Long-Term Follow-Up in Composite Tissue Allotransplantation: In-Depth Study of Five (Hand and Face) Recipients
Abstract
Composite tissue allotransplantations (CTAs) have clinically shown little, if any, evidence of chronic rejection. Consequently, the effect of chronic rejection on bones, joints, nerves, muscles, tendons and vessels may still have undescribed implications. We thoroughly assessed all allograft structures by histology, magnetic resonance imaging, ultrasonography and high resolution peripheral quantitative computed tomography scan in four bilateral hand-grafted patients (10, 7, 3 and 2 years of follow-up, respectively) and in one facial allotransplantation (5 years of follow-up). All the recipients presented normal skin structure without dermal fibrosis. Vessels were patent, without thrombosis, stenosis or intimal hyperplasia. Tendons and nerves were also normal; muscles showed some changes, such as a variable degree of muscular hypotrophy, particularly of intrinsic muscles, accompanied by fatty degeneration that might be related to denervation. In the majority of hand-grafted patients graft radius and recipient tibia showed a decrease in trabecular density, although in the graft radius the alterations also involved the cortices. No deterioration of graft function was noted. In these cases of CTA no signs of chronic graft rejection have been detected. However, the possibility that chronic rejection may develop in CTA exists, highlighting the necessity of close continuous follow-up of the patients.
Abbreviations:
-
- CTA
-
- composite tissue allotransplantation
-
- CTAs
-
- composite tissue allotransplantations
-
- HR-pQCT
-
- high resolution peripheral quantitative computed tomography
-
- MRI
-
- magnetic resonance imaging
Introduction
Composite tissue allotransplants involve transplantation of various tissues including vessels, nerves, skin, bones, and immune cells and entail a significant antigenic load. Different immunosuppressive protocols are used for experimental and clinical composite tissue allotransplantation (CTA) that allow survival of the different components of composite tissue allografts.
Although chronic rejection is difficult to induce in rodent models of CTA (1,2), a recent study using a rat hind-limb allotransplantation model showed pathological changes secondary to multiple acute rejection episodes, involving skin, muscles, bones and finally vessels (3).
On the basis of clinical experience (4) CTAs have shown so far little evidence of chronic rejection and in the ‘Chronic Rejection’ section of the Banff 2007 working classification of skin-containing CTA pathology (5), it is stated that ‘currently, insufficient data are available to define specific changes of chronic rejection in a CTA’.
In solid organ transplantation chronic rejection is characterized by a gradual deterioration of graft function months to years after transplantation accompanied by typical pathological features (6,7). The remarkable pathological feature of the chronic rejection process is intimal hyperplasia accompanied by organ specific lesions. The development of this transplant vasculopathy, referred as transplant arteriosclerosis, is a multifactorial process but its precise pathogenetic mechanisms remain undefined (8).
In order to search for changes related to chronic rejection we have investigated all tissues composing CTAs including skin, bones, joints, nerves, muscles, tendons and vessels as well as graft function. This study was performed in four bilateral hand transplantations and one partial facial allotransplantation, which did not show any apparent clinical signs of chronic rejection.
Material and Methods
Patients
The study included four bilateral hand-grafted patients and one facial transplantation. The follow-up period was 10, 7, 3 and 2 years for the hand-grafted patients and 5 years for the face grafted one. Patient characteristics are summarized in Table 1. The immunosuppressive therapy included induction and maintenance treatment. All the bilateral hand-grafted patients received antithymocyte globulins as induction and a triple maintenance therapy based on prednisone (5 mg/day after the first year), tacrolimus (blood through level between 5 and 10 ng/mL) and mycophenolate mofetil (2 g/day).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Type of CTA | Bilateral hand Tx | Bilateral hand Tx | Bilateral hand Tx | Bilateral hand Tx | Face Tx |
| Sex | M | M | F | M | F |
| Recipient age (years) | 43 | 28 | 29 | 29 | 40 |
| Donor age (years) | 18 | 45 | 40 | 29 | 46 |
| Body weight (kg) | 60 | 49 | 61 | 54 | 50 |
| Height (cm) | 167 | 167 | 174 | 170 | 165 |
| Date of Tx | 13.01.2000 | 30.04.2003 | 19.02.2007 | 03.07.2008 | 27.11.2005 |
| Follow-up (years) | 10 | 7 | 3 | 2 | 5 |
| IMS regimen | TAC-MMF-S | TAC-MMF-S | TAC-MMF-S | TAC-MMF-S | SRL-MMF-S |
| N° acute rejection episodes | 2 | 2 | 4 | 1 | 2 |
| Banff score | 2, 2 | 2, 2 | 2, 2, 3, 2 | 2 | 2, 2 |
- IMS = immunosuppressive; TAC = tacrolimus; MMF = mycophenolate mofetil; S = steroids; SRL = sirolimus.
In the case of facial transplantation the immunosuppressive therapy included antithymocyte globulins, associated with tacrolimus, mycophenolate mofetil and prednisone. In addition, prior to surgery, bone marrow was collected from the donor's iliac crests. Half of the bone marrow cells were infused into the recipient on days 4 and 11 after face allotransplantation. Tacrolimus was replaced by sirolimus 11 months after transplantation and the patient's maintenance therapy consisted in sirolimus (targeted through level: 8–12 ng/mL), mycophenolate mofetil (1.5 g/day) and prednisone (5 mg/day).
During the follow-up period, all recipients experienced at least one episode of acute rejection (Table 1), which was easily reversed by increasing steroid oral dose and by topical immunosuppressants (clobetasol and tacrolimus ointments) in patients 1 and 2; in patient 3 the first two episodes were successfully treated with intravenous steroids, the third episode with antithymocyte globulins and the fourth episode by increasing the oral steroid dose; patient 4 presented only one rejection episode which was successfully treated with intravenous steroids.
Anti-HLA antibodies were monitored by LUMINEX each year after transplantation in all recipients. Only patient 2 developed transiently anti-HLA class II antibodies in November 2009, which were not detectable in the peripheral blood 1 year later.
Histology
Protocol 4 mm punch skin biopsies were obtained from the transplanted forearms/hands and oral mucosa and/or skin biopsies (chin and sentinel skin graft) from the facial transplantation at regular time points postgraft, including 1, 3, 6 and 12 months during the first year, thereafter every 6 months during the following 2 years, then once a year until the end of the follow-up period. Furthermore, skin and/or mucosal biopsies were obtained at various time points postgraft whenever rejection was suspected. The specimens for histology were formalin-fixed and paraffin-embedded; 5 μm thick sections were stained with hematoxylin-eosin and examined namely for the presence of pathological changes suggestive of rejection. Sections were also immunolabeled with antibodies detecting various antigens of normal or inflamed skin, including (among others) CD3, CD4, CD8, FoxP3, TIA-1, CD1a, CD207, Ki67, MART-1, factor XIIIa, CD34, S100 protein and neurofilaments. A muscle biopsy was performed at the last time point of the follow-up in all hand-grafted patients to search for signs of rejection.
HR-pQCT system
All the recipients of bilateral hand transplantation underwent high-resolution peripheral quantitative computed tomography scan (HR-pQCT, XtremeCT, Scanco Medical AG, Bruttisellen, Switzerland). Trabecular architecture and volumetric bone mineral density were assessed at the distal radius (donor bone) and tibia (control recipient bone) with a nominal isotropic voxel size of 82 μm. At each skeletal size, 110 CT slices were obtained, thus delivering a three-dimensional representation of 9 mm (9).
Ultrasonography
Hand grafts were studied by ultrasonography (Siemens Elegra, Erlangen, Germany). Duplex ultrasound assessment was performed every year during the follow-up in the bilateral hand-grafted patients to investigate patency of the vessels and thickness of arterial walls.
Ultrasonographic measurements of the median and cubital nerve cross-sectional area were performed at several points of their course.
Ultrasonography was also used in the facial transplantation to study soft tissues, particularly adipose and connective tissues, from both recipient and graft.
MRI
All recipients underwent magnetic resonance imaging (MRI, Magnetom Symphonie, Erlangen, Germany) to detect neural and other soft tissue changes, bony details, luminal stenosis and/or morphological features of recipient and graft vessels.
Functional results
Hand transplantation recipients were evaluated each post-transplant year using the International Registry Score System, which allows evaluating both objective and subjective parameters (4) and DASH score (10).
In the face transplant recipient motor recovery was evaluated using motion images, which were periodically captured by a video camera, and recording the phonetic exercises. Thermal (cold and hot) and Semmes–Weinstein tests were performed to evaluate sensibility recovery
Results
Macroscopic aspect of the grafts
As shown in Table 1, during the follow-up period, all recipients experienced at least one episode of acute rejection, manifesting clinically with erythematous macules over the graft skin. Apart from episodes of graft rejection, during the follow-up the skin looked normal, as assessed by temperature, color, texture and hair and nail growth during the follow-up.
Histological aspect of the grafts
Acute rejection episodes were characterized mainly by the presence of a dermal perivascular lymphoid infiltrate made predominantly of CD3+/CD4+ T cells occasionally reaching and penetrating the epidermis. Some FoxP3+Treg cells and TIA-1+ cytotoxic cells were found within the perivascular dermal infiltrate (Fig. 1)
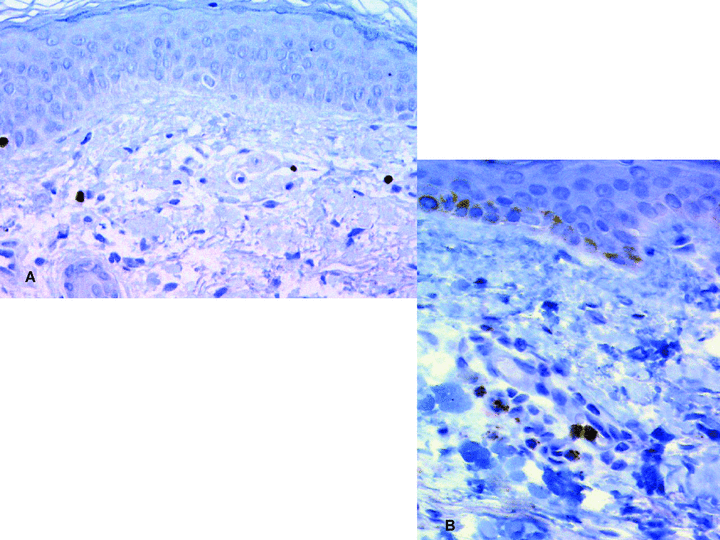
Immunoperoxidase revealed with diaminobenzidin (original magnification ×250). A: FoxP3+ T-reg cells in a human hand allograft 5 years postgraft. B: TIA-1+ cytotoxic T cells in the sentinel skin graft of the face-transplant recipient 3 years postgraft.
Apart from episodes of acute rejection during the protocol follow-up, skin biopsies did not show significant changes, or showed only minimal ones. Figure 2 shows the pathological aspects of skin biopsies of each bilateral hand-grafted patient taken at two time points of the follow-up. Immunohistochemically, the skin also showed a normal structure, and contained all its normal cell components, including keratin+ keratinocytes, CD1a/CD207 epidermal Langerhans cells, MART-1+ melanocytes, keratin 20+ Merkel cells, factor XIIIa+ dermal dendrocytes, CD34+ blood endothelial cells, S100+ Schwann cells and neurofilament+ axons, suggesting that the skin was both immuno- and melano-competent. Several basal keratinocytes of the epidermis and its appendages (hair follicles and sweat glands) expressed the proliferation antigen Ki67. Deep biopsies showed a sparse interstitial lymphocytic infiltrate in patients 1, 2 and 4, while in patient 3 a mild dermal perivascular lymphocytic infiltrate coming occasionally in contact with the epidermis and a mild interstitial lymphocytic infiltrate between muscle fibers were found.
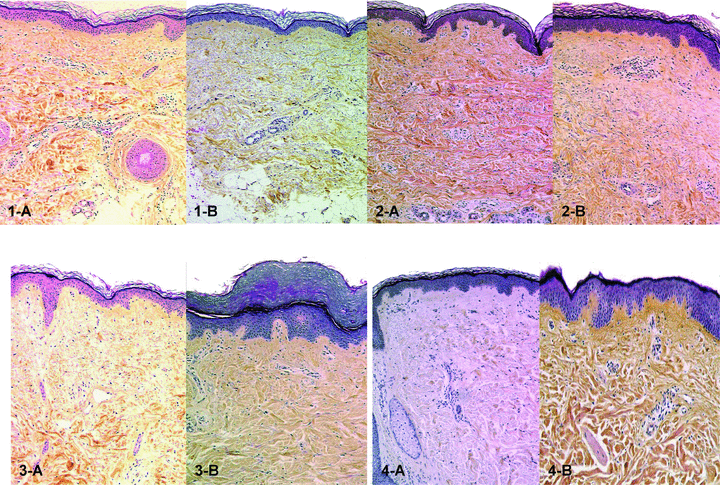
Histology of skin biopsies in bilateral hand-grafted patients (original magnification: ×100). 1-A and 1-B: patient 1 at 5 and 10 years after transplantation respectively. Normal appearance of epidermis and dermis. A very mild perivascular lymphocytic infiltrate is seen in 1-A; 2-A and 2-B: patient 2 at 3 and 7 years after transplantation, respectively. Normal-looking epidermis and dermis with sparse perivascular lymphocytic infiltrate in 2-B; 3-A and 3-B: patient 3 at 1 and 3 years after transplantation, respectively. Epidermis is slightly thickened in 3-B. Normal looking dermis with mild perivascular lymphocytic infiltrate in both 3-A and 3-B; 4-A and 4-B: patient 4 at 1 and 2 years after transplantation. Normal-appearing skin with sparse perivascular lymphocytic infiltrate in both figures.
The sentinel skin flap of the partial face transplantation looked histologically normal (Figure 3).
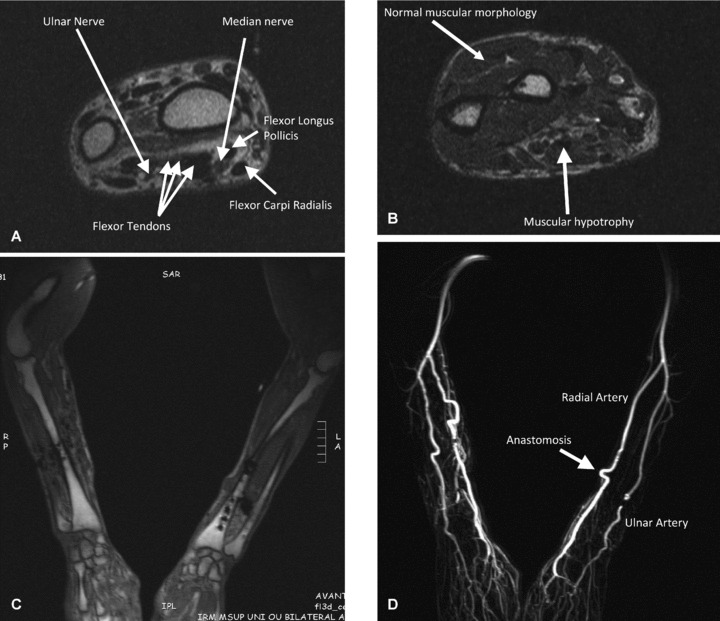
MRI images of patient 2. A–C: At wrist level tendons and nerves are normal; muscle analysis shows atrophy of some muscles and fatty degeneration. Bones are normal without any difference between donor and recipient bone signal. D: MRI with gadolinium injection. In this case there is no void caused by the metallic material and the arteries are well visualized without any abnormality.
HR-pQCT system
The presence of metallic plates and screws caused some artifacts in the median part of forearms, particularly in patients 1 and 4.
As reported in Figure 4, all male recipients showed alterations at level of distal tibia and distal radius: a slight decrease in trabecular density and microarchitecture at the distal tibia level, and in patient 2 also at cortices level; these alterations were more pronounced at the distal radius level. Patient 3, a woman, showed normal bone structure at level of distal tibia and radius.
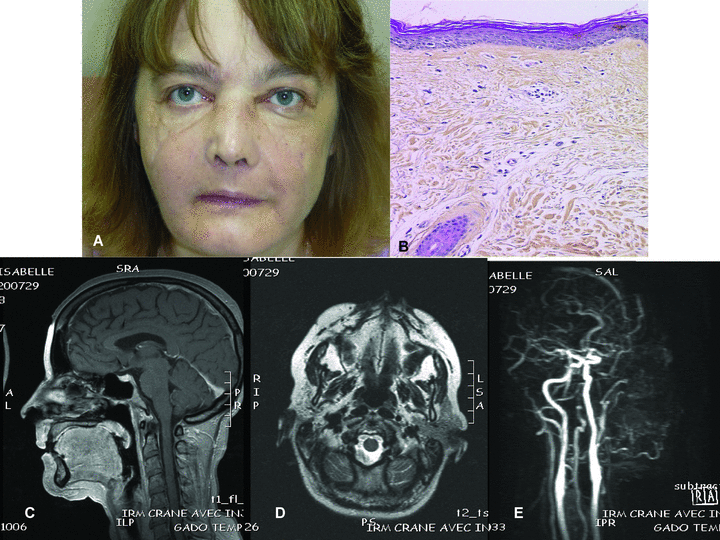
Partial face transplantation. A: Macroscopic aspect of the graft: no signs of rejection. B: Histology of the sentinel skin flap (original magnification: ×100): the epidermis is normal. The dermis contains a sparse lymphocytic perivascular infiltration. C: Grafted tissues look normal except for slight oedema of orbicularis muscle in the MRI image. No signs of fibrosis. D: MRI images do not show any difference between graft and recipient tissues. No signs of graft fibrosis. E: MRI with gadolinium injection. The recipient carotid arteries are patent as well as the facial artery, which is tortuous.
Ultrasonography
Duplex ultrasounds did not show any alteration of flow and vessel wall thickness during the follow-up in the four hand-grafted patients.
Size and morphology of nerves were normal except for swelling at the anastomosis level in all hand-grafted patients.
In the facial transplant no significant differences between recipient and graft soft tissues were detected.
MRI
In the bilateral hand transplantations the presence of metallic plates and screws caused some artifacts in the median part of forearms. The results achieved in these patients are summarized in Table 2. They showed normal structure of bones, nerves and tendons. The muscles (particularly the intrinsic ones) showed a slight hypotrophy and some degree of fatty degeneration (Figure 5).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Bones | Normal | Normal | Normal | Normal |
| Tendons | Normal | Normal | Normal | Normal |
| Muscles | Discrete hypotrophy of intrinsic muscles with fatty degeneration | Trophysm of muscles on left side. Signs of fatty degeneration on right side | Discrete trophysm but with signs of fatty degeneration | Hypotrophy of intrinsic muscles with fatty degeneration |
| Nerves | Normal | Normal | Normal | Normal |
| Vessels | Tortuous aspect of ulnary arteries | Normal | Normal | Normal (? Many artifacts) |
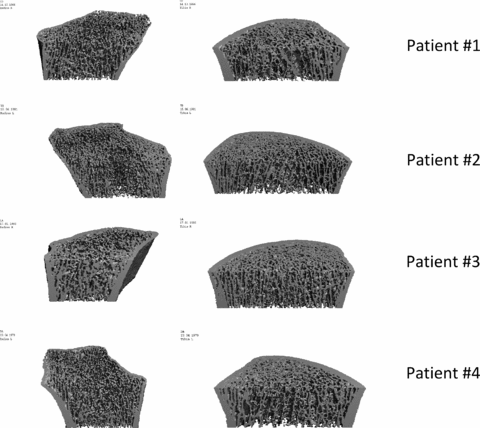
HR-pQCT images of distal radius (left) and tibia (right). Tibia of patients 1, 2 and 4: early impairment of trabecular compartment; in patient 2 there is also involvement of cortical thickness. Radius of patients 1, 2 and 4: thin cortices and degradation of the trabecular microarchitecture. Patient 3: normal bone structure at radius and tibia levels.
The vessels were not well appreciable in patients 1 and 4 because of the metallic material; patient 1 showed a slight tortuosity of the ulnar arteries.
In the facial transplant all structures were normal without significant difference between recipient and graft tissues (Figure 3); the vessels looked normal.
Functional results
In all bilateral hand transplanted patients an increase in both systems of evaluation occurred in the first 5 years after transplantation at least, without any decrease in the sensorimotor recovery in the longer follow-up period.
In the case of face transplantation during the follow-up period the patient recovered normal opening and complete closure of the mouth, and disappearance of inferior lip hypotonus.
Discussion
In the present study the histological and clinical features of chronic graft rejection have been investigated in two types of CTAs, four bilateral hand allografts and a partial facial transplantation, for a period ranging from 2 to 10 years.
The term CTA indicates simultaneous transplantation of skin, muscles, nerves, tendons, bones, vessels and other tissues. These multiple tissues are of ectodermal and mesodermal origin and represent a different antigenic load. The skin has been considered the main target of rejection process and for this reason it was strictly studied during the whole period of follow-up; moreover skin can be easily observed macroscopically and biopsied, thus allowing early detection and consequent prompt treatment of the acute rejection episodes. Apart from them, all patients showed a normal skin structure at level of epidermis, dermis and hypodermis. The main skin cell types (keratinocytes, melanocytes, Langerhans cells, Merkel cells, endothelial cells, dermal dendrocytes, Schwann cells, axons, fat cells) were detectable by routine staining and/or immunohistochemistry. The presence of cycling (Ki67+) basal cells in the epidermis and its appendages (hair follicles and sweat glands) shows that these structures are viable and have the potential of regeneration. The presence of melanocytes and dendritic cells (Langerhans cells, dermal dendrocytes) shows that the skin is able to protect itself from UV light and antigenic challenges, respectively. The presence of normal vasculature and innervation accounts for the normal trophicity of the skin (noted clinically) and its ability to perceive sensitive stimuli. In some patients, skin biopsies occasionally disclosed a mild dermal perivascular lymphocytic infiltrate; however, neither significant vascular changes nor dermal fibrosis were observed. Muscle biopsies disclosed a sparse perivascular lymphocytic infiltrate.
On the basis of these results we also studied all the other tissues of the CTAs at the last time point of the follow-up.
In the bilateral hand-grafted patients MRI and HR-pQCT system were used to study some tissues of CTAs although in hand transplantation these exams showed some limits due to the artifacts caused by the presence of metallic materials.
MRI allowed a qualitative study of the bones and did not show any difference between recipient and graft bones, while the HR-pQCT, which allowed a quantitative study of the bones, showed in male patients a decrease in trabecular density in the recipient's tibia as well as in graft bones, even though in graft radius the alterations involved also the cortices. The reported alterations of bone structure seem to be correlated with several factors, such as nutrition and recipient BMI, immunosuppressive treatment (steroid dose), recipient and donor age.
MRI and ultrasonography were used to study nerves and tendons and did not show any specific alteration in any hand-grafted patient. In addition, electromyography was performed each year after the transplantation showing a reinnervation process at the level of median and ulnar nerves, which improved significantly during the first years after transplantation (11).
Muscles were the only tissue that showed some modifications. Indeed, MRI evidenced a variable degree of muscular hypotrophy, particularly of intrinsic muscles, accompanied by fatty degeneration. These alterations were not considered as signs of chronic rejection as it is known that after denervation rapid degeneration of muscles occurs (12); biochemical (enzyme activities) and morphological changes in muscle fibers are permanent after the first months (13). Changes in muscle activation pattern also lead to muscular atrophy. In addition, ischemia/reperfusion injury could contribute to muscle hypotrophy and fatty degeneration since oxidative damage can induce muscle cell death and degeneration.
The major histomorphological feature of chronic rejection in solid organ transplantation is endoarteritis. During the last meeting of the International Society of Hand and Composite Tissue Allotransplantation (Valencia, Spain, September 2009) an acute arterial thrombosis in a unilateral hand-grafted recipient was reported to occur 275 days after transplantation but its cause was not clearly established: was it a case of chronic graft rejection? At present it is not possible to affirm that acute ischemia of the grafted upper extremity without any other previous signs is a clinical manifestation of chronic graft rejection; however, this case prompted us to investigate particularly the vessels of CTAs. Although a recent experimental study (3) showed that graft vasculopathy is the last lesion to occur during the chronic rejection process, in this study macro- and micro-circulation were investigated by Duplex ultrasounds, MRI and histology. Moreover, all patients underwent nailfold capillaroscopy, which did not detect any changes (data not shown), and at the last time point of the follow-up an angiography in patient 1 at 1 and 10 years after transplantation (data not shown). These exams showed that the main vessels were patent without any stenosis or significant alterations of arterial silhouette, except for some tortuosity of ulnar arteries in patient 1. Histological examination of deep biopsies did not show any arteriolar thrombosis. Despite these reassuring results in the future an angiography will be performed in all recipients at 1 year and each 5 years after transplantation. In addition, on the basis of the results achieved using intravascular ultrasounds in heart transplantation, this interventional procedure might be used to study intimal thickening in CTAs.
Patient 2 developed transiently anti-HLA class II antibodies without showing any vascular alteration and his muscular degeneration was inferior compared with the other hand-grafted patients. We did not detect C4d deposition in CTA specimens (14) including the biopsies of this patient. This is consistent with the fact that during acute rejection, the cellular infiltrate consists mainly of CD4 and CD8 lymphocytes, with only sparse B lymphocytes, and that the rejection process is mediated more by the cellular than the humoral arm of the immune response. Whether clinically significant humoral rejection occurs in CTAs is still unknown.
In these four bilateral hand transplantations neither fibrosis, intimal hyperplasia nor alterations of other tissues, which could be considered as signs of chronic rejection, were detected. In addition, no deterioration of graft function but instead an increase of their ability, at least during the first 5 years after transplantation, was noted.
In the face transplantation no differences between recipient and graft tissues were found upon MRI and ultrasonographic examination. The patient experienced continuous sensorimotor recovery during the follow-up period (15).
Up to now the authors did not detect any sign that could suggest a process of chronic graft rejection by any of the reported procedures. Thus, despite a high incidence of acute rejection, the occurrence of chronic rejection in CTA might be much lower than that in solid organ transplantation, suggesting that mechanisms of chronic rejection might differ from that of solid organ transplantation. Some features of the CTA graft might explain this discrepancy: (i) the graft contains hematopoietic stem cells in the transplanted bones that might be involved in the acceptance of the graft, as reported in rodent models of vascularized bone marrow transplantation, where mixed chimerism is associated with tolerance to CTA (16). However, we did not detect any chimerism in the peripheral blood of our hand transplanted patients; (ii) CD4+CD25+ T-regulatory cells were found in the skin graft of the first bilateral transplant patient (1) 6 years after the graft (17). However, this high percentage of T-regulatory cells was only transient in this patient and was not found in the other patients; (iii) the absence of chronic rejection of the skin might also be related to the ability of the skin graft to heal without fibrosis after the prompt diagnosis and treatment of acute rejection; (iv) the absence of circulating anti-HLA antibodies might explain the lack of evidence of transplant vasculopathy; (v) the persistence of donor derived immunocompetent cells (Langerhans cells) in the grafted skin could also favor graft nonrejection (18).
The use of a triple immunosuppressive therapy in these patients and their adherence to the treatment may have also contributed to these findings. The low incidence of chronic rejection in our CTA cohort prompted us to maintain our patients on triple immunosuppression and not to wean it off in the absence of complications.
In conclusion, a close continuous follow-up and an appropriate study of all tissues composing the CTAs (above all the skin that proved to be the main target of the rejection process) are of paramount importance.
Acknowledgments
This study was supported by Centaure Foundation. The Authors wish to thank Viet Vo Hoang, MD, and Pascale Bureau du Colombier, MD, for their help. A particular thanks to Barbara Trudu for her careful English proofreading and Mrs Celine Dagot for her precious assistance.
Disclosure
The manuscript was not prepared in any part and was not funded in any part by a commercial organization, including educational grant. All the authors of the manuscript AJT-0-10-01004 have no conflicts of interest to disclose as described by the American Journal of Transplantation.




