The Importance of Portal Venous Blood Flow in Ischemic-Type Biliary Lesions after Liver Transplantation
Abstract
Ischemic-type biliary lesions (ITBL) are the most frequent cause of nonanastomotic biliary strictures after liver transplantation. This complication develops in up to 25% of patients, with a 50% retransplantation rate in affected patients. Traditionally, ischemia-reperfusion injury to the biliary system is considered to be the major risk factor for ITBL. Several other risk factors for ITBL have been identified, including the use of liver grafts donated after cardiac death, prolonged cold and warm ischemic times and use of University of Wisconsin preservation solution. In recent years however, impaired microcirculation of the peribiliary plexus (PBP) has been implicated as a possible risk factor. It is widely accepted that the PBP is exclusively provided by blood from the hepatic artery, and therefore, the role of the portal venous blood supply has not been considered as a possible cause for the development of ITBL. In this short report, we present three patients with segmental portal vein thrombosis and subsequent development of ITBL in the affected segments in the presence of normal arterial blood flow. This suggests that portal blood flow may have an important contribution to the biliary microcirculation and that a compromised portal venous blood supply can predispose to the development of ITBL.
Abbreviations:
-
- ITBL
-
- ischemic-type biliary lesions
-
- PBP
-
- peribiliary vascular plexus
-
- DCD
-
- donation after cardiac death
-
- UW
-
- university of wisconsin
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- ERCP
-
- endoscopic retrograde cholangiopancreatography
-
- PSC
-
- primary sclerosing cholangitis
-
- HTK
-
- histidine–tryptophan–ketoglutarate
-
- AIH
-
- auto-immune hepatitis
Introduction
Biliary complications are a significant cause of morbidity and even mortality in patients after liver transplantation (1). Early biliary complications, either leakage or stenosis at the anastomotic site, are usually caused by surgical failure and occur within the first week after liver transplantation. Nonanastomotic strictures can occur in the context of hepatic artery thrombosis (2,3), or with an open hepatic artery. Nonanastomotic strictures occurring with and without a patent hepatic artery share many radiological similarities and thus the latter have been called ischemic-type biliary lesions (ITBL). They are characterized by bile duct destruction, subsequent stricture formation and sequestration. Several studies have identified factors influencing the incidence of ITBL, including cold ischemia time (4), ABO incompatibility (5), the use of different preservation solutions (6), the use of grafts donated after cardiac death (DCD) (7), bile salt toxicity (1) and arterial back-table pressure perfusion (8). In recent years, interest in impaired biliary microcirculation as a possible cause of ITBL has increased (9,10). Previous studies have concluded that the peribiliary plexus (PBP), which is responsible for the blood supply to the bile ducts, is exclusively provided by blood from the hepatic artery (11,12). Therefore, the role of the portal venous blood supply has not been considered as a possible cause for the development of ITBL. In this study we describe three patients with partial portal vein thrombosis and intact arterial blood supply who develop isolated ITBL in the segments affected by portal vein thrombosis. The findings in these patients strongly suggest that portal blood flow has an important contribution to the biliary microcirculation and that a compromised portal venous blood supply can predispose to the development of ITBL.
Case 1
A 57-year-old male, diagnosed with nonalcoholic steatohepatitis, was transplanted for cirrhosis and three intrahepatic hepatocellular carcinomas smaller than 2 cm in diameter. A blood group compatible liver from a 51-year-old heart-beating donor was transplanted. Cold storage was in the University of Wisconsin (UW) preservation solution (ViaSpan, Bristol-Myers Squibb, Belgium). The surgical procedure was uncomplicated. The liver was reperfused after the side-to-side cavocavostomy and portal vein anastomosis resulting in a cold ischemia time of 510 min and a warm ischemia time of 23 min. The arterial anastomosis was performed after portal reperfusion in 19 min. An end-to-end biliary anastomosis, without the use of a T-tube, was performed. The total blood loss was estimated at 2300 mL but no packed red blood cells were administered. Intra- and direct postoperative Doppler color ultrasonography showed intact arterial, hepatic and portal venous blood flow. On the first postoperative day however, routine Doppler ultrasound examination showed a turbulent flow in the right portal vein branch, without abnormalities in arterial flow. Liver function was improving quickly, measured by decreasing (from 400 IU/L) transaminases, recovering coagulation, and decreasing serum bilirubine and γ-GT. Therefore surgical intervention was considered unnecessary. Ultrasound imaging in the following days showed persistent turbulent flow in the right portal vein branch but normal hepatic artery flow. Postoperative recovery was complicated by a wound infection, which was treated by draining the wound. Immunosuppressive therapy consisted of tacrolimus (Prograft, Astellas Pharma, The Netherlands) and a low-dose prednisone withdrawal scheme. The patient was discharged from the hospital on the 24th postoperative day. However, over a course of several months he suffered from recurrent cholangitis with bilirubine levels up to 150 μmol/L (normal < 16 μmol/L). On subsequent magnetic resonance cholangiopancreatography (MRCP) studies and endoscopic retrograde cholangiopancreatographic (ERCP) interventions, multiple biliary strictures in the right anterior and posterior biliary tree were seen, while bile ducts in the left liver half remained unaffected (Figure 1). Portal venous flow was uncompromised in the main left and right portal branches, but showed tapering at the right anterior and posterior portal bifurcation and diminished portal flow to the complete right hemiliver. Several procedures of biliary dilatation and stenting were only successful for short periods of time. Additional treatment with ursodeoxycholic acid did not resolve symptoms. Eventually, 15 months after the initial transplantation, a right hemihepatectomy was performed. Histopathological examination of the explanted right hemiliver showed an intact arterial circulation, but extensive circulatory impairment and thrombosis of the portal system (Figure 2A). It furthermore showed typical microscopic characteristics of ITBL, including extensive damage and irregularity of the biliary epithelium accompanied by concentric fibrosis, ductular reaction and complete ductopenia in several portal tracts (Figure 2B). The patient recovered uneventfully from surgery and is currently asymptomatic with normal bilirubine levels and otherwise intact liver function.
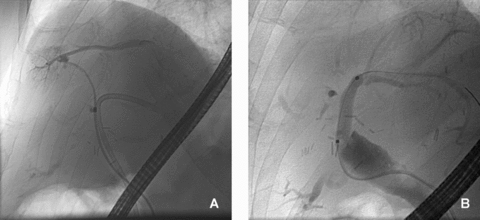
ERCP imaging showing (A) abnormal structured bile ducts on the right side of the liver in patient 1 with isolated right-sided portal vein thrombosis and (B) normal bile ducts in the same patient in the left hemiliver.
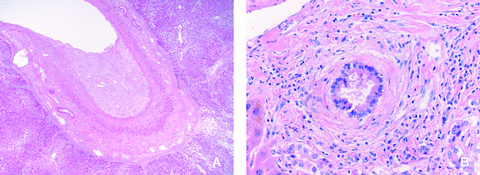
Hematoxyline and eosin-stained biopsy of the resected right hemiliver of patient 1 showing (A) extensive thrombosis of a large portal vein branch in the liver and (B) irregular biliary epithelium with concentric fibrosis and separation from the outer wall as typically seen in ITBL.
Case 2
A 53-year-old female diagnosed with primary sclerosing cholangitis (PSC) was transplanted for severe cholestasis and recurrent cholangitis despite years of endoscopic stenting and dilatations. For her, a 66-year-old, blood group compatible, liver from a heart-beating donor became available. Cold storage was in UW preservation solution. The surgical procedure was uncomplicated, with cold and warm ischemia times of 541 and 28 min respectively. Arterial anastomosis was performed in 25 min and bile flow was restored with a Roux-en-Y hepaticojejunostomy. Total blood loss was estimated at 2000 mL and 3 units of packed red blood cells were administered. Arterial, hepatic and portal venous blood flow was intact intra- and postoperatively measured by Doppler color ultrasonography. Routine Doppler ultrasound the following day showed normal perfusion patterns. On the third postoperative day however, liver transaminases were highly elevated (AST 3682 IU/L and ALT 1880 IU/L) and subsequent ultrasonography showed the absence of portal flow in the left portal vein branch while a normal triphasic arterial flow was present in all parts of the liver. Contrast enhanced three-phase CT confirmed these findings. A relaparotomy was performed due to fever and abdominal pain and the suspicion of bile leakage, during which the perfusion defect of the left liver segments was confirmed, showing a perfusion defect of the left liver segments with intact right portal flow and normal flow in the left and right hepatic arteries. Concomitantly, a small bile leak, which was diagnosed on the anterior side of the hepaticojejunostomy, was sutured. The patient was put on intravenous heparin and could be discharged 30 days after transplantation after an otherwise uneventful postoperative course. Immunosuppressive therapy consisted of cyclosporine (Neoral, Novartis Pharma, The Netherlands) and a low-dose withdrawal scheme of corticosteroids. Within 2 months the patient's condition deteriorated due to recurrent periods of cholangitis with bilirubine levels constantly exceeding 100 μmol/L. MR imaging showed atrophy and diffuse biliary strictures limited to the left hemiliver (Figure 3). However the patient was not considered eligible for a left hemihepatectomy because of uncertainty of extension of ITBL into the right liver lobe. Finally she was retransplanted 3 months after the initial transplantation and symptoms alleviated after this second transplantation. Examination of the first graft showed extensive damage and irregularity of the biliary epithelium with concentric fibrosis and complete loss of bile ducts in several portal tracts, characteristic for ITBL, confined to the left lobe (Figure 4). In this part of the liver the portal vein was obstructed while the artery was patent as in the previous patient.
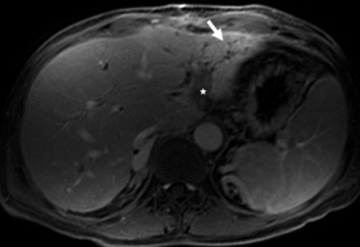
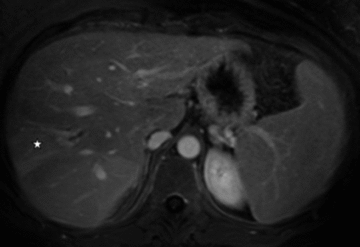
T1-weighted postcontrast MR image showing atrophy of the left liver lobe with focal irregular dilatation of bile ducts and local arterial compensatory hyperperfusion (arrow) in patient 2. Thrombosis of the left portal vein can be appreciated (star).
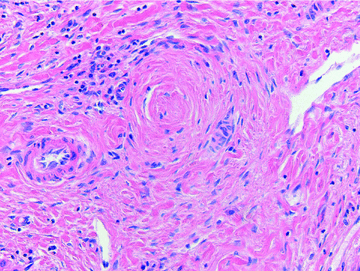
Hematoxyline and eosin-stained biopsy from the affected part of the explanted liver showing complete replacement of biliary epithelium by concentric fibrosis in a portal tract typical for severe ITBL pathology in patient 2.
Case 3
The third patient, a 44-year-old female, was transplanted with a liver from a blood group compatible heart-beating donor, for primary biliary cirrhosis. Cold storage of the liver graft was in UW preservation solution. Reperfusion was performed after the side-to-side cavocavostomy and portal vein anastomosis. Cold and warm ischemia times measured 506 and 27 min respectively, and the hepatic artery anastomosis was performed in 25 min. The bile duct was anastomosed end-to-end and due to blood loss 4 units of packed red cells were administered. Intra- and postoperative ultrasonography showed normal hepatic and portal flow patterns. Postoperative course was complicated by the formation of a biloma, which needed drainage. The patient was discharged in good health 15 days postoperatively with a mycophenolate mofetil (CellCept, Roche Pharma, The Netherlands) and low-dose tacrolimus immunosuppressive regime. During a routine Doppler ultrasound examination 6 months after transplantation however, a partial portal vein thrombosis was found at the bifurcation in the right portal vein and portal flow to segments 5 and 8 was partially compromised. Portal flow to segments 6 and 7 remained uncompromised and arterial flow was normal in the entire liver. The patient at that moment presented with slightly elevated transaminase levels (up to 150 IU/L) and was treated with phenprocoumon (Marcoumar, Roche Pharma, The Netherlands). However,3 weeks later a complete thrombosis of the right anterior portal branch was diagnosed and anticoagulant therapy was discontinued. Currently the patient is in good clinical condition, however liver function tests show persistent elevated levels of bilirubine (30 μmol/L), alkaline phosphatase (> 200 IU/L) and γ-GT (> 400 IU/L), while diffuse segmental biliary dilatation can be noted on imaging studies (Figure 5).
Thrombosis of the segmental portal vein of 5/8 (not shown) with segmental dilatation of the bile ducts and local compensatory arterial hyper perfusion (star) in patient 3.
Discussion
ITBL are a serious complication that generally occurs at 1–12 months after liver transplantation and can affect up to 25% of patients (1,8,9,13,14). Patients developing ITBL have a significantly worse graft and overall survival and ITBL has become a leading cause of liver retransplantation (8,13). Reported risk factors for the development of ITBL include prolonged ischemia times, bile toxicity, inadequate flushing of the graft and PBP, blood group incompatibility, DCD grafts and PSC in the recipient (1,4–8,13,15). The real pathogenesis of ITBL however, remains to be elucidated.
Recently, a retrospective study in over 1750 transplants identified six risk factors for ITBL, but in the multivariate analysis only cold ischemia time and organ recovery without high pressure hepatic artery perfusion remained significant (14). Ischemia-reperfusion injury has further been suggested, as it has been shown that the length of the cold ischemia time correlates with the magnitude of ischemia-reperfusion injury (16). Cold ischemia times in excess of 13 h have been reported to cause a 50% incidence of ITBL in patients (4). Damage to the endothelium of peribiliary arterioles could lead to ischemic damage of the biliary epithelium. This is in line with the findings that ITBL closely resembles the biliary pathology occurring after hepatic artery thrombosis and that additional arterial back table perfusion could significantly reduce the rate of ITBL (8). Gravity perfusion through the hepatic artery may not completely rinse the PBP, which causes blood to stagnate locally in the small, incompletely flushed vessels, leading to formation of microthrombi in these arterioles. Consequently, local ischemia may occur, resulting in biliary fibrosis and strictures, as seen in ITBL. In contrast, flushing the hepatic artery with fibrinolytic drugs has been reported to decrease the incidence of ITBL (9). An incomplete rinsing of the PBP due to the high viscosity of UW might provide an explanation why ITBL develops more often after UW preservation than after preservation with the much less viscous histidine–tryptophan–ketoglutarate (HTK) preservation solution (6).
In our three patients, risk factors for ITBL were also present: cold storage with UW in all liver grafts, cold ischemia times approaching 10 h and two recipients with a history of immune-mediated end-stage liver disease. Patient 2 and 3 were transplanted for PSC and PBC respectively. In the literature it is known that immune-mediated liver disease as the cause of liver transplantation is related to a higher incidence of ITBL. Patients transplanted for PSC and auto-immune hepatitis (AIH) were found to have a 3.7- and 3.0-fold increased risk respectively for developing ITBL versus PBC, in a report of 749 patients and 842 liver grafts (17). In the same report, the incidence of ITBL did not differ significantly between patients transplanted for PBC or transplanted for alcoholic, viral, cryptogenic or other liver disease. Similarly, an earlier report describes a higher percentage of nonanastomotic strictures in patients transplanted for PSC compared to non-PSC recipients (27 vs. 13% for intrahepatic and 6 vs. 2% for extrahepatic strictures) (18). In addition, Feller et al. have reported that PSC was more often the indication for transplantation in patients developing nonanastomotic strictures compared to controls (31 vs. 9%) (19). Similar results were also published more recently by Buis et al. (20). The latter study again did not find a correlation between ITBL and PBC as the indication for liver transplantation. Based on the literature it can be concluded that patients with PSC, and AIH, but not PBC, are at greater risk of developing ITBL. However, none of the original manuscripts and reviews on ITBL in PSC and AIH disease report such a segmental ITBL pattern, as noticed in our three patients. In cases 2 and 3, generalized immune-mediated disease was found in the native explant livers, but in case 2, the second explant liver did not show any signs of biliary disease in the nonaffected lobes, making ITBL more likely than recurrent immune-mediated biliary injury.
Regarding the blood flow in the PBP, traditionally the hepatic artery is considered the sole provider of blood and responsible for oxygenation of the cholangiocytes (11,12). In all three patients, the hepatic artery blood flow was proven to be preserved by Doppler US and yet ITBL developed in segments of the liver that were deprived of portal venous blood. In patients 3 and 2 additional studies in the form of contrast enhanced MRI and CT confirmed the patency of the hepatic artery. Based on the literature the latter should serve as the golden standard for detecting vascular complications after liver transplantation (21). Doppler US and contrast enhanced MRI have a reported sensitivity and specificity of up to 91.3% and 100% (22,23), and ranging between 67–100% and 90–100% respectively (23,24), for detecting hepatic artery thrombosis following liver transplantation. However these studies do not consider small arterial lesions, which one can contemplate, could be undetectable on the conducted imaging studies as well as CT-angiography performed in this study. Therefore a possibility exists that such undetectable small arterial lesions caused the biliary complications seen in the presented cases, which could have been present in the three patients in conjunction with the portal vein thrombosis.
The portal vein thrombi in the presented patients were diagnosed using Doppler US, which is routinely conducted upon arrival at the ICU, day 1 and day 7 after liver transplantation at our center and is reported to have a sensitivity and specificity of 100% in diagnosing generalized portal vein thrombosis after liver transplantation (22). The conducted study does not apply to segmental portal vein thrombosis, thus one can contemplate that the sensitivity for diagnosing segmental portal vein thrombosis might be lower. However, all ultrasonographies in this study were performed or supervised by two dedicated hepatologists, both with over 10 years of experience on liver ultrasonography in liver transplantation, and additional imaging studies discussed earlier also confirmed the partial portal vein thrombi in cases 2 and 3.
In the literature, 1.7% of the patients receiving a whole liver graft develop total portal thrombosis after liver transplantation. In this published series however, the development of ITBL was not described (25). In contrast to the presented patients though, (immediate) restoration of the portal circulation was attempted in these reports. In our patients no risk factors for portal vein thrombosis, such as pre-existing portal vein thrombosis, earlier splenectomy, use of venous conduits, and small portal vein size (25), were present. Other postoperative complications capable of causing portal vein thrombosis as well as biliary strictures such as periportal edema, cholangitis, hepatitis, abscess formation, hepatic artery thrombosis and hepatic vein outflow obstruction, were ruled out on repeated imaging and laboratory studies.
In conclusion, our case presentation indicates that the portal circulation may play an important role in the integrity of the biliary tree and especially in development of ITBL after liver transplantation. Our findings suggest that in the case of partial intrahepatic portal vein thrombosis, even with sufficient liver function, a thrombectomy or thrombolysis should be considered to prevent late biliary complications.
Acknowledgment
This work has been supported by the Foundation for Liver and Gastrointestinal Research (SLO) Rotterdam.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. This manuscript was not prepared, nor funded, in any part by a commercial organization.




