Crosstalk between Complement and Toll-like Receptor Activation in Relation to Donor Brain Death and Renal Ischemia-Reperfusion Injury
Abstract
Two central pathways of innate immunity, complement and Toll-like receptors (TLRs), play an important role in the pathogenesis of renal injury inherent to kidney transplantation. Recent findings indicate close crosstalk between complement and TLR signaling pathways. It is suggested that mitogen activated protein kinases (MAPKs) might be the key molecules linking both the complement and TLR pathways together. Complement and TLRs are important mediators of renal ischemia-reperfusion injury (IRI). Besides IRI, complement C3 can also be upregulated and activated in the kidney before transplantation as a direct result of brain death (BD) in the donor. This local upregulation and activation of complement in the donor kidney has been proven to be detrimental for renal allograft outcome. Also TLR4 and several of its major ligands are upregulated by donor BD compared to living donors. Important and in line with the observations above, kidney transplant recipients have a benefit when receiving a kidney from a TLR4 Asp299Gly/Thr399Ile genotypic donor. The role of complement and TLRs and crosstalk between these two innate immune systems in relation to renal injury during donor BD and ischemia-reperfusion are focus of this review. Future strategies to target complement and TLR activation in kidney transplantation are considered.
Abbreviations:
-
- AR
-
- acute rejection
-
- ARF
-
- acute renal failure
-
- BD
-
- brain death
-
- C3aR
-
- C3a receptor
-
- C5aR
-
- C5a receptor
-
- C5aRA
-
- C5a receptor antagonist
-
- CAN
-
- chronic allograft nephropathy
-
- CR1
-
- complement receptor 1
-
- CS
-
- complement system
-
- DAF
-
- decay accelerating factor
-
- DAMPs
-
- danger associated molecular patterns
-
- GF
-
- graft function
-
- GS
-
- graft survival
-
- HMGB1
-
- high-mobility group protein 1
-
- IR
-
- ischemia-reperfusion
-
- IRI
-
- ischemia-reperfusion injury
-
- LPS
-
- lipopolysaccharide
-
- MAC
-
- membrane attack complex
-
- MAPK
-
- mitogen activated protein kinase
-
- MASP1
-
- MBL-associated serine protease 1
-
- MASP2
-
- MBL-associated serine protease 2
-
- MBL
-
- Mannan binding lectin
-
- MyD88
-
- myeloid differentiation factor 88
-
- NA
-
- not assessed
-
- PAMPs
-
- pathogen associated molecular patterns
-
- ROS
-
- reactive oxygen species
-
- SAP
-
- serum amyloid P
-
- SCR1
-
- soluble complement receptor 1
-
- siRNA
-
- small interfering RNA
-
- SNP
-
- single nucleotide polymorphism
-
- TLR
-
- Toll-like receptor
-
- TRIF
-
- TIR-domain-containing adaptor protein including IFN-β
Introduction
Long-term renal allograft survival after kidney transplantation remains unsatisfactory, which can mainly be attributed to the development of chronic allograft nephropathy (CAN). Noxious stimuli such as donor brain death (BD) and age, cold ischemia, organ preservation methods, HLA-matching, reperfusion and acute rejection are independent risk factors for the development of CAN (1–4). Different pathophysiological mechanisms are triggered by these noxious insults leading to loss of allograft function. The complement system (CS) and Toll-like receptors (TLRs), as part of the innate immune system, are two important effector mechanisms that play a crucial role in the pathogenesis of renal injury related to renal transplantation. The CS and TLRs, both critical for first-line host defense, can be promptly activated upon threat from outside, by invading microorganisms, or from within, by tissue ischemia-reperfusion (IR). The contribution of the CS and TLRs as a response on IR on tissue injury has been demonstrated in various animal models, however to date its role in clinical transplantation remains unclear. Recently, complement activation induced by BD in renal allograft donors has also been suggested to contribute to loss of allograft function after transplantation (5,6). These findings could have major implications for intervention strategies. Thus far, activation of TLRs during BD is unknown. However, by the emerging body of publications on crosstalk between the CS and TLRs, activation of the TLR system could be anticipated.
This review discusses the role and crosstalk between complement and TLRs in relation to pretransplant renal injury by donor BD and early posttransplant renal ischemia-reperfusion injury (IRI). The reader is referred to more detailed publications regarding complement and/or TLR activation in relation to adaptive immunity (7,8).
The Complement System
The CS consists of more than 30 proteins in the plasma and on the cell surface. Activation of complement is amongst others involved in the elimination of pyogenic bacteria, the interaction between innate and adaptive immunity, and the clearance of cellular debris and immune complexes. There are three known pathways of complement activation: The classical, lectin and alternative pathway (Figure 1).
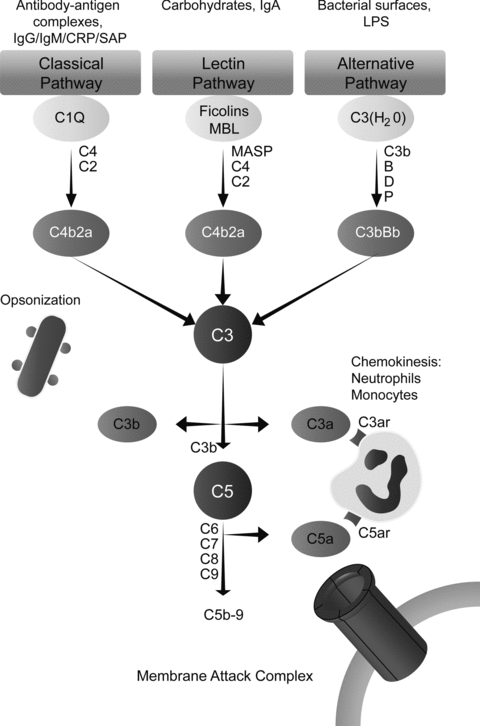
Schematic overview of the three known pathways of complement activation and its major ligands. There are three known pathways of complement activation: The classical, lectin and alternative pathway. Classical pathway activation occurs after binding of the C1 complex to antibody-antigen complexes, cell particles or certain acute phase proteins such as CRP or serum amyloid P (SAP). Once activated, the C1 complex can cleave respectively C4 and C2 leading to the formation of a C3 convertase and thereby C3 activation. The lectin pathway is activated when MBL and/or ficolins interact with carbohydrate ligands. Consequently, this activates MBL-associated serine proteases (MASPs), cleaving C4 and C2 and activating C3. The alternative pathway is activated by lowgrade spontaneous hydrolysis of systemic C3 (‘C3 tickover’). Hydrolyzed C3 [C3(H2O)] can associate with factor B, which is subsequently activated by factor D, finally cleaving C3. The cleavage product of C3, C3b, can bind to hydroxyl groups on cell-surface carbohydrates and proteins thereby enhancing alternative pathway activation. Alternative pathway activation can also be initiated as an amplification loop when bound C3b, generated by classical, alternative and lectin pathway activation, binds factor B. This C3bBb complex is stabilized by factor P or properdin. Activation of the three pathways results in the formation of a C3- and subsequently a C5 convertase eventually leading to the formation of the membrane attack complex (MAC). In addition to the capacity to generate the MAC, the split products of C3, C4 and C5 are known to have immunomodulating functions (9,10).
Toll-Like Receptors
TLRs are transmembrane receptors which can be activated by danger associated molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs). TLRs are present on a variety of cell types including epithelial cells, endothelial cells, leukocytes and dendritic cells. Once TLRs are activated, downstream signaling pathways are initiated leading to enhanced expression of cytokines and chemokines thereby bridging innate and adaptive immunity (Figure 2).
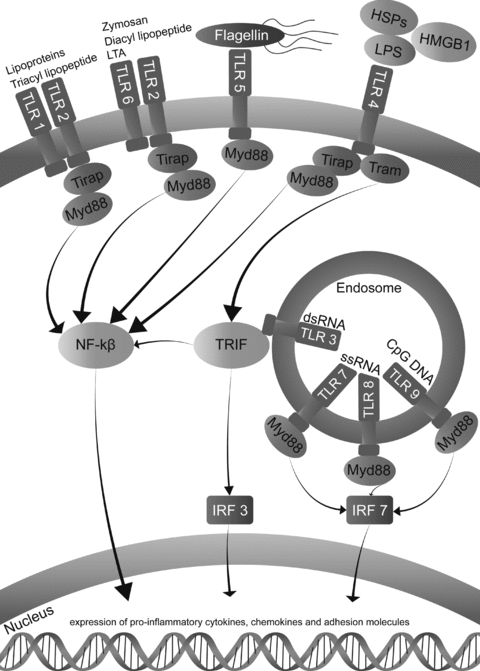
Toll-Like receptor signaling and its major ligands. Unique PAMPs are exogenous ligands present on groups of pathogens and include LPS, flagellin, lipoteichoic acid and double stranded RNA (viruses). DAMPs are intracellular proteins which are released outside the cell upon cellular injury, such as heat-shock proteins and HMGB1 (11,12). Different TLRs recognize specific PAMPs and DAMPs thereby orchestrating immune responses with a high specificity. Downstream activation of TLR signaling can couple five known adaptor molecules: MyD88, Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP), TIR-domain-containing adaptor protein including IFN-β (TRIF or TICAM-1), TRIF-related adaptor molecule (TRAM or TICAM2) and sterile α- and armadillo motif-containing protein (SARM; Ref. 13). MyD88 is used by all TLRs except for TLR3, which signals through TRIF. TLR2 and TLR4 are able to couple MyD88 through TIRAP subsequently activating NF-kβ leading to the expression of several proinflammatory cytokines, chemokines and adhesion molecules. Activation through TRIF by TLR3 also activates NF-kβ as well as IFN regulatory factor 3 (IRF3) (14). TLR 3, 7, 8 and 9 are specific for detection of viral or bacterial nucleic acid and are localized inside the endosome.
Complement and Renal IRI
Renal IR is an inevitable consequence of kidney transplantation. The immunological process is among others characterized by recruitment of leukocytes, upregulation of vascular proinflammatory molecules and formation of reactive oxygen species (ROS; Refs. 15–17). Complement activation has shown to be one of a number of factors to participate in renal IRI. During the last decade, several studies have focused on the role of complement activation in renal IRI in a variety of animal models and in different species as summarized in Table 1.
| CS or TLR | Study | Species | Knock-out or treatment | IRI |
|---|---|---|---|---|
| CS | Zhou et al. (12) | Mouse | C3–/–C5–/–C6–/– | ↓ |
| Zhou et al. (12) | Mouse | C4–/– | – | |
| De Vries et al. (13) | Mouse | C5a mAb | ↓ | |
| Arumugam et al. (14) | Rat | C5aR antagonist | ↓ | |
| De Vries et al. (15) | Mouse | C5aR antagonist | ↓ | |
| Castellano et al. (21) | Swine | C1-inhibitor | ↓ | |
| Moller-Kristensen et al. (23) | Mouse | MBL-A–/–MBL-C–/– | ↓ | |
| Thurman et al. (26) | Mouse | Factor B–/– | ↓ | |
| Thurman et al. (74) | Mouse | Factor B mAb | ↓ | |
| Zheng X et al. (72.73) | Mouse | siRNA against C3, C5aR | ↓ | |
| TLR | Leemans et al. (32) | Mouse | TLR2–/–TLR4–/– | ↓ |
| Leemans et al. (32) | Mouse | TLR2 antisense oligonucleotides | ↓ | |
| Shigeoka et al. (33) | Mouse | TLR2–/–MyD88–/–MyD88 × TRIF–/– | ↓ | |
| Shigeoka et al. (33) | Mouse | TRIF | – | |
| Wu et al. (34) | Mouse | TLR4–/–MyD88–/– | ↓ | |
| Pulskens et al. (35) | Mouse | TLR4–/– | ↓ | |
| Pulskens et al. (35) | Mouse | MyD88–/–TRIF–/– | – |
- Reduced renal IRI (↓), no effect on renal IRI (–).
A central role for the terminal complement cascade in renal IRI was demonstrated in C6–/– mice, which were protected from renal IRI. A less important role was ascribed to the release of C5a and subsequent recruitment of neutrophils into the kidney since treatment with C5a antibodies did not show additional protection against renal IRI in C6–/– mice (18). In contrast, de Vries et al. also found that inhibition of C5 with a monoclonal antibody protects against renal IRI with reduced neutrophil influx into the kidney (13). In line with these findings, treatment with a C5a receptor antagonist (C5aRA) showed a marked reduction in renal IRI (14). However, de Vries et al. found that the protective effects of a C5aRA occurs independent of neutrophil influx (15). Therefore, other functions of C5a rather than inflammation through chemokinesis have been suggested. Possibly, C5a has a direct effect on renal vascular endothelium or tubular epithelium thereby enhancing kidney immunogenicity. In vitro studies support this hypothesis since there is a direct proinflammatory effect of C5a on endothelial as well as tubular epithelial cells through C5a receptor (C5aR) signaling (16–18). Thus it can be concluded from these studies that both C5a as well as the terminal complement cascade are the primary mediators of complement dependent renal IRI in mice. On the other hand, Zhou et al. found no prominent role of C5a during renal IRI, which can possibly be explained by the fact that these investigators used a more severe IRI model in contrast to others (12). A short period of renal IRI has shown to be dependent on neutrophil influx while longer time periods of IRI seem to be associated with apoptosis (19,20). Therefore, differences in contribution of the membrane attack complement (MAC) in renal IRI could be ascribed to models of severe or moderate renal injury. Notably, the differences between studies in mice might also depend on the use of different strains, different models of renal IRI (duration, bi/unilateral clamping) and the use of knock-outs with unknown secondary effects.
Elucidation of the pathways of complement that are activated is crucial to obtain insight in the initiating factors during renal IRI. In mice, it was found that absence of C4 does not protect against renal IRI and therefore, classical pathway activation is unlikely to be involved in the course of renal IRI (12). On the other hand, in a swine model of renal IRI, Castellano et al. found evidence for activation of the classical, lectin and alternative pathways (21). Besides classical pathway activation, also lectin pathway activation was demonstrated in a mouse model of renal IRI and in patients suffering from posttransplant acute renal failure (22). Others found that mannan binding lectin (MBL)-A and MBL-C deficient mice are protected from renal IRI (23). Possibly, since C4–/– mice were not protected against renal IRI, MBL mediated injury may act through direct activation of C3 by MBL-associated serine protease 1 (MASP1) and not through C4. The important role of MBL is supported by studies in kidney transplant recipients, in which high serum MBL was found to be associated with inferior transplant survival (24,25).
Following classical pathway activation, the alternative pathway amplification loop might be triggered, or is activated by endogenous or exogenous ligands. In line with Castellano et al. who found activation of the alternative pathway in their swine model, also others have shown less renal IRI, a decline of tubulo-interstitial C3 deposition and neutrophil influx in factor B deficient mice (26). Moreover, increased alternative pathway activation was observed in kidney biopsies from patients with acute tubular necrosis (27).
Altogether, these studies emphasize that there is a clear difference between complement activation patterns among species. This questions whether the results in animals are also applicable to humans. There is a great demand for clinical studies analyzing the expression and activation of complement in human kidney transplantation. Ultimately, clinical trials have to be initiated targeting complement activation. The C1-esterase inhibitor, which is already widely used in humans to threat hereditary angioedema seems to be the obvious first choice (28). However, downstream C5aR blockade is more favorable, leaving most of the complement pathways intact because of its major role in host defense.
TLRs and Renal IRI
Similar to complement, also TLRs (TLR 1, 2, 3, 4 and 6) are primarily expressed by tubular epithelial cells and mesangial cells (29). Wolfs et al. were the first to demonstrate that TLR2 and TLR4 are constitutively expressed by the kidney in proximal and distal epithelial cells. Moreover, enhanced expression of TLR2 and TLR4 was found after renal IRI in renal epithelial cells of distal tubules, the thin limb of the loops of Henle and collecting ducts. Induction of both TLR2 and TLR4 was shown to be largely dependent on TNF-alpha and IFN-gamma (30). Comparable findings of TLR2 and TLR4 induction were found in a rat model of renal IRI (31). Interestingly, both studies found almost identical kinetics of TLR2 and TLR4 induction, 3–5 days after reperfusion. To investigate the role of TLRs in renal IRI in a more physiological manner, many groups have performed experiments using knock-outs as summarized in Table 1.
Investigating the role of TLR2 in renal IRI, Leemans et al. demonstrated that TLR2 deficient mice have less renal inflammation and injury after renal IRI. They clearly demonstrated that not TLR2 expression on leukocytes but renal TLR2 expression is the main mediator of TLR2 dependent renal IRI. Furthermore, treatment of mice with TLR2 antisense oligonucleotides resulted in protection against renal IRI (32).
Since several intracellular signaling pathways are linked to TLR signaling, Shigeoka et al. investigated the deficiency of TLR2, myeloid differentiation factor 88 (MyD88), TIR-domain-containing adaptor protein including IFN-β (TRIF) and MyD88 × TRIF in a mouse renal IRI model. They found that deficiency of respectively TLR2, MyD88 and MyD88 × TRIF protected against renal IRI (33). However, protection in TLR2–/– mice was more profound compared to MyD88–/– animals, suggesting TLR2/MyD88 independent pathways of TLR mediated renal injury. However, mice deficient in TRIF remained susceptible for renal IRI. Therefore, other TLR2 signaling pathways independent from MyD88 and TRIF signaling have been proposed.
Similar to TLR2, Wu et al. found that also TLR4 knock-outs are protected against renal IRI. This seems to be regulated through activation of MyD88 since MyD88 deficient mice were also protected against renal IRI. Moreover it was shown that not TLR4 expressed by leukocytes but intrinsic kidney cells are responsible for renal IRI. The same study shows that endogenous ligands for TLR such as high-mobility group protein 1 (HMGB1), hyaluronan and biglycan are upregulated after IRI, providing evidence that one of these ligands might be responsible for TLR4 activation (34). Unfortunately, this study did not examine the co-expression of these ligands with TLR4 activation. Pulskens et al. found similar results in the protection of IRI in a TLR4 knock-out (35). However, in contrast to Wu et al., no protection was found against renal IRI in MyD88 or TRIF deficient mice. This suggests the existence of another signaling pathway, besides MyD88 and TRIF-signaling, as suggested in TLR2 signaling. Another explanation could be the enhanced activation of parallel pathways that also make use of MyD88, thereby neutralizing the proinflammatory signal of TLR4. Besides, the use of a more severe IRI model by Pulskens et al. might explain the difference in findings. This may also clarify why there was no difference in protection of leukocyte or renal TLR deficient cells after renal IRI. Abundant infiltration of leukocytes into the kidney in the severe model of Pulskens et al. might thereby abolish TLR4 dependent leukocyte infiltration.
Future studies have to explore the unknown, MyD88 and TRIF independent pathways underlying TLR2 and TLR4 activation in renal IRI. Also, co-expression studies of TLR ligands with TLRs are needed since this could unravel the initiating factors contributing to TLR mediated damage. Finally, expression and activation of TLR2, TLR4 and possibly other TLRs have to be analyzed in human renal transplantation.
Crosstalk Between Complement and TLRs
Complement and TLRs are both activated by common ligands suggesting a potential crosstalk between the two systems. Both complement and TLRs can be activated in response to infection or by microbial structures such as lipopolysaccharide (LPS) and CpG DNA (36). The two systems might be able to synergize each other thereby shaping and validating the innate immune response against genuine danger signals. Today, however, to our knowledge no studies have been published which analyzed the interplay between complement and TLR activation in renal injury, let alone in renal IRI or transplantation. Therefore, our current knowledge on this interaction is based on studies performed either in models of experimental sepsis or in vitro cell culture systems.
In vivo, Zhang and colleagues found that decay accelerating factor (DAF) deficient mice, when treated with LPS (TLR4 agonist), showed increased levels of IL-6, TNF-alpha, IL-1beta, IL-10 and decreased levels of IL-12 compared to wild-type mice. The LPS-induced cytokine release was absent in dual knockouts for both DAF and C3, which indicates that this response was strongly dependent on complement. Besides, enhanced cytokine production was found in wild-type mice which were co-treated with LPS and cobra venom factor, a potent activator of complement. Dual knockouts for DAF and TLR4 were non-responsive to LPS stimulation, indicating that the LPS-induced cytokine production was also TLR4 dependent. Interestingly, also zymosan (TLR2/6 agonist) and CpG DNA (TLR9 agonist) enhanced systemic cytokine levels in DAF deficient mice. These results suggest that the crosstalk between TLR and complement occurs mainly through MyD88 since only TLR4 can act independently from MyD88 (Figure 2). Downstream, the synergistic effect of complement and TLR4 activation was found to be mainly dependent on C5aR and less on C3a receptor (C3aR) signaling. In whole spleen lysates, increased activation of NF-kβ and phosphorylation of the mitogen activated protein kinases (MAPKs) ERK1/2 and JNK was found in LPS stimulated DAF -/- mice (37).
Further evidence for TLR4 signaling through C5aR was found in a model of experimental sepsis. Absence or blockade of the C5aR significantly reduced serum levels of IL-6 during sepsis. Furthermore, in vitro, it was shown that the enhanced effects of C5a on LPS-induced IL-6 from neutrophils is dependent on the phosphorylation of p38 and ERK/12 MAPKs (38). Recently Wang et al. observed similar responses of C5a on the release of IL-8 in vivo during experimental sepsis and in vitro in human whole blood cells (39). Interestingly, C5a induced IL-8 generation was enhanced by several TLR agonists. TLR4 enhanced C5a induced IL-8 production was dependent on ERK1/2 and p38 but independent of JNK signaling. These studies clearly show that the signaling pathways underlying the enhanced effects of C5a on the LPS-induced inflammatory responses are dependent on the type of cell and the cytokines involved.
In line with the findings in vivo by Wang et al, Hawlisch et al. found that complement (C5a) negatively regulates TLR4 induced synthesis of IL-12 family members such as IL-12, IL-23 and IL-27 from inflammatory macrophages (40). Also decreased IL-12 production by macrophages after C5a treatment was found by others (41). Because the IL-12 family members are involved in the regulation of T-cell differentiation and development, the interplay between complement and TLR might play a role in triggering adaptive immune responses. For a more extensive review about the crosstalk between TLRs and complement and regulation of T-cell immunity we refer to a recent publication by Hajishengallis and Lambris (42).
Altogether, the above-mentioned reports suggest that MAPKs might be the key molecules linking both complement and TLR pathways together. Although no studies have focused on the interplay between complement and TLR activation in renal IRI, a recent publication shows that such an interaction is evident in intestinal IRI. Complement C3 and factor B were upregulated in the intestine after IRI in wild type mice but not in TLR4 knock-out mice. Furthermore, C5a in the serum and intestinal C3 deposition were markedly decreased in TLR4 deficient compared to wild-type mice after intestinal IRI. Treatment with CR2-Crry, which inhibits complement locally, significantly reduced TLR4 gene expression after intestinal IRI, suggesting that complement also regulates TLR4 expression (43). Whether such a crosstalk between complement and TLRs also occurs during renal IRI has to be investigated.
Complement Activation by the Donor
In their landmark paper, Pratt et al. clearly showed that local C3 expression by the donor kidney is detrimental for renal allograft survival. Absence of local C3 production in the donor kidney greatly improved kidney allograft survival after transplantation. Complement C3 deficient kidneys survived up to more than 100 days compared to wild type kidneys, which were rejected from 10 days on. In contrast, when kidneys from wild-type mice were transplanted into C3 -/- mice, renal allograft rejection could not be prevented (44). Also, absence of local C3 synthesis by the donor kidney, instead of circulating C3 by the recipient, was found to prevent complement-mediated reperfusion damage (45). These results indicate that not circulating C3 in the recipient but locally produced C3 by the donor kidney is an important mediator of early post-reperfusion and late rejection associated allograft injury. This can be explained by the large molecular size of C3 and the consequent poor penetration of C3 from the circulation through the basal membrane into the kidney. Besides the main production of C3 by the liver, the kidney itself is a prominent producer of complement. Endothelial cells, mesangial cells, glomerular epithelial cells and especially tubular epithelial cells are capable of producing complement components in vitro (46–52).
Although the study by Pratt and colleagues is interesting, it is not taken into account that in clinical practice, most kidneys are recovered from deceased donors. Still, the majority of the kidneys that are finally transplanted are recovered from brain-dead donors. It is known that these kidneys have an inferior transplant function and survival when compared to living donors (53). Recently, our group showed local renal upregulation of C3 in a rat model of BD before organ recovery prior to transplantation (5). Also, Kusaka et al. demonstrated the presence of complement deposition in kidneys of rat brain-dead donors (54). In humans, we also demonstrated a higher gene expression of C3 and increased deposition of C3d in kidney biopsies taken before organ recovery from brain-dead donors. Interestingly, no additional renal C3 expression was found after cold ischemia or reperfusion upon the induction of C3 by BD (5). In line with our findings, Naesens et al. showed induction of complement components in kidneys from deceased donors after cold ischemia. Furthermore, renal C3 expression in pre-implantation biopsies was associated with both early and late graft function in terms of superior glomerular filtration rates after transplantation (6). Our findings, however, discriminate between the significance of BD induced C3 rather than C3 induction due to cold ischemia. In future studies, BD induction should be performed in C3 knock-out animals to investigate the contribution of complement dependent renal injury during donor BD. Furthermore, C3 deficient brain-dead donor kidneys transplanted into wild-type recipients would indicate the significance of BD-induced renal C3 in the donor. In humans, a first approach would be the analysis of gene expression rates of complement components in a large set of deceased donor and reperfusion biopsies to assess the routes of complement activation and its association with clinical outcome.
Previous studies have focused on the influence of local complement activation by the donor kidney. However, it was shown in a mouse model of BD that also systemic complement becomes activated after BD in the donor (55). Moreover, in human deceased donors, a high C5b-9 level is associated with a higher rate of acute rejection in the recipient (56). Since activation of local and systemic complement occurs already before organ donation in brain-dead donors, strategies targeting complement activation should be considered shortly after the diagnosis of BD.
Besides local and systemic complement activation by donor BD, the important role of donor complement is also illustrated by functional polymorphism studies. After kidney transplantation, Brown et al. found an increased long-term graft survival when donors carrying the F allele were transplanted into SS recipients (57). However, a recent study, comprising a larger transplant cohort with a superior follow-up, demonstrated no difference in allograft survival when a F allele carrying donor kidney was transplanted into a SS carrying recipient (58). It is possible that the initial study by Brown and colleagues was a false positive result as can be encountered in genetic studies. These findings are supported by a recent publication in which no association was found between donor and recipient C4 allotype and renal allograft outcome (59). Therefore it can be concluded that chronic renal allograft deterioration is a multifactoral process which is not affected by functional polymorphisms in the C3 and C4 gene. Table 2 summarizes studies on the role of complement activation in deceased donors on renal allograft outcome in the recipient.
| CS or TLR | Study | Species | Model | Outcome |
|---|---|---|---|---|
| CS | Pratt et al. (44,45) | Mouse | Allograft transplant: C3–/– to C3 wild type | Improved GS Less ARF |
| Pratt et al. (44,45) | Mouse | Allograft transplant: C3 wild type to C3–/– | No difference in GS or ARF | |
| Naesens et al. (6) | Human | C3 induction in cold biopsies | Worse GF | |
| Damman et al. (5) | Rat | C3 induction in brain-dead donor kidneys | NA | |
| Damman et al. (5) | Human | C3 induction and activation in BD donor and cold biopsies | Worse GF | |
| Kusaka et al. (54) | Rat | Complement deposition in brain-dead donors | NA | |
| Brown et al. (57) | Human | F allele donors into S allele recipients | Improved GS | |
| Varagunam et al. (58) | Human | F allele donors into S allele recipients | No difference in GS | |
| Wahrmann et al. (59) | Human | C4 donor and recipient gene copy number | No difference in GS | |
| Atkinson et al. (55) | Mouse | Systemic complement activation after donor BD | NA | |
| Damman et al. (56) | Human | High deceased donor plasma C5b-9 | Increased risk of AR | |
| TLR | Palmer et al. (61) | Human | TLR4 299/399 donor genotype in deceased donors | Decreased risk to develop AR |
| Nogueira et al. (62) | Human | TLR4 299/399 donor genotype in deceased donors | No influence on the risk to develop AR | |
| Krüger et al. (66) | Human | TLR4 upregulation in pretransplant cold biopsies vs living. | NA | |
| Krüger et al. (66) | Human | TLR4 299/399 donor genotype in deceased donors | Higher incidence of direct GF |
- GS, graft survival; ARF, acute renal failure; NA, not assessed; GF, graft function; AR, acute rejection.
TLR Activation by the Donor
Today, the role of TLRs in human kidney transplantation is mainly assessed by analysis of functional single nucleotide polymorphisms (SNPs) in the donor and recipient. Two functional SNPs have been described in the TLR4 gene, affecting the extracellular domain of the receptor. The occurrence of both variants, Asp299Gly and Thr399Ile, leads to a blunted immunological response after inhalation of LPS (60). Although Palmer et al found no association between recipient TLR4 299/399 polymorphism and transplant outcome, an association between donor TLR4 genotype and outcome was demonstrated. It was found that TLR4 genotype of deceased donors protects the allograft from developing biopsy proven acute rejection after transplantation (61). However, the protective effect of this TLR4 mutant genotype could not be confirmed by Nogueira and colleagues (62). This is likely explained by the relative large proportion of living donors in their transplant cohort. One of the proposed mechanisms is the activation of TLRs in deceased donors by PAMPs and DAMPs that are released and locally upregulated upon brain damage and finally BD in the donor. Our group showed that BD leads to an increased intestinal permeability with subsequently elevated levels of circulating LPS (63). Furthermore, also other ligands of TLRs such as HSP70 are locally upregulated in the kidney after experimental and clinical donor BD (64,65). Recently, Krüger et al. found that TLR4 was significantly upregulated as a consequence of BD and/or cold ischemia in pretransplant cold biopsies compared to living donors. Besides, a stronger staining for the endogenous TLR ligand HMGB1 was found in kidney biopsies from deceased donors compared to biopsies from living donors (66). HMGB1 could therefore be a candidate protein to serve as an endogenous ligand, contributing to TLR4 signaling in kidneys from brain-dead donors. Besides, Krüger et al. found that kidneys of donors with a 299/399 TLR4 genotype have lower gene expression rates of TNF-alpha, MCP-1 and a higher expression of HO-1 compared to wildtype. Moreover, deceased donors with the 299/399 TLR4 genotype have a higher incidence of immediate graft function after transplantation. Thus, these studies indicate that kidney transplant recipient might benefit from receiving a kidney from a TLR4 299/399 donor. Confirmative studies are needed to validate the protective findings of the donor 299/399 TLR4 genotype in a second independent cohort of patients to better assure this association with transplant outcome. Also, renal upregulation of TLR2, TLR4 and co-expression with their ligands in deceased donor kidneys has to be confirmed in other donor cohorts. Table 2 summarizes studies on the role of TLR4 in deceased donors on renal allograft outcome in the recipient.
Intervention Strategies
Pharmacological intervention in complement and TLR pathway activation of both the donor and recipient might be a promising strategy to improve renal allograft outcome. Today, most studies have focused on the inhibition of complement activation to reduce renal injury, while targeting TLR activation is emerging.
Targeting complement
Soluble complement receptor 1 (SCR1) is a complement regulator protein and accelerates the decay of C3 and C5 convertases and serves as a cofactor for factor I mediated degradation of C3b and C4b, thereby inhibiting C3 and C5 convertase. In a rat allograft model, Pratt et al. demonstrated less vascular injury and leukocyte infiltration in allografts of recipient rats pretreated with recombinant humanized sCR1 (TP10) (67). Also, a modified form of complement receptor 1 (CR1) which was targeted to renal endothelium and epithelium reduced the amount of complement deposition, histological signs of renal injury and improved renal function after transplantation (68). Further, administration or targeting of other complement regulator proteins such as CD59, CD55 or CD46 might be a potential way to reduce renal injury during renal transplantation.
Since complement also plays a role in tolerance and clearance of pathogens, ideally one would inhibit complement activation at the level of C5 or C5aR, thereby leaving most of the complement pathway intact. Blocking the conversion of C5 consequently prevents the release of C5a and formation of the MAC. As described previously, blocking C5 activation or the C5aR reduces renal IRI (13–15). In addition, improved graft survival was observed of mice kidneys treated with a C5aR inhibitor during preservation (69). Others showed improved long term graft survival in mice when recipients were treated with C5aR inhibitor before or even after transplantation (70).
An alternative strategy to target complement-mediated injury is by the administration of small interfering RNA (siRNA) thereby silencing C3 or C5aR and consequently attenuating renal IRI (71–73). Furthermore, blockade of the alternative complement pathway improved renal function and decreased apoptosis after renal IRI (74).
Targeting TLRs
Currently, several drugs are being developed to inhibit TLR2 and/or TLR4 expression or signaling. Since LPS is a specific ligand for TLR4, particular attention has been made to TLR4 in sepsis, These drugs have successfully been used to treat sepsis in animal models or in patients with sepsis (reviewed in (75)). Only one study has performed a TLR4 inhibition study in renal IRI. It was shown that blockade of TLR4 by eritoran reduces renal IRI in terms of renal function and histology (76). Furthermore, in a mouse model of myocardial IRI, pretreatment with the same drug decreased infarction size and cytokine expression (77). Future experiments have to investigate whether inhibitors of TLR2 and TLR4 activation, currently being used to treat experimental sepsis, can also prevent renal IRI or transplant related injury. The ultimate goal would be the use of such agents in clinical renal transplantation.
Conclusion
Two pathways of the innate immune system namely complement and TLRs, play an important role in the pathogenesis of renal injury inherent to kidney transplantation. Recent studies have indicated an important crosstalk between both systems thereby resulting in immune responses with a high specificity. Both complement and TLR activation play an important role in the pretransplant renal injury induced by donor BD and early posttransplant injury as a result of renal IRI. Therefore, modulating complement and TLR activation in kidney transplantation could be a useful strategy to improve graft outcome. Pretreatment of brain-dead donors as well as the recipient before transplantation might be beneficial for renal allograft outcome after transplantation. Whether the combined use of complement and TLR blocking agents will be successful in humans remains to be investigated.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




