Impact of Cytomegalovirus Disease in D+/R– Kidney Transplant Patients Receiving 6 Months Low-Dose Valganciclovir Prophylaxis
Abstract
Late-onset cytomegalovirus (CMV) disease remains common in CMV serology naïve kidney transplant patients of CMV serology positive organs (D+/R–) despite the use of antiviral prophylaxis. We studied clinical efficacy of 6-month low-dose valganciclovir (VGCV) prophylaxis, risk factors for late-onset CMV disease and its impact on kidney transplant outcomes. Between October 2005 and December 2009, 166 consecutive D+/R– kidney alone and simultaneous pancreas and kidney transplant patients received VGCV 450 mg daily for 6 months after transplantation. After a median follow-up of 3.2 years, 30 cases of CMV disease occurred within the first 2 years after transplantation with a cumulative incidence of 11.5 and 18.1% at 1 and 2 years, respectively. The use of an induction agent with rabbit antithymocyte globulin and older donor age were factors associated with the risk of late-onset CMV disease (AHR 2.91, 95% CI 1.18–7.20, p = 0.021 and AHR 1.03, 95% CI 1.01–1.06, p = 0.016, respectively). Late-onset CMV disease was associated with increased risk for death-uncensored graft loss (AHR 2.95, 95% CI 1.15–7.61, p = 0.025). In conclusion, late-onset CMV disease continues to negatively impact kidney transplant outcome despite 6-month low-dose VGCV prophylaxis. Investigations focusing on novel preventive approaches should be emphasized.
Abbreviations:
-
- AA
-
- African American
-
- AHR
-
- adjusted hazard ratio
-
- AOR
-
- adjusted odds ratio
-
- CI
-
- confidence interval
-
- CMV
-
- cytomegalovirus
-
- CNI
-
- calciuneurin inhibitor
-
- CrCl
-
- creatinine clearance by Cockcroft-Gault equation
-
- CsA
-
- cyclosporine
-
- D+/R–
-
- donor CMV serology positive and recipient CMV serology negative combination
-
- DGF
-
- delayed graft function
-
- ECD/DCD
-
- expanded criteria donor/donation after cardiac death
-
- G-CSF
-
- granulocyte colony stimulating factor
-
- GCV
-
- ganciclovir
-
- HCV
-
- hepatitis C virus
-
- MMF
-
- mycophenolate mofetil
-
- PCR
-
- polymerase chain reaction
-
- PRA
-
- panel reactive antibodies
-
- rATG
-
- rabbit antithymocyte globulin
-
- Tac
-
- tacrolimus
-
- VGCV
-
- valganciclovir
Introduction
With the widespread adoption of universal prophylaxis in cytomegalovirus (CMV) serology naïve organ transplant recipients of serology positive organs (D+/R–), late-onset CMV disease, defined as the occurrence of CMV disease after the cessation of prophylaxis, remains as a concern (1–4). CMV disease can cause significant morbidity, increases mortality and is associated with inferior transplant outcomes, particularly, in kidney transplantation (5–7).
Valganciclovir (VGCV) is an effective anti-CMV drug used in the prevention and treatment of CMV disease in solid organ transplant recipients (1,8). A recently completed randomized study has shown clinical benefit of prolonged VGCV prophylaxis up to 200 days after transplantation in D+/R– kidney transplant patients (4). Nevertheless, late-onset CMV disease continues to affect 21–37% of D+/R– kidney transplant patients within 2 years after transplantation (9,10). The routinely recommended dose of VGCV for prophylaxis is 900 mg daily based on the original clinical trial (1). However, in clinical practice, a dose of 450 mg daily is frequently used (11–14). Yet, there are only few reports of clinical efficacy of low-dose VGCV on late-onset CMV disease in D+/R– kidney transplant population, most with a relatively short follow-up period (13,15). A recent meta-analysis suggested that the clinical efficacy of universal prophylaxis with VGCV either 900 mg or 450 mg daily was similar (16). Furthermore, it remains unknown whether late-onset CMV disease negatively impacts patient and graft outcome in D+/R– kidney transplant patients receiving extended low-dose VGCV prophylaxis.
This study is based on a single center experience involving D+/R– kidney alone (KA) and simultaneous pancreas and kidney (SPK) transplant patients who received low-dose VGCV for 6 months following transplantation. This retrospective study aims to assess clinical efficacy and safety of such regimen in the prevention of late-onset CMV disease and to identify potential risk factors as well as effects on patient and graft outcomes associated with late-onset CMV disease.
Materials and Methods
All D+/R– KA and SPK transplant patients from October 1, 2005 to December 31, 2009 who survived through at least the period of prophylaxis with a functioning kidney graft were included. All patients received prophylaxis with VGCV 450 mg daily, if their creatinine clearance (CrCl) was equal or greater than 60 mL/min, for 6 months after transplantation. A dose reduction was required if CrCl was lower than 60 mL/min such that VGCV 450 mg every other day was given for patients with CrCl between 25 and 59 mL/min, VGCV 450 mg twice weekly for patients with CrCl below 25 mL/min, and VGCV 450 mg three times a week after each hemodialysis treatment for patients who experienced initial delayed graft function. Patients were followed up to the time of graft loss, death or December 31, 2010. The study was approved by the institutional review board (IRB).
Immunosuppression regimens, including induction and maintenance, were provided according to the institutional protocols. For induction regimen, rabbit antithymocyte globulin (rATG; Thymoglobulin®, Genzyme, Cambridge, MA, USA) or basiliximab, an anti-IL-2 receptor antibody (Simulect®, Novartis, East Hanover, NJ, USA) were utilized where dictated by the specific center protocols. More specifically, patients with panel reactive antibodies (PRAs) titer equal or greater than 20, African American racial identification, living unrelated kidney transplant or pancreas transplant were given rATG whereas patients with delayed or slow graft function were given basiliximab. For maintenance immunosuppression, a triple drug regimen which consisted of a calcineurin inhibitor (CNI; cyclosporine or tacrolimus), an antiproliferative agent (mycophenolate mofetil—MMF or others) and prednisone was usually used. Target trough levels for cyclosporine (CsA) and tacrolimus (Tac) were 150–300 and 4–15 ng/mL, respectively, during the first 3 months. Subsequently, CsA and Tac trough levels were maintained at 100–200 and 4–8 ng/mL, respectively. Prednisone was tapered to 10 mg/day at about 8 weeks posttransplant and remained at 5–10 mg daily thereafter over the study period.
The primary endpoints were the incidence of late-onset CMV disease and kidney graft loss, censored and uncensored for death, respectively.
The diagnosis of CMV disease was based on a constellation of symptoms and positive CMV DNA viremia determination using polymerase chain reaction (PCR) technique during the entire duration of follow-up. CMV/PCR testing was requested based upon clinical suspicion by transplant physicians. The test was performed on the platform of COBAS Amplicor instrument with all reagents purchased from the Roche Diagnostics (Indianapolis, IN, USA). The most common clinical suspicions for ordering such determination were gastroenteric symptoms, viral like symptoms such as fever and malaise or the presence of leukopenia, particularly neutropenia. The tissue diagnosis to document the presence of tissue invasion was obtained in some patients on a case-by-case basis as determined by physicians. Patients with a positive CMV DNA viremia, with or without symptoms, were treated with an additional course of either intravenous ganciclovir (GCV) or oral VGCV and temporary discontinuation of antiproliferative agents for up to 2–3 weeks (induction therapy phase).
The secondary endpoint was the incidence of leukopenia and/or neutropenia, the only adverse effect of prophylaxis considered, defined as peripheral blood leukocyte and neutrophil counts of less than 4000/μL and 1400/μL, respectively. The lowest leukocyte and neutrophil counts were recorded during the initial 6 months after transplantation. The management of leukopenia/neutropenia, including the use of granulocyte colony stimulating factor (G-CSF) was documented.
Student's t-tests and χ2 tests were used to compare continuous and categorical variables, respectively, for baseline demographic and clinical characteristics between patients with and without late-onset CMV disease. The Kaplan–Meier method was used to estimate the incidence of late-onset CMV disease during the study period. A generalized estimating equation (GEE) model based on the Poisson distribution was utilized to compare the cumulative incidence rate of kidney graft loss between patients with and without late-onset CMV disease. Multivariate Cox proportional hazard regression analysis was used to assess the effects of late-onset CMV disease on kidney graft loss and to identify the risk factors associated with late-onset CMV disease. In order to account for changes in disease status during the follow-up period, late-onset CMV disease was included as a time-dependent covariate. In this way, patients were allowed to contribute time at risk to the disease-free group until the time of disease diagnosis, at which point they switched to the disease group. Initial model selection was via backward selection. Because overfitting was a concern in the Cox models, models were further reduced with the goal of optimizing the Bayesian Information Criteria (BIC) (17). Multivariate logistic regression analyses were performed investigating factors associated with leukopenia.
Analyses were performed using SAS 9.2, with statistical significance set at a two-sided α≤ 0.05.
Results
A total of 182 consecutive CMV D+/R– KA and SPK transplant patients were identified initially as meeting inclusion criteria for the study. The median follow-up was 3.2 years from the time of transplant with range between 1 and 5 years. A mean of 3.8 ± 4.2 diagnostic CMV viremia determinations per patient was carried out during the study period and 30 patients (16.5%) were found to have developed late-onset CMV disease within the first 2 years after transplantation. An additional 16 patients (8.8%) were found to have positive CMV DNA viremia without symptoms suggestive of disease. Due to the lack of systematic viremia monitoring during the entire study period, these 16 cases of asymptomatic CMV infection could represent an underestimated phenomenon with possibly different clinical implication and were thus excluded from further analyses (1,18,19).
Of 30 patients with late-onset CMV disease, 22 had probable or confirmed CMV gastroenteric disease (fever, abdominal pain, vomiting and diarrhea at presentation, only four patients had a tissue diagnosis through endoscopic procedures) with or without signs of hepatitis and pancreatitis, and eight had CMV syndrome. The cumulative incidence of late-onset CMV disease was 11.5% at 1 year and 18.1% at 2 years after transplantation, respectively (Figure 1A). The median time to CMV disease was 293 days from the time of transplant with a range from 114 to 639 days posttransplant. Four patients developed CMV disease during the period of prophylaxis. A careful chart review revealed that two patients had their VGCV on hold for more than 1 month (for leukopenia/neutropenia) and other two patients had their dose of VGCV reduced to twice and thrice weekly, respectively, due to reduced renal function, prior to the diagnosis. Furthermore, another five patients (16.7%) had recurrent CMV viremia requiring additional courses of antiviral treatment. CMV resistance was not detected in any of these cases.
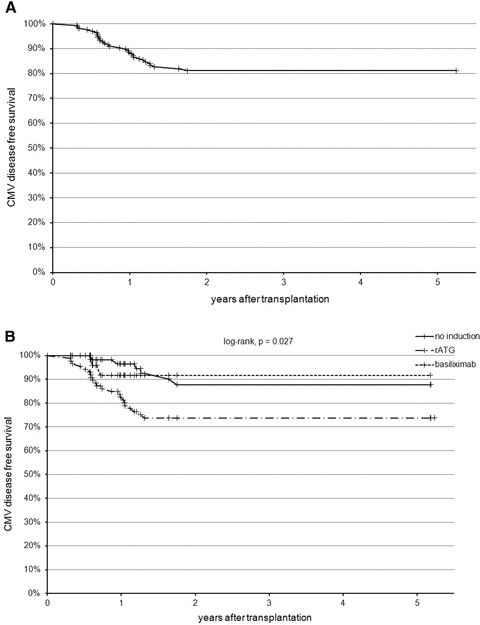
Kaplan–Meier late-onset CMV disease-free survival: (A) among all patients; (B) stratified by the use of different induction regimens.
Demographic and baseline clinical characteristics of patients with and without late-onset CMV disease are presented in Table 1. Patients were comparable with regard to the majority of baseline characteristics including recipient age, gender, positive hepatitis C (HCV) serology, the use of expanded criteria donor or donation after cardiac death kidneys (ECD/DCD) and incidence of delayed graft function and acute rejection. The only baseline variables that were significantly different between patients with and without late-onset CMV disease are the differential use of induction regimens, donor age and the numbers of CMV/PCR determination per patient. In fact, patients who developed late-onset CMV disease were given induction more frequently with rATG (73.3% vs. 47.0%, p = 0.02), received more often kidney from older donors (44.0 ± 14.5 vs. 37.1 ± 15.0 years, p = 0.02) and had more CMV/PCR tests per patient (8.7 ± 8.0 vs. 2.6 ± 1.6, p < 0.001).
| No CMV N = 136 | CMV disease N = 30 | p | |
|---|---|---|---|
| Recipient age, years (SD) | 46.1 (11.9) | 46.5 (14.6) | 0.87 |
| Recipient gender, male, n (%) | 97 (71.3) | 23 (76.7) | 0.55 |
| Recipient race, AA, n (%) | 16 (11.8) | 5 (16.7) | 0.46 |
| HCV positive serology, n (%) | 6 (4.4) | 1 (3.3) | 1.00 |
| Acute rejection, n (%) | 0.75 | ||
| Banff 1a or less | 16 (11.8) | 4 (13.3) | |
| Banff 1b or higher | 12 (8.8) | 1 (3.3) | |
| PRA, n (%) | 0.06 | ||
| < 10 | 110 (80.9) | 21 (70.0) | |
| 10 – 80 | 24 (17.6) | 6 (20.0) | |
| > 80 | 2 (1.5) | 3 (10.0) | |
| Induction, n (%) | 0.02 | ||
| None | 50 (36.8) | 6 (20.0) | |
| rATG | 64 (47.0) | 22 (73.3) | |
| basiliximab | 22 (16.2) | 2 (6.7) | |
| CNIs, Tac, n (%) | 28 (20.6) | 8 (26.7) | 0.46 |
| Living donor, n (%) | 51 (37.5) | 14 (46.7) | 0.35 |
| Donor age, years (SD) | 37.1 (15.0) | 44.0 (14.5) | 0.02 |
| Donor gender, male, n (%) | 73 (53.7) | 11 (36.7) | 0.09 |
| Pancreas and kidney transplant, n (%) | 12 (8.8) | 2 (6.7) | 0.70 |
| First transplant, n (%) | 123 (90.4) | 26 (86.7) | 0.65 |
| Diabetes mellitus, n (%) | 47 (34.6) | 10 (33.3) | 0.90 |
| CrCl, mL/min. (sd) | 77.2 (19.9) | 72.8 (23.5) | 0.35 |
| CMV PCR test per patient, n (SD) | 2.6 (1.6) | 8.7 (8.0) | <0.001 |
We further studied the effects of induction on the development of late-onset CMV disease in this cohort of patients. Six of 56 patients (10.7%) with no induction, 2 of 24 patients (8.3%) with basiliximab induction and 22 of 86 patients (25.6%) with rATG induction developed late-onset CMV disease during the study period (Figure 1B). All five patients with recurrent CMV viremia received induction with rATG. Multivariate Cox proportional hazard regression analysis with the use of backward selection demonstrated that the use of rATG, but not basiliximab, was associated with a statistically significant increase in the risk for late-onset CMV disease (AHR 2.91, 95% CI 1.18–7.20, p = 0.021). The only other variable associated with an increased risk for late-onset CMV disease is donor age, as a 1-year increase in donor age was associated with 3% higher risk of late-onset CMV disease (AHR 1.03, 95% CI 1.01–1.06, p = 0.016).
During the study period, there were 19 kidney graft losses including seven deaths with functioning graft. The incidence rate was significantly higher among patients with late-onset CMV disease than without late-onset CMV disease (12.3 cases vs. 2.7 cases per 100 person-years for uncensored graft loss, p = 0.001, and 12.3 cases vs. 1.1 cases per 100 person-years for death censored graft loss, p < 0.001, respectively; Figure 2). Multivariate analysis using late-onset CMV disease as a time-dependent covariate suggested increased hazards for graft loss among patients with late-onset CMV disease (AHR 2.95, 95% CI 1.15–7.61, p = 0.025 for uncensored graft loss, and AHR 7.50, 95% CI 2.32–24.30 p < 0.001 for death censored graft loss). Other risk factors associated with increased hazards for graft loss included lower baseline renal function and older recipient age (uncensored only; Table 2).
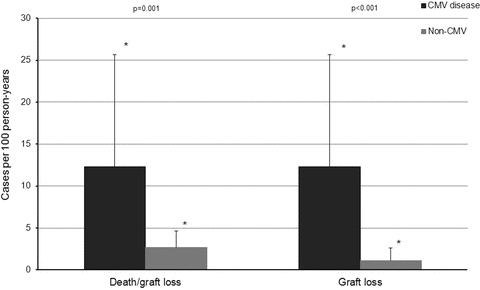
Cumulative incidence of kidney graft loss (overall and death censored) between patients with and without late-onset CMV disease.*Upper limit of 95% CI.
| Death or graft loss | Death-censored graft loss | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Late-onset CMV disease | 2.95 | 1.15, 7.61 | 0.025 | 7.50 | 2.32, 24.3 | < 0.001 |
| Recipient age (years) | 1.09 | 1.04, 1.14 | < 0.001 | NS | ||
| Baseline renal function | 0.95 | 0.92, 0.98 | 0.004 | 0.94 | 0.90, 0.99 | 0.013 |
Finally, we assessed the safety aspect of 6 months prophylaxis with low-dose VGCV. One of the major adverse effects related to the use of VGCV is bone marrow suppression, manifested most frequently as leukopenia/neutropenia. Of 166 patients, 105 patients (63.3%) experienced at least one episode of leukopenia during the period of prophylaxis. Among them, 49 patients (46.7%) had neutrophil counts of less than 1400/μL necessitating the use of G-CSF (filgrastim) in 18 patients. Otherwise, patients were managed with temporary reduction or discontinuation of antiproliferative agents, and occasionally of VGCV, by individual caring physicians. Logistic regression analyses identified the use of rATG (AOR 3.49, 95% CI 1.55–7.85, p = 0.003) and African American race (AOR 18.6, 95% CI 2.34–147.7, p = 0.006) as two risk factors that were individually and independently associated with the development of at least one episode of leukopenia during the period of prophylaxis.
Discussion
CMV disease and/or infection were associated with poor clinical outcome among kidney transplant patients (5,6,19). The use of universal prophylaxis appeared to improve the clinical outcome and was thus recommended, particularly for D+/R– patients (20–24). Nonetheless, late-onset CMV disease remains common affecting 21–36% of D+/R– kidney transplant patients despite the use of prophylaxis (7,9,25). The similar clinical efficacy of VGCV 900 mg daily compared to oral GCV 1000 mg three times daily has established its role in the prevention of primary CMV infection in the field of solid organ transplantation (1). However, the optimal duration of prophylaxis of VGCV remains a matter of debate, despite the fact that a recently completed clinical trial has demonstrated the additional benefit in reducing late-onset CMV disease with longer duration of VGCV prophylaxis at 900 mg daily (4,16,25,26). Little is known about the clinical efficacy of 6-month low-dose VCGV at 450 mg daily, a dose frequently used in the clinical practice, in preventing late-onset CMV disease and in reducing the deleterious effects of CMV disease on graft loss. The drug exposure of VCGV 450 mg daily was similar or higher than that achieved by oral GCV 1000 mg three times daily (12–15,27). Our study thus provides evidence of clinical efficacy of low-dose VGCV at 450 mg daily given for 6 months in preventing late-onset CMV disease in D+/R– KA and SPK transplant patients. Although a direct comparison between our study and other studies including a recently completed randomized clinical trial cannot be made and was not the objective of our study for obvious reasons, the cumulative incidence rate of 11.5% and 18.1% at 1 and 2 years is lower than what was reported in various studies in which a dose of VGCV 900 mg daily was used for 200 days or 6 months (4,9,25). Whether such difference is of any clinical significance or related to the fact of using lower dose of valganciclovir is beyond our speculation. On the other hand, our results pointed out persistent deleterious effects of late-onset CMV disease on kidney transplant outcome despite 6 months prophylaxis. Furthermore, after a median follow-up of 3.2 years, we did not detect new cases of disease beyond 2 years from the date of transplantation.
CMV infection is one of the most common opportunistic infections following kidney transplantation. Previous studies, where no prophylaxis or prophylaxis for 3 months were used, have shown deleterious effects of CMV disease and/or infection on the clinical outcome of kidney transplantation, including reduced graft survival, increased economic burden and shortened patient life (6,7,13,20). Patients with CMV D+/R– serology combination represent the highest risk and the use of universal prophylaxis has been recommended for this group of patients by various guidelines (3,24,28). The duration of prophylaxis varies although increasing evidence suggests that longer is better (4,13,15). However, the incidence of late-onset CMV disease remains unacceptably high (4,9). This phenomenon could be partly caused by increasing use of induction agents, particularly, T-cell depleting antibodies, such as rATG (29–31). Our study further confirmed that such negative effect of rATG persists despite prolonged period of prophylaxis as 2 years cumulative incidence of late-onset CMV disease was more than two and a half folds higher among patients with than without the use of rATG induction (25.6% vs. 10.5%).
Leukopenia, in particular neutropenia, is a well-known adverse effect related to the use of valganciclovir. We report 63.3% incidence of at least one episode of leukopenia during the period of prophylaxis which is higher than that reported by the Impact study despite the fact that we used lower dose of valganciclovir (4). There are several possible explanations, including a slightly different definition of leukopenia used between our study and that of the Impact study, different use in rATG, and/or difference in frequency of laboratory testing leading to overdetection in our patient population. Nevertheless, leukopenia remains common in our study population with 10.8% of patients requiring administration of G-CSF (filgrastim).
The major strengths of our study rely on the sample size, which to the best of our knowledge, is one of the largest involving only D+/R– KA and SPK transplant recipients, sizable cases with late-onset CMV disease and long duration of follow-up. In addition, in our study, the diagnosis of late-onset CMV disease was uniformly confirmed through the determination of DNA viremia and follow-up was complete with only three patients lost to follow-up after 345–635 days. We were therefore able to provide a complete picture of late-onset CMV disease including its deleterious effects on the clinical outcome in the era of 6 months universal prophylaxis as practiced in a single academic transplant center. Even with only 19 kidney graft losses observed during the 5-year study period, the negative impact of late-onset CMV disease remains clearly visible.
Major limitations of our study are related to its retrospective observational nature and its single center setting. The diagnosis of late-onset CMV disease was based upon clinical suspicions by individual physicians, thus there might be some missing cases with subtle clinical symptoms and/or signs of CMV disease. On the other hand, a sizable number of asymptomatic cases (8.8%) argues for heightened awareness of such infection among clinicians involved in the patient care. In addition, single center experience may not necessarily be generalized due to some uniqueness of patient population and/or clinical practice pattern. Finally, due to above reasons, a direct comparison with other studies (clinical trial and/or other observational studies) should not be made (4,9,25).
In conclusion, late-onset CMV disease continues to negatively impact kidney transplant outcome despite a 6-month low-dose VGCV prophylaxis. Investigations of novel preventive approaches are urgently needed.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.