epstein-Barr virus.
| Treatment modalities and outcome | n (%) |
|---|---|
| Reduction of immunosuppression | 163 (95) |
| Missing | 10 |
| Antiviral therapy | 31 (17) |
| Missing | 4 |
| Rituximab alone | 45 (24) |
| Missing | 3 |
| Chemotherapy alone | 39 (22) |
| Missing | 2 |
| Rituximab+chemotherapy | 63 (35) |
| Missing | 3 |
| Surgery | 261 (15) |
| Missing | 3 |
| Radiotherapy | 22 (12) |
| Missing | 3 |
| Remission | 136 (82) |
| Missing | 15 |
| Death | 73 (40) |
- 1Eighteen transplantectomies.
Univariate analyses (Table 4 and Figure 3) revealed that the factors associated with increased risk of PTLD were older recipient age (>46 years), deceased donor, simultaneous kidney-pancreas transplantation, year of transplant (before 2001), duration of dialysis (<5 years), more HLA mismatches (5 or 6), recipient EBV seronegativity and EBV mismatch, induction therapy by polyclonal antibodies or OKT3 and azathioprin. Recipient and donor gender, CMV donor and recipient serostatus and CMV mismatch, primary kidney disease, hepatitis B and C recipient infection, ABO group, PRA, first versus subsequent transplantation, cold ischemia time, cyclosporine, tacrolimus, MMF and steroids were not significantly associated with the occurrence of PTLD in the entire cohort.
| Variables | Modalities | Total | N | HR | CI (95%) | p-Value |
|---|---|---|---|---|---|---|
| Recipient gender | Male | 21 349 | 13 179 | 1 | 0.89 | |
| Female | 8 170 | 0.98 | 0.72–1.32 | |||
| Recipient age | Continuous variable | 21 349 | 1.02 | 1.01–1.04 | <0.0001 | |
| 18–32 years | 21 349 | 3 505 | 0.99 | 0.60–1.65 | 0.004 | |
| 33–46 years | 6 394 | 1 | – | |||
| 47–60 years | 8 028 | 1.54 | 1.05–2.24 | |||
| >60 years | 3 422 | 2.03 | 1.31–3.16 | |||
| Donor type | Deceased | 21 349 | 20 054 | 3.49 | 1.12–10.9 | 0.032 |
| Living | 1 295 | 1 | 0.14–1.03 | |||
| Transplant organ | Kidney | 21 349 | 20 703 | 1 | – | 0.041 |
| SKP | 646 | 1.94 | 1.03–3.68 | |||
| Year of transplant | 1998–1999 | 21 349 | 3 480 | 3.39 | 1.78–6.44 | 0.0004 |
| 2000–2001 | 3 672 | 2.83 | 1.48–5.41 | |||
| 2002–2003 | 4 120 | 1.79 | 0.90–3.54 | |||
| 2004–2005 | 4 714 | 1.72 | 0.86–3.45 | |||
| 2006–2007 | 5 363 | 1 | – | |||
| Duration of dialysis before transplantation | <5 years | 18 144 | 16 214 | 2.04 | 1.00–4.16 | 0.049 |
| >5 years | 1 930 | 1 | – | |||
| Recipient EBV serostatus | Negative | 20 256 | 723 | 4.77 | 3.14–7.25 | <0.0001 |
| Positive | 19 533 | 1 | – | |||
| EBV matching | Donor + Recipient − | 19 414 | 604 | 5.16 | 3.32–8.04 | <0.0001 |
| Others | 18 810 | 1 | – | |||
| HLA matching | 5 or 6 MM | 21 304 | 7 777 | 1.4 | 1.04–1.88 | 0.027 |
| 0 to 4 MM | 13 527 | 1 | – | |||
| Induction therapy (polyclonal Ab or OKT3) | No | 21 349 | 9 706 | 1 | – | 0.011 |
| Yes | 11 643 | 1.50 | 1.10–2.05 | |||
| Cyclosporine | No | 20 728 | 10 072 | 1 | – | 0.39 |
| Yes | 10 656 | 1.14 | 0.84–1.55 | |||
| Tacrolimus | No | 20 667 | 11 329 | 1 | – | 0.12 |
| Yes | 9 338 | 0.78 | 0.57–1.07 | |||
| Azathioprin | No | 20 640 | 18 366 | 1 | – | 0.03 |
| Yes | 2 274 | 1.51 | 1.04–2.19 | |||
| MMF | No | 20 722 | 3 212 | 1 | – | 0.54 |
| Yes | 17 510 | 0.89 | 0.62–1.29 | |||
| Number of IS drugs | < 4 | 21 349 | 6 819 | 1 | – | 0.11 |
| 4 | 14 530 | 1.32 | 0.94–1.87 |
- HR = hazard ratio; CI = confidence interval; SKP = simultaneous kidney pancreas; MM = mismatches; IS = immunosuppressive.
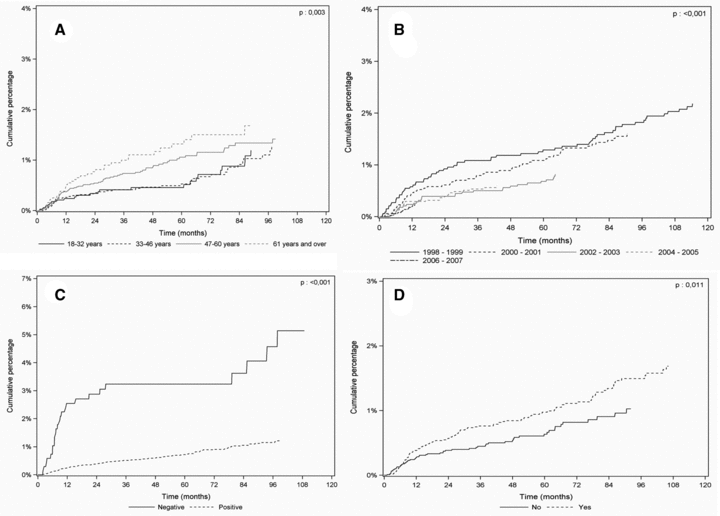
Time to diagnosis for posttransplant lymphoproliferative disorders after kidney transplant stratified by age, period of transplant, EBV recipient status and induction therapy. (A) Patients over 60 years of age (n = 3422), 47–60 years (n = 8028), 18–32 years (n = 6394), versus 33–46 (n = 3505), p = 0.003 by the log-rank test. (B) Period of transplant 1998–1999 (n = 3480), 2000–2001 (n = 3672), 2002–2003 (n = 4120), 2004–2005 (n = 4714) versus 2006–2007 (n = 5363), p < 0.001 by the log-rank test. (C) EBV seronegative recipients at time of transplant (n = 723) versus EBV positive recipients (n = 19 533), p < 0.001 by the log-rank test. (D) Patients with antilymphocyte globulins or OKT3 treatment (n = 11 643) versus patients without (n = 9706), p = 0.011 by the log-rank test.
Multivariate analysis (Table 5) showed that factors associated with lymphoma occurrence were older age (>46 years), SKP transplantation, period of transplant (before 2001), having 5 or 6 HLA mismatches, EBV mismatch (vs. all other EBV matching) and induction therapy with T-cell depleting antibodies.
| Variables | Modalities | AHR | IC 95% | P |
|---|---|---|---|---|
| Recipient gender | Female | 1.05 | [0.76–1.46] | 0.76 |
| Male | 1 | - | ||
| Recipient age | 18–32 years | 1.06 | [0.60–1.87] | <0.0001 |
| 33–46 years | 1 | - | ||
| 47–60 years | 1.87 | [1.22–2.86] | ||
| >60 years | 2.80 | [1.73–4.55] | ||
| Period of transplant | 1998–1999 | 3.36 | [1.64–6.87] | 0.003 |
| 2000–2001 | 3.08 | [1.55–6.15] | ||
| 2002–2003 | 1.90 | [0.93–3.91] | ||
| 2004–2005 | 1.64 | [0.79–3.40] | ||
| 2006–2007 | 1 | - | ||
| SPK transplantation | No | 1 | - | 0.008 |
| Yes | 2.52 | [1.27–5.01] | ||
| EBV matching | All others | 1 | - | <0.0001 |
| Donor + recipient− | 5.31 | [3.36–8.39] | ||
| HLA matching | 0–4 mismatches | 1 | – | 0.008 |
| 5 or 6 mismatches | 1.54 | [1.12–2.12] | ||
| Induction therapy (polyclonal Ab or OKT3) | No | 1 | – | 0.05 |
| Yes | 1.42 | [1.00–2.02] | ||
| Cyclosporine | No | 1 | – | 0.17 |
| Yes | 0.66 | [0.36–1.19] | ||
| Tacrolimus | No | 1 | – | 0.19 |
| Yes | 0.66 | [0.36–1.22] | ||
| Azathioprin | No | 1 | – | 0.34 |
| Yes | 1.30 | [0.76–2.19] | ||
| MMF | No | 1 | – | 0.44 |
| Yes | 1.22 | [0.74–2.02] |
- AHR = adjusted hazard ratio; CI = confidence interval; SKP = simultaneous kidney-pancreas; MM = mismatches.
Of note, when SKP recipients (n = 10) were excluded from the cohort, risk factors for PTLD were the same by univariate and multivariate analyses (data not shown).
Analyzing risk factors of lymphoma according to specific subgroups revealed different levels of risk for the above-mentioned factors (Figures 4A and B).
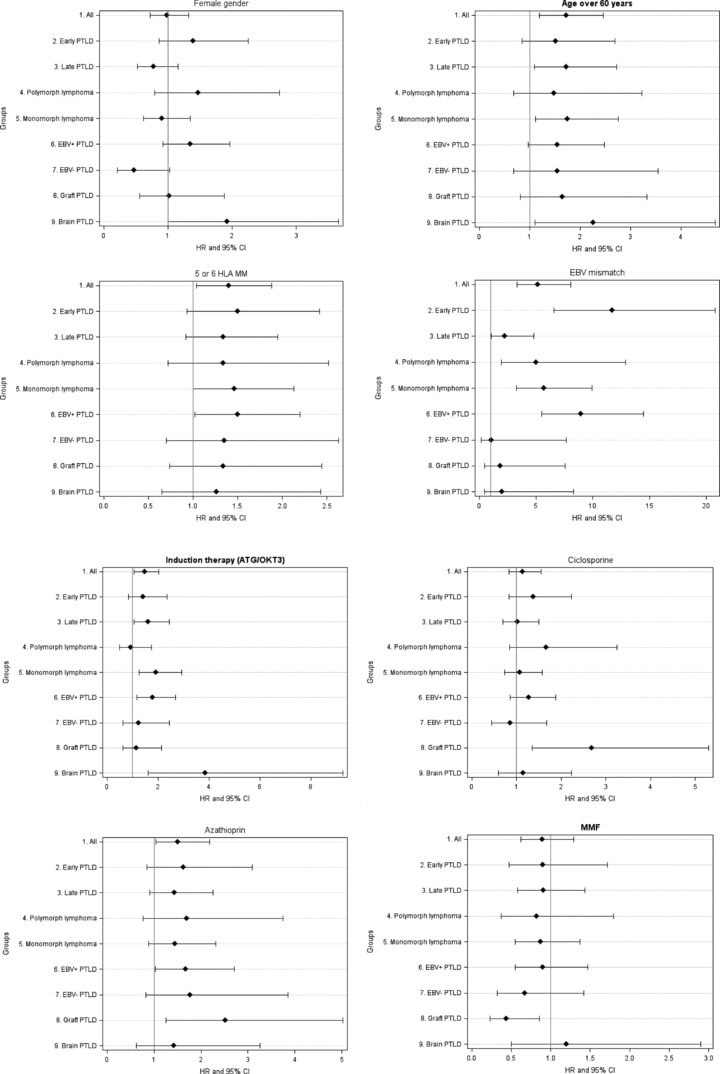
Risk factors for PTLD occurrence in different homogeneous lymphoma subgroups. (A) Distribution of HR according to gender, recipient age above 60 years, 5–6 HLA mismatches and EBV D+/R− mismatch in all PTLD, early and late PTLD, polymorphic and monomorphic PTLD, EBV positive and EBV negative PTLD and graft and cerebral PTLD. (B) Distribution of HR according to T-cell depleting agents, cyclosporine, azathioprin and MMF therapy in all PTLD, early and late PTLD, polymorphic and monomorphic PTLD, EBV positive and EBV negative PTLD and graft and cerebral PTLD.
Early-onset PTLD (n = 69) were linked to the earlier transplant period (1998–1999 and 2000–2001 vs. 2006–2007, HR = 2.94, CI = 1.37–6.31 and HR = 2.34, CI = 1.06–5.15, respectively, p = 0.024), simultaneous kidney–pancreas transplantation (vs. kidney alone, HR = 2.6, CI = 1.04–6.4, p = 0.04), first graft (vs. subsequent transplantation, HR = 2.84, CI = 1.03–7.8, p = 0.043), and 1 or 2 HLA-B mismatches (vs. 0, HR = 2.21, CI = 1.1–4.46, p = 0.027). Furthermore, EBV seronegativity (HR 10.3, CI = 5.9–18, p < 0.0001) and EBV mismatch (HR = 11.7, CI = 6.6–20.8, p < 0.0001) were shown to be very strong risk factors for early-onset PTLD, highlighting the role of the virus in this process. In our series, 18 out of 67 patients (27%) with an early-onset PTLD were EBV negative at transplantation and at least 15 received a transplant from an EBV positive donor. On the other hand, risk factors for late-onset lymphomas (n = 112) were recipient age (HR = 1.03 for each year, p = 0.0004), >60 and 47–60 years vs. 33–46 years (HR = 2.19, CI = 1.25–3.85 and HR = 1.62, CI = 1–2.61, respectively, p = 0.02), EBV seronegativity (HR = 2.3, CI = 1.16–4.57, p = 0.017), EBV mismatch (HR = 2.2, CI = 1.03–4.8, p = 0.042), induction therapy by polyclonal antibodies or OKT3 (HR = 1.56, CI = 1.04–2.33, p = 0.032) and there was a trend for azathioprin therapy (HR = 1.43, CI = 0.91–2.25, p = 0.12).
Risk factors for EBV positive PTLD (n = 107) were year of transplantation 1998–1999 and 2000–2001 vs. 2006–2007 (HR = 3.77, CI = 1.79–7.96 and HR = 2.5, CI = 1.15–5.42, respectively, p = 0.001), older age (HR = 1.02 for each year, CI = 1–1.03, p = 0.017), SPK transplantation (HR = 2.3, CI = 1.07–4.94, p = 0.034), recipient EBV seronegativity (HR = 8.24, CI = 5.16–13.2, p < 0.0001), EBV mismatch (HR = 8.93, CI = 5.5–14.5, p < 0.0001), cold ischemia time (HR = 1.02 for each hour, CI = 1–1.04, p = 0.015), 5 or 6 HLA mismatches (vs. 0 to 4, HR = 1.5, CI = 1.02–2.2, p = 0.038), induction therapy by polyclonal antibodies or OKT3 (HR = 1.78, CI = 1.17–2.69, p = 0.007) and azathioprin treatment (HR = 1.67, CI = 1.03–2.71, p = 0.037) whereas EBV negative tumors (n = 36) were associated with male gender (HR = 2.13, CI = 0.97–4.68, p = 0.06), recipient CMV seronegativity (HR = 1.97, CI = 1–3.87, p = 0.05) and CMV mismatch (HR = 1.97, CI = 0.94–4.13, p = 0.07).
Regarding PTLD histology, time to polymorphic PTLD was shorter than time to monomorphic PTLD, but the difference was not statistically significant (26 ± 30 vs. 35 ± 30 months, p = 0.2). Monomorphic PTLD were more often localized in the gastro-intestinal tract than polymorphic PTLD (22% vs. 9%, p = 0.046). Conversely, polymorphic PTLD occurred more often in the graft than monomorphic lymphomas (40.5 vs. 18.5%, p = 0.046). Risk factors associated with polymorphic (n = 41) or monomorphic PTLD (n = 109) did not differ from the total population except for cold ischemia time which was associated with polymorphic PTLD (HR = 0.44, CI = 0.23–0.81 for CIT <24 h, p = 0.009) and induction therapy which was associated with monomorphic lymphomas (HR = 1.92, CI = 1.26–2.93, p = 0.002).
Finally, when risk factors were analyzed according to the location of PTLD, some specific features appeared. Graft PTLD occurred in 44 patients, three were transplanted with EBV mismatch, mean delay of diagnosis was 11 ± 21 months, 32 (73%) were early-onset PTLD, 35 (80%) were localized only in the graft, 84% were EBV induced, 78% were monomorphic, all were treated with
IS reduction, seven with antiviral therapy, 20 with rituximab alone, four with chemotherapy alone and 10 with Rituximab+chemotherapy, 16 were treated by graft removal, only three showed progression of the disease after treatment and 10 died, only one from lymphoma progression. Graft PTLD were closely associated with period of transplant (1998–1999 and 2000–2001 vs. 2006–2007, HR = 24.9, CI = 3.31–186 and HR = 15.6, CI = 2.03–120, respectively, p = 0.0005), cold ischemia time (HR = 1.03 for each hour, CI = 1–1.06, p = 0.06), cyclosporine (HR = 2.68, CI = 1.35–5.3, p = 0.005) and azathioprin (HR = 2.52, CI = 1.26–5, p = 0.002) whereas tacrolimus (HR = 0.33, CI = 0.16–0.68, p = 0.003) and MMF (HR = 0.44, CI = 0.23–0.86, p = 0.015) were associated with a lower risk of graft PTLD. Brain lymphomas occurred in 37 patients (18M, 19F), six were transplanted with EBV mismatch, mean delay of diagnosis was 35 ± 26 months, only eight (22%) were early onset PTLD, 34 (92%) were localized only in the central nervous system, all tumors tested for EBV (n = 31) were EBV positive, 73% were monomorphic, all but two were treated with IS reduction, five with rituximab alone, 15 with chemotherapy alone and 11 with Rituximab+chemotherapy, five were treated by surgery, 23 showed complete remission after treatment and 20 died, the majority from lymphoma progression. Risk factors for brain PTLD were female gender (HR = 1.92, CI = 1–3.66, p = 0.049), older age (HR for each year = 1.06, CI = 1.03–1.09, p = 0.0001), especially in recipients>60 years old (HR = 2.81, CI = 1.11–7.12, p = 0.18), donor CMV seropositivity (HR = 2.25, CI = 1.11–4.58, p = 0.025) and induction therapy by T-cell depleting agents (HR = 3.86, CI = 1.61–9.26, p = 0.002).
Discussion
Analysis of the French registry enabled us to determine the incidence and risk factors of PTLD in a vast population of adult renal transplant patients with a follow-up of more than 10 years. We showed that lymphomas continue to be a long-term risk after transplantation with incidences of 0.35% 1 year, 1% 5 years and up to 2.1% 10 years post-transplant. These rates are close to those reported in the US, CTS and ANZDATA registries (2,4,9,12,15). Of note, US and CTS cohorts included pediatric patients whereas the French registry recorded only cases in adult patients. We chose to enroll only adults because the epidemiology of PTLD in pediatric patients has been shown to be different from that of the adult population (5,18). Whereas US registries only provide data on the first 3 years after transplantation, our registry includes outcomes up to 10 years after transplantation. Regarding the increase in life expectancy after kidney transplantation, providing long-term data is of interest. We observed a bimodal distribution pattern of PTLD incidence: high during the first year posttransplant, reduced from the second to the seventh year and high again after the eighth posttransplant year. These findings are in accordance with reports from the ANZDATA registry and suggest a role for specific factors in the pathogenesis of early and late lymphomas (15). The first peak could be explained by a higher incidence of EBV-induced lymphoma, particularly in cases of primo-infection, and by the higher occurrence of graft PTLD. The second peak is less likely to be linked to EBV lymphoproliferations but could be the consequence of aging and of the duration of immunosuppression exposure.
In our study, we observed a decrease in the incidence of lymphomas in the more recent period. In 2002–2005, patients were treated less frequently with T-cell depleting induction (51 vs. 66%, p < 0.001) and cyclosporine (45 vs. 65%, p < 0.0001), and more frequently with tacrolimus (51 vs. 33%, p < 0.0001), less frequently with azathioprin (4 vs. 25%, p < 0.0001) and more frequently with MMF (92 vs. 70%, p < 0.0001). These changes probably played a role in the decrease in the incidence of lymphoma. Nevertheless, the transplant period was also shown to be a risk factor after adjustment for immunosuppressive drugs, arguing for the existence of other contributing factors that could not be easily identified in the French registry. We could hypothesize that the generalization of EBV monitoring in patient management provided guidance for the intensity of immunosuppression and hence reduced the occurrence of PTLD.
To our knowledge, this is the first time that PTLD incidence has been analyzed according to the location of the tumor, as previous registries did not record lymphoma topography. We showed that graft PTLD were more frequent in the first 2 years, cerebral lymphomas occurred from the 2nd to the 7th year, while gastro-intestinal PTLD mostly occurred later, from the 6th to the 10th year posttransplant. The shorter time of occurrence of graft PTLD could be explained by a specific pathogeny, especially by the development of lymphoproliferations from donor passenger lymphocytes as we have already reported (19), the implication of chronic antigenic stimulation (20), and the role of EBV proliferation (21). The long-term higher incidence of digestive PTLD should encourage physicians to rapidly investigate digestive symptoms in kidney recipients.
Risk factors associated with PTLD in our global cohort were mostly well-known factors: age, EBV seronegativity and transplant period, simultaneous kidney pancreas transplantation, HLA mismatches, T-cell depleting agents and azathioprin. Other risk factors like recipient gender, cold ischemia time, HLA-B mismatches, cyclosporine and MMF were not associated with risk of PTLD in the whole cohort but only in particular subgroups of lymphomas. As the French registry recorded a large number of PTLD, we were able to analyze risk factors according to their clinical or histological characteristics. Here our aim was to improve our understanding of the specificity of the pathogenesis of lymphomas in homogeneous subgroups of PTLD as it is known that the spectrum of posttransplant lymphoproliferations is very wide and ranges from ‘benign’ early nodal B-cell hyperplasias to the frankly malignant late occurring and disseminated extranodal lymphomas. Moreover, risk factors, presentation and outcomes of particular topography of PTLD such as graft and brain lymphomas differ significantly, suggesting specific triggers and pathogeny. We showed that age is particularly associated with late lymphomas and cerebral PTLD. Association of age with late lymphomas was previously reported (22). This association was assumed to be due to age-related loss of immunosurveillance based on the natural history of lymphomas in the general population (23), the immunodepression being accentuated by the duration of immunosuppression in the case of late lymphomas.
In our study, recipient gender was unequally distributed in the subgroups of PTLD. Male gender was not a risk factor for the global cohort but there was a trend toward male predominance in late and EBV-negative lymphomas. This was already reported in data from the Scientific Registry of Transplant Recipients for late lymphomas (22). Surprisingly, female gender was associated with an almost twofold higher risk of brain lymphoma in our series whereas gender distribution was almost equal in immunocompetent patients (24). This increased risk has never previously been reported, probably because in the majority of previous registries, the location of PTLDs was not taken into account. The reason is not known, but hormonal or genetic causes are possible.
The link between EBV and PTLD was established in the early 1980s by Hanto and the Minneapolis group (25,26) and is now widely recognized (6,27). We showed that the risk of early-onset PTLD was much greater in mismatched EBV donor/recipient pairs, with a RR of 12, and in recipients with EBV-positive lymphomas, with a RR of 9, highlighting the role of EBV primo-infection in the development of early and EBV+PTLD (6). Fortunately, this situation is rare in adult transplantation but is still a matter of concern in pediatric populations (28) in which measures like EBV vaccination before transplantation should be investigated (29,30). Moreover, the choice of immunosuppressive therapy should be guided by knowledge of donor and recipient EBV antibody status. In contrast, EBV-negative lymphomas were not associated with EBV but with CMV mismatch arguing for a putative role of another virus in the development of EBV-negative lymphomas. Positive donor CMV serostatus was also associated with a greater risk of brain lymphoma suggesting a possible interaction between EBV and CMV infections.
In our series, HLA mismatches, particularly for recipients with five or six mismatches, were associated with PTLD as described in a USRDS report (4). Subgroup analysis showed that the association was stronger for EBV-positive PTLD. This finding might be explained by the larger amount of IS drugs used in this population. However, the higher risk persisted after adjusting for immunosuppressive medication, suggesting that factors such as reduced MHC-restricted antigen-specific antiviral activity may be involved. Moreover, we underlined the role of HLA-B mismatch in the subgroup of early-onset PTLD, where the risk in recipients with one or two HLA-B mismatches was more than two times higher than in patients with no HLA-B mismatch. A link between HLA-B mismatch and non-Hodgkin lymphomas was already reported (31). Opelz reported an increased incidence of graft lymphomas in kidney recipients with two HLA-B mismatches (32), highlighting the role of a strong local antigenic stimulus exerted by the allograft that could influence lymphomagenesis. In addition to an immunological trigger, nonimmunological activation of the allograft could influence the risk of lymphoma. For the first time, we showed that there is a link between the duration of cold ischemia time and the development of lymphoproliferation. This was particularly notable for polymorphic, EBV and intragraft lymphoproliferations.
Immunosuppressive drugs are another well-known risk factor associated with the development of lymphoproliferations. Even though the total amount of immunosuppression plays a key role in the risk of malignancy, some authors focused on the specific role of each immunosuppressive molecule. In the French registry, multivariate analysis showed that polyclonal induction was associated with a 1.4-fold increase in the risk of PTLD. This is in line with the majority of published data (2,4,10,12,15,33) but some authors found no link between R-ATG and PTLD (9,34). Of note, use of polyclonal antibodies for induction therapy is much more frequent in French centers (around 70% of patients) than in US centers (between 20 to 45%). Our subgroups analysis revealed that risk of developing brain lymphomas is particularly high (fourfold higher) in patients who received T-cell depleting agents. To our knowledge, this has never previously been reported and thus deserves particular attention. On the contrary, graft lymphomas were not associated with depleting induction therapy in our series. Cyclosporine was associated with an increased risk of graft lymphomas (RR = 2.7) but not with other subtypes of PTLD, suggesting a potential role for this drug in the development of donor-derived PTLD, since donor-derived lymphoproliferation occurs preferentially in the graft itself (19). Regarding antimetabolites, we found that azathioprin was associated with the development of lymphomas, particularly graft PTLD and EBV positive lymphoproliferations. The role of azathioprin in DNA instability could explain the effect of the drug on lymphomagenesis. On the other hand, despite its potent immunosuppressive effect, MMF did not increase the risk of lymphoma in the French renal transplant population as reported in previous studies (4,9,11,12,35,36). Moreover, graft PTLDs were less frequent in patients who were treated with MMF than in patients who were not. A possible explanation is that fewer cases of acute rejection resulted in less frequent use of antirejection therapies in the MMF-treated group than in AZA-treated patients (25 vs. 16%, p = 0.0001).
The limitations of our study concerned missing data, especially data concerning the control group and particularly on EBV serostatus and immunosuppressive therapy. Nevertheless, EBV records are more complete in the French database than in other nationwide registries since only 5% data on EBV recipient serostatus were missing in the French database compared to 75% in ANZDATA and 87% in USRDS. As no data concerning race are included in French databases, we were unable to analyze incidence and risk factors for specific ethnic groups. Data on immunosuppressive therapy in the French registry were incomplete, particularly on the exact type of induction (polyclonal antilymphocyte antibodies or OKT3), so we were unable to analyze the exact role of each induction drug. In addition, information on the dose and duration of therapy was incomplete in the Cristal database and no precise information was available on PSI treatment.
Conclusion
The long recruitment period of the French registry and the large number of enrolled patients enabled us to produce strong results on the epidemiology and risk factors of PTLD. We showed that incidence has decreased slightly in recent years but that PTLD is still a major issue as long as 10 years posttransplant. We confirmed and extended our previous knowledge on risk factors for lymphomas, especially for specific subgroups of lymphomas leading to a better understanding of pathogenesis.
Funding source: None.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Appendix
Collaborators:
PF Westeel (CHU Amiens), F Villemain, JF Subra (CHU Angers), JM Rebibou (CHU Besançon), D Morel, P Merville (CHU Bordeaux), B Bourbigot, MC Moal (CHU Brest), B Hurault de Ligny (CHU Caen), AE Heng, P Deteix (CHU Clermont-Ferrand), Y Tanter, C. Mousson (CHU Dijon), F Bayle, B Janbon (CHU Grenoble), F Provot, C Noel (CHU Lille), JP Szelag, M Essig (CHU Limoges), JL Garnier, E Morelon, C Pouteil Noble, R Cahen (CHU Lyon), R Purgus, V Moal, T Legris (CHU Marseille), S Delmas, G Mourad (CHU Montpellier), E Renoult, M Kessler, L Frimat (CHU Nancy), E Cassuto (CHU Nice), R Snanoudj, C Hiesse, A Durrbach (Paris, Hôpital Bicêtre), C Antoine, D Glotz (Paris, Hôpital Pompidou), MF Mamzer, MN Peraldi (Paris, Hôpital Necker), B Viron (Paris, Hôpital Bichat), J Bedrossian, E Thervet, C Legendre (Paris, Hôpital Saint-Louis), E Rondeau, N Ouali (Hôpital Tenon), M Pastural, M Delahousse (Paris, Hôpital Foch), V Leblond, S Choquet (Paris, Hôpital La Pitié-Salpétrière), G Touchard, A Thierry (CHU Poitiers), O Toupance (CHU Reims), J Rivalan (CHU Rennes), I Etienne (CHU Rouen), B Bourgeon, R Genin (CHR St Denis de la Réunion), E Alamartine (CHU St Etienne), B Ellero (CHU Strasbourg), N Kamar, L Rostaing, D Durand (CHU Toulouse), A Al Najar, M Buchler (CHU Tours).




