Subclinical Rejection in Stable Positive Crossmatch Kidney Transplant Patients: Incidence and Correlations
Abstract
We reviewed 116 surveillance biopsies obtained approximately 1, 3, 6 and 12 months posttransplantation from 50 +XM live donor kidney transplant recipients to determine the frequency of subclinical cell-mediated rejection (CMR) and antibody-mediated rejection (AMR). Subclinical CMR was present in 39.7% of the biopsies at 1 month and >20% at all other time points. The presence of diffuse C4d on biopsies obtained at each time interval ranged from 20 to 30%. In every case, where histological and immunohistological findings were diagnostic for AMR, donor-specific antibody was found in the blood, challenging the long-held belief that low-level antibody could evade detection due to absorption on the graft. Among clinical factors, only recipient age was associated with subclinical CMR. Clinical factors associated with subclinical AMR were recipient age, positive cytotoxic crossmatch prior to desensitization and two mismatches of HLA DR 51, 52 and 53 alleles. Surveillance biopsies during the first year post-transplantation for these high-risk patients uncover clinically occult processes and phenotypes, which without intervention diminish allograft survival and function.
Introduction
Many transplant recipients face immunological barriers including anti-HLA donor-specific antibodies (DSA), which have traditionally precluded opportunities for kidney transplantation. Recently, utilizing ‘desensitization’ protocols, transplantation has become feasible in these circumstances (1–5). Short-term patient and allograft survival rates of these high immunologic risk recipients have generally been good but the long-term outcomes for these allografts are unknown. Pathological processes such as acute rejection, drug toxicity and viral nephropathy can occur and impact kidney allograft outcome long before there is a rise of serum creatinine. If these processes were detected earlier, intervention might ultimately improve allograft outcomes especially in populations at high risk for subclinical renal injury.
The incidence and determinants of subclinical cell-mediated rejection (CMR) and antibody-mediated rejection (AMR) following kidney transplantation in positive crossmatch (+XM) recipients is unknown. Surveillance biopsies of kidney transplant recipients are often included in studies of new immunosuppressive regimens. They, however, are not routinely performed in stable patients due to the anticipated low yield of findings and the risks of complications associated with this procedure (6). In 1994, Rush et al. (7) reported a 30% prevalence of subclinical CMR in biopsies performed 3 months posttransplantation among recipients with stable kidney function treated with prednisone, cyclosporine and azathioprine. Recent observations from several protocol biopsy series, however, document a much lower incidence, 3–8%, of subclinical CMR at 3 and 6 months (8–11). This lower rate is likely due to newer clinical protocols of immunosuppression with more frequent use of antibody induction and also maintenance therapy consisting of prednisone, tacrolimus and mycophenolate.
There is only one study describing the incidence of subclinical histological processes that occur at 12 months following transplantation of +XM kidney transplant recipients (12). Detection of subclinical rejection could be important as subclinical rejection has been predictive of poor long-term allograft function (13–18). Given the paucity of published data, the current study was undertaken to determine the prevalence throughout the first year following surgery of subclinical CMR and AMR. A high frequency of subclinical rejection would justify the incorporation of protocol surveillance biopsies into management protocols of these patients.
Materials and Methods
Patients
This is a retrospective case series of surveillance biopsies performed on 95 consecutive +XM patients who underwent live donor kidney transplantation in the Incompatible Kidney Transplant Program at Johns Hopkins between February 10, 1998 and January 27, 2006. +XM patients were defined by having donor-specific anti-HLA antibody (DSA) detectable by solid-phase immunoassay and/or reactivity in a cytotoxic or flow cytometric crossmatch assay. Four patients were both blood group incompatible (ABOi) and +XM with their donors. Following informed consent, all patients were treated with a standardized desensitization protocol approved by the Johns Hopkins Institutional Review Board, which included pre- and posttransplant plasmapheresis/low-dose intravenous immunoglobulin (PP/IVIg) and quadruple, sequential immunosuppression
Biopsies
Surveillance biopsies were performed at approximately 1, 3, 6 and 12 months following transplantation. Biopsies were excluded from this analysis if they were performed at a time the recipient had concomitant clinical criteria that justified the performance of the biopsy for cause: serum creatinine (SCr) ≥ 1.8 mg% 1 month postsurgery, SCr ≥ 20% from baseline, treatment of rejection within 2 weeks of a biopsy or new onset proteinuria. Fifty-one of the 177 surveillance biopsies performed were excluded by these criteria. Biopsies were evaluated using updated Banff’97 criteria (19,20). Based on this, an additional 10 biopsies were excluded because they were inadequate by Banff’97 criteria to grade for rejection. The final number of biopsies included in this series was 116 from 50 individuals. There were 14 participants with one biopsy, 14 participants with two biopsies, 14 participants with three biopsies and 8 participants with four biopsies during the study period.
Allograft biopsies were performed using an 18-gauge spring-loaded biopsy needle utilizing real-time ultrasound guidance (21). A 3–4 mm portion of renal cortical and/or medullary tissue was frozen from each biopsy in OCT compound (Tissue-Tek; Sakura Finetek, Torrance, CA) at –20°C for immunofluorescence studies. This was performed by indirect immunofluorescence on cryostat sections by using mouse anti-human C4d antibody (Quidel, San Diego, CA) at 1:40 dilution, followed by fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA). The remaining tissue was fixed in 10% buffered formalin and processed for routine histological examination of paraffin sections stained with hematoxylin–eosin (H &E), periodic acid-Schiff (PAS), silver methenamine and Masson's trichrome stains.
Immunosuppression
All patients were treated prior to transplantation with alternate-day plasmapheresis (PP) and 100 mg/kg of intravenous hyperimmune globulin (IVIg) (CMVIg: Cytogam, Medimmune, Inc., Gaithersburg, MD) to remove DSA and received quadruple-drug immunosuppression (daclizumab, tacrolimus or sirolimus, mycophenolate and steroids) as previously described (22). Some +XM recipients were also treated with anti-CD20 therapy (n = 12), splenectomy (n = 1) or both (n = 1) to suppress DSA. Rejections, both clinical and subclinical, were managed with steroids, T-cell depletional therapy, plasmapheresis and low-dose IVIg, anti-CD20 antibody and/or high-dose IVIg depending upon the biopsy grade and response to therapy and also based on the course of kidney function, strength of DSA and follow-up biopsies.
Compatibility testing
HLA typing was performed by the basic complement-dependent lymphocytotoxicity (HLA-A, -B, -C) (23) and by sequence-specific primer amplification (HLA-DR, -DQ) (MicroSSP™, LABType®, One Lambda, Canoga Park, CA). Donors and recipients were typed for all serologically defined antigens at the HLA-A, -B, -C, -DRB1, DRB3–5 and -DQB1 loci except A903, A43, A80, B46, B59, B75–78 and B81. Lymphocyte crossmatch tests were performed by complement-dependent cytotoxicity (AHG-enhanced for T cells and one-wash for B cells) (23) and by flow cytometry (24). Tests of HLA-specific antibodies were by solid-phase immunoassay on the ELISA (Quik-ID class I and class II, GTI, Brookfield, WI) and/or Luminex platforms (Lifematch ID and LifeScreen kits, Tepnel LifeCodes, Stamford, CT; Single Antigen Bead kits, One Lambda). All sera were tested for the presence of HLA-specific antibodies of the IgG class against targets comprising pooled HLA class I and class II antigens. Specificity determination was based on the results of tests with a panel of phenotypes and, when necessary for clarification, a panel of single, purified antigens.
Outcome variables and covariates
We defined CMR and AMR by using the most recently updated Banff criteria (20). Demographic variables included were age, gender and race. The clinical variables included were donor and recipient cytomegalovirus (CMV) status, recipient hepatitis C antibody status, primary diagnosis of end-stage renal disease prior to transplant: diabetes (DM), glomerulonephritis (GN), familial, congenital, hypertension (HTN), other or unknown, delayed graft function and previous acute clinical rejection prior to the time of biopsy. Immunological variables included were primary or retransplantation, number of mismatches among five groups of HLA antigens (class I: HLA- A, -B; class II: DR 1–18, DQ 1–9; and analyzed separately DR 51, 52, 53), number of repeat mismatches from prior transplant, initial cytotoxicity crossmatch (positive or negative) and cytotoxicity crossmatch (positive or negative) at the time of transplant. For many recipients, assays of DSA were performed within 1 week of surveillance biopsies and when measured included in the analysis as being either present or absent.
Statistical analysis
Descriptive analyses were conducted to determine differences in demographic and clinical characteristics for the 50 transplant recipients at baseline and follow-up by the Student's t-test, chi-square test and one-way analysis of variance. Descriptive analyses were also performed for all 116 biopsies among all transplant recipients. Univariate analyses were conducted to determine the odds ratios for subclinical CMR for demographic and clinical variables by logistic regression. Odds ratios for subclinical AMR were computed for demographic, clinical and biochemical variables in a univariate analysis. Statistical tests were conducted to determine if there was a linear trend for association with categorical variables having ≥3 categories. Due to the small sample size, only parsimonious multivariate analyses were conducted with variables that were borderline or significant by univariate analyses. To account for repeated biopsies among the same individual, the analyses were clustered by person. We also conducted a separate logistic regression analysis to determine if a prior clinical rejection was associated with subclinical rejection in subsequent surveillance biopsies. The analyses were restricted to the surveillance biopsy immediately following the prior clinical rejection. All analyses were conducted using STATA statistical software (Version 9.0, College Station, TX). A p <0.05 was considered as statistically significant.
Results
Patients
Fifty of the 95 +XM recipients (53%) had one or more protocol surveillance kidney transplant biopsies performed at times when they were clinically stable and form the study population for this biopsy series. Their demographics and baseline characteristics are shown in Table 1. African Americans made up 14% of the cohort. Donor characteristics, CMV status, HLA match and frequency of DGF are also listed in Table 1.
| Recipient characteristics | |
| Age recipient (mean in years ± SD) | 43.7 ± 11.1 |
| Male recipient, N (%) | 17 (34) |
| African American, N (%) | 7 (14) |
| Primary diagnosis, N (%) | |
| Diabetes | 3 (6) |
| Glomerulonephritis | 11 (22) |
| Familial | 8 (16) |
| Congenital | 8 (16) |
| Hypertension | 2 (4) |
| Other | 10 (20) |
| Unknown | 3 (6) |
| Previous transplants, N (%) | 28 (56) |
| HCV +, N (%) | 2 (4) |
| Donor characteristics | |
| Age donor (mean in years ± SD) | 42.7 (11.9) |
| Male donor, N (%) | 25 (50) |
| African American | 5 (10) |
| CMV donor+ recipient−, N (%) | 9 (18) |
| Living related,2 N (%) | 27 (53) |
| HLA mismatch (mean ± SD) | |
| Class I (A and B) | 2.5 ± 1.1 |
| Class II (DR1–18 and DQ1–9) | 2.2 ± 1.2 |
| Class II (DR51, 52, 53) | 0.66 ± 0.63 |
| Total mismatch | 4.7 ± 2.0 |
| Cytotoxicity crossmatch positive, N (%) | 27 (54) |
| DGF3, N (%) | 5 (10) |
| Repeat mismatches with prior transplants, N (%) | |
| 1 | 4 (8) |
| 2 | 7 (14) |
| 3 | 1 (2) |
- 1Includes four +XM recipients who are also ABOi.
- 2Parents, siblings, children.
- 3Two patients received hemodialysis for hyperkalemia.
Subclinical CMR
One hundred sixteen surveillance biopsies met inclusion criteria. The number of biopsies in each time interval, serum creatinine and the Banff grading for individual biopsies are shown in Table 2.
| Number of biopsies | 116 | |||
| Biopsy time in months | 0—2 | 2.1–4 | 4.1–9 | >9.1 |
| Median time in months | 1.2 | 3.1 | 6 | 12.2 |
| Range in months | 0.7–1.9 | 2.3–4.0 | 4.2–8.9 | 9.9–15.2 |
| Number of biopsies in each time period (%) | 28 (24) | 27 (23) | 31 (27) | 30 (26) |
| Serum creatinine, mg/dL (SD) | 1.1 (0.2) | 1.1 (0.3) | 1.1 (0.2) | 1.3 (0.2) |
| Banff grade, N | ||||
| Borderline | 2 | 8 | 9 | 12 |
| 1a | 2 | 3 | – | 2 |
| 1b | – | 1 | 3 | – |
| 2a | 8 | 2 | 3 | 3 |
| 3 | – | – | 1 | – |
| Total rejection ≥ 1a, N (%) | 10 (35.7) | 6 (22.2) | 7 (22.6) | 5 (16.7) |
| Total rejection ≥ borderline, N (%) | 12 (42.9) | 14 (51.9) | 16 (51.6) | 17 (56.7) |
- 1Includes four recipients who are both ABOi and +XM.
The prevalence of subclinical CMR for each time interval is shown in Figure 1. The highest frequency of subclinical CMR Banff grade ≥ 1a occurred between months 0 and 2 (40%). This remained high between months 2 and 4 and 4 and 9, 22 and 23%, decreasing to 17% after 9 months. If borderline inflammation is included, the frequency of subclinical CMR increases but continues to show a similar pattern across the year of follow-up (Table 2).

The prevalence of subclinical cellular rejection between 0–2, 2.1–4, 4.1–9 and >9.1 months following transplantation for +XM recipients.
By univariate analysis (Table 3), only older age was associated with subclinical CMR Banff ≥ 1a. However, after multivariate adjustment, this was no longer statistically significant as the confidence interval crossed 1 but the point estimates were similar. Clinical variables such as recipient gender and race, CMV status, HLA mismatch, repeat mismatches with prior transplants, cytotoxicity crossmatch positive before desensitization therapy or at the time of surgery and the presence of DSA at the time of biopsy were not associated with subclinical rejection in all biopsies. Also, by univariate analysis, the occurrence of a biopsy-proven clinical rejection prior to the protocol surveillance biopsy was not predictive of subclinical cellular rejection Banff ≥ 1a (data not shown).
| Odds ratio (95% CI) | p-Value | p trend | |
|---|---|---|---|
| Age (per 10 years) | 1.0 (1.0–1.001) | 0.01 | |
| Gender(male vs. female) | 1.3 (0.5–3.1) | 0.5 | |
| Caucasian (vs. African American) | 2.3 (0.6–10.9) | 0.2 | |
| CMV status: Donor+ recipient− | 0.6 (0.3–1.3) | 0.2 | |
| DGF (vs. none) | 2.8 (0.8–9.0) | 0.09 | |
| HLA class I (A and B) | |||
| 1 mismatch (vs. none) | 1.1 (0.2–5.5) | 0.9 | 0.6 |
| 2 mismatches (vs. none) | 0.4 (0.1–1.9) | 0.3 | |
| 3 mismatches (vs. none) | 0.8 (0.2–3.6) | 0.8 | |
| 4 mismatches (vs. none) | 0.5 (0.1–3.8) | 0.5 | |
| HLA class II (DR1–18 and DQ1–9) | |||
| 1 mismatch (vs. none) | 0.5 (0.2–1.5) | 0.2 | 0.4 |
| 2 mismatches (vs. none) | 0.7 (0.3–1.9) | 0.5 | |
| 3 mismatches (vs. none) | 5 (0.7–36.7) | 0.1 | |
| 4 mismatches (vs. none) | 0.8 (0.3–2.1) | 0.7 | |
| HLA class II (DR51–52–53) | |||
| 1 mismatch (vs. none) | 0.6 (0.3–1.4) | 0.3 | 0.7 |
| 2 mismatches (vs. none) | 1.3 (0.5–3.6) | 0.6 | |
| Repeat mismatch | |||
| 1 mismatch (vs. none) | 0.9 (0.2–4.6) | 0.9 | 0.6 |
| 2 mismatches (vs. none) | 1.6 (0.6–4.7) | 0.4 | |
| Initial cytotoxicity crossmatch (positive vs. negative) | 1.0 (0.4–2.3) | 1 | |
| Cytotoxicity crossmatch at transplant (positive vs. negative) | 1.4 (0.5–3.4) | 0.5 | |
| DSA+ at biopsy (present vs. absent) | 1.1 (0.4–3.0) | 0.9 | |
C4d+
We have previously shown that surveillance biopsies from patients who are ABOi frequently show C4d deposition without the histological features of AMR (21). For this reason, we have excluded nine biopsies from four patients who were ABOi, in addition to having a +XM with their donors. The frequency of C4d+ was therefore evaluated in 107 of the 116 surveillance biopsies obtained from +XM-only recipients. As shown in Figure 2, among these individuals the prevalence of diffuse C4d+ ranged from 20 to 30% for all time intervals posttransplantation. Among the 17 participants who had repeated biopsies at four time periods, diffuse C4d positivity was present in seven participants (41%) at approximately 1 month and remained positive in 29% (n = 5) of the protocol biopsies at approximately 3, 6 and 12 months. There was no difference in the specificity of DSA associated with C4d+ although having class I-specific antibody alone appears to be less (only 20% of biopsies where DSA was class I were C4d+ whereas 52% of biopsies where DSA included class II antibody were C4d+, p = 0.06).
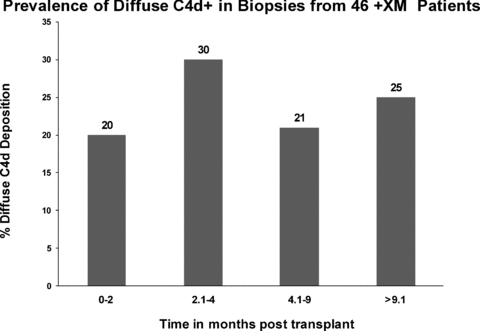
The prevalence of diffuse C4d deposition in biopsies from +XM recipients between 0–2, 2.1–4, 4.1–9 and >9.1 months following transplantation. Recipients who were both +XM and ABOi are excluded from this figure.
Thirty-one percent of biopsies with C4d+ had a subclinical cellular rejection Banff grade ≥ 1a (50% of these rejections being grade 2a or greater). Twenty-two percent of biopsies without C4d+ had a subclinical cellular rejection Banff grade ≥ 1a (61% of these rejections being grade 2a or greater). There was no significant difference between the frequency of subclinical cellular rejections with and without a vascular component between C4d+ and C4d– biopsies.
Subclinical AMR
DSA was measured at the time of biopsy for 75 of the 107 surveillance biopsies obtained from +XM recipients (Table 4). DSA was found to be present at the time of 44 of the 75 biopsies. Twenty-one of these 75 biopsies (28%) had diffuse C4d+. Diffuse C4d+ deposition in the peritubular capillaries (ptc) was 100% specific for the presence of DSA in the blood in as much as this pattern of C4d was not observed on any surveillance biopsies when DSA was absent. Of these 21 C4d+ biopsies, all had margination of leukocytes in peritubular capillaries (3 with ptc = 1; the remainder with ptc scores ≥ 2) and 15 had glomerulitis (4 with g>1). Thus, all C4d+ biopsies from +XM recipients where DSA status was known met Banff criteria for subclinical AMR.
| DSA | |||
|---|---|---|---|
| Present N = 44 | Absent N = 31 | ||
| C4d | Positive | 21 (47.7%) | 0 (0%) |
| Negative | 23 (52.3%) | 31 (100%) | |
| Cellular rejection by Banff grade | No | 19 (43.2%) | 20 (64.5%) |
| Borderline | 13 (29.6%) | 3 (9.7%) | |
| 1a | 4 (9.1%) | 2 (6.5%) | |
| 1b | 1 (2.3%) | 0 (0%) | |
| 2a | 7 (15.9%) | 5 (16.1%) | |
| 2b | – | – | |
| 3 | 0 (0%) | 1 (3.2%) | |
In the absence of diffuse C4d deposition, margination of leukocytes in peritubular capillaries (ptc ≥ 1) with glomerulitis (g > 1) was observed in 17% (4/23) of the biopsies in the presence of DSA and 13% (4/31) of the biopsies in the absence of DSA. These were not diagnosed as subclinical AMR by Banff criteria.
DSA status at the time of biopsy did not alter the frequency of subclinical cellular rejection Banff grade ≥ 1a (Table 4). Subclinical cellular rejection was present in 27.3 and 25.8% of the biopsies in the presence or absence of DSA, respectively. The proportion of these subclinical cellular rejections with a vascular component (Banff grade ≥2a) was likewise similar between the two groups; however, the numbers of biopsies showing rejection are very small in these groups.
Clinical variables associated with a recipient having at least one surveillance biopsy consistent with subclinical AMR included age, two mismatches of HLA DR 51, 52 and 53 and a positive initial cytotoxicity crossmatch prior to starting desensitization therapy (Table 5). Multivariate adjustment was not possible given the small sample size.
| Odds ratio (95% CI) | p-Value | p trend | |
|---|---|---|---|
| Age (per 10 years) | 1.02 (1.01–1.03) | 0.0001 | |
| Gender (male vs. female) | 1.0 (0.3–3.4) | 0.9 | |
| Caucasian (vs. African American) | 0.7 (0.2–3.0) | 0.6 | |
| CMV status: donor + recipient- | 0.8 (0.2–2.3) | 0.6 | |
| DGF (vs. none) | 1.6 (0.3–8.6) | 0.6 | |
| HLA class I (A and B) | |||
| 1 mismatch (vs. none) | 0.2 (0.02–3.2) | 0.3 | 0.7 |
| 2 mismatches (vs. none) | 0.6 (0.1–3.1) | 0.6 | |
| 3 mismatches (vs. none) | 0.8 (0.1–4.2) | 0.8 | |
| 4 mismatches (vs. none) | 0.7 (0.1–5.0) | 0.7 | |
| HLA class II (DR1—18 and DQ1–9) | |||
| 1 mismatch (vs. none) | 1.8 (0.1–25.5) | 0.7 | 0.07 |
| 2 mismatches (vs. none) | 0.8 (0.1–11.4) | 0.8 | |
| 3 mismatches (vs. none) | 12 (0.5–263.6) | 0.1 | |
| 4 mismatches (vs. none) | 3.9 (0.3–55.5) | 0.3 | |
| HLA class II (DR51–52–53) | |||
| 1 mismatch (vs. none) | 2.2 (0.6–7.7) | 0.2 | 0.04 |
| 2 mismatches (vs. none) | 6.9 (1.3–35.9) | 0.02 | |
| Repeat mismatch | |||
| 1 mismatch (vs. none) | 0.3 (0.02–4.2) | 0.4 | 0.2 |
| 2 mismatches (vs. none) | 0.3 (0.04–2.1) | 0.2 | |
| Initial cytotoxicity crossmatch (positive vs. negative) | 7.9 (2.1–29.1) | 0.002 | |
| Cytotoxicity crossmatch at transplant (positive vs. negative) | 2.2 (0.6–8.3) | 0.2 | |
Clinical follow-up
Serum creatinine 1 year posttransplantation was not different comparing patients having no SCR (n = 20) with patients having SCR (n = 30) (1.43 ± 0.69 mg% vs. 1.47 ± 0.27 mg%, p = ns). Serum creatinine at 1 year was also similar between patients with only subclinical CMR (n = 11) and patients with subclinical AMR with or without subclinical CMR (n = 19) (1.41 ± 0.30 mg% vs.1.49 ± 0.72 mg%, p = ns). Delta serum creatinine between months 1 and 12 did not differ between patients with and without SCR (0.22 ± 0.45 mg% vs. 0.21 ± 0.75 mg%, p = ns). Delta serum creatinine was also not significantly different between patients with no SCR, patients with only subclinical CMR (–0.13 ± 0.87 mg%) and patients with subclinical AMR ± subclinical CMR (0.41 ± 0.62 mg%) (p = 0.085).
No grafts were lost due to complications of surveillance biopsies in this cohort. Complications occurred in a total of 6 of the 116 biopsies in this series (5%). The complications included pain (n = 1), cutaneous hematoma (n = 1), macroscopic hematuria (n = 1), AV fistula (n = 1) and hematuria with obstructive uropathy from clot retention (n = 2). Only the patients with obstructive uropathy required hospitalization for observation, and only one patient required significant intervention with bladder irrigation and antegrade drainage prior to resolution. Thus, the rate of significant morbidity was 4.3%.
Discussion
We performed surveillance biopsies of +XM kidney transplant recipients to evaluate the frequency of subclinical AMR and CMR. Compared with the 3–8% prevalence of subclinical cellular rejection reported in recent years from ‘non-high risk’ kidney transplant recipients (8–11), we observed a much higher frequency of 40% within 2 months postsurgery. The frequency of subclinical cellular rejection decreased at later time points with a prevalence ranging between 17 and 23% at 3, 6 and 12 months.
We also observed a 20–30% prevalence of C4d+ in protocol surveillance biopsies at all time points following transplantation, similar to what has been reported by others in 12 months surveillance biopsies (12). Although C4d+ has been reported to occur in the absence of DSA (25), we did not observe this to be the case in this series. In all cases where diffuse C4d was present on biopsy, DSA was detectable at the time of biopsy in peripheral blood making this one of the most notable findings of this study. For biopsies for which DSA data were available, the presence of diffuse C4d staining in the peritubular capillaries was in all cases accompanied by both the presence of DSA and the presence of margination of neutrophils and/or mononuclear leukocytes in the peritubular capillaries, thus meeting Banff criteria for AMR. This is the first evidence from a large study of high immunologic risk patients that the lack of detectable DSA in peripheral blood is a very reliable indicator of absence of ongoing antibody-mediated injury in the graft. This finding corroborates data showing DSA elimination in desensitized patients and refutes the concept that DSA can elude detection by absorption on the graft (5). It also may further strengthen epidemiologic estimates of the prevalence of de novo DSA after transplantation.
In recent reports, the decline of subclinical cellular rejection from the 30% prevalence reported by Rush et al. in 1994 (7) to 3–8% may reflect both changes of immunosuppressive protocols, such as the increased use of antibody induction therapy and substitution of mycophenolate for azathioprine, as well as the increase of live donor kidney transplantation. Maintenance immunosuppressive therapy used in this current series of +XM recipients is similar to regimens used in more recent reports. Despite this and the increased intensity of immunosuppression used perioperatively for the desensitization protocol, we observed a frequency of subclinical cellular rejection in this high-risk population similar to what was reported in the 1990s.
Similar studies of +XM recipients from The Mayo Clinic (12,26) report lower frequencies of subclinical cellular rejection than we have observed. The reasons for this difference are not easily discernable by comparison with these studies but could reflect donor and recipient factors and also immunosuppressive regimens. The two groups do differ with regard to baseline demographics. In particular, the patients in this study may be at higher risk for cellular rejection given the higher prevalence of African Americans and those with prior transplantation. Also, HLA mismatching might be a factor but cannot be directly compared with the data given in these reports. Differences in DSA specificity and strength could also account for the differences in these outcomes. Desensitization protocols appear comparable; however there are slight differences between the two groups as to the final permissible DSA titer prior to surgery. Both groups used similar maintenance immunosuppression protocols but at The Mayo Clinic, T-cell depletion rather than IL-2 receptor antibody induction therapy was utilized (12,26). Future investigations with larger numbers and pooled data from multiple centers will be required to address these factors.
In recent studies of recipients, who are not +XM, factors found to impact the incidence of subclinical cellular rejection included type of immunosuppression (use of tacrolimus and mycophenolate), donor–recipient relationship (living related versus living unrelated), cause of donor death, HLA-DR mismatch and having a prior acute rejection (11,13,15,27). We studied similar predictors of subclinical cellular rejection but with the small sample size were not able to determine if these predictors were consistently associated with rejection while controlling for other risk factors. Using univariate analysis, even with the small sample size, age was associated with increased odds of having subclinical cellular rejection Banff grade ≥ 1a in surveillance biopsies. The mechanism for the observation of a rising risk of subclinical cellular rejection with age is unclear. This might reflect an increased risk for sensitization due to factors such as prior transplantation, transfusion, pregnancy and exposure to infections. Due to small numbers this could not be determined from this analysis. Patients in the present series also differ from those in these other studies by virtue of having DSA and having received desensitization therapy for this. These small numbers could account for the lack of association observed here between subclinical cellular rejection and both HLA mismatch and having a prior acute rejection.
We evaluated for associations between donor and recipient factors and the finding of subclinical AMR in one or more subsequent protocol surveillance biopsies. We found that antibody strength (reflected by a positive cytotoxicity crossmatch) prior to desensitization therapy and two mismatches of HLA DR 51, 52 and 53 antigens were associated with increased odds of having a C4d+ surveillance biopsy. We also observed that C4d+ biopsies occurred less often if the recipient had only class I DSA, which is similar to other published studies; however, due to the small sample size, we could not confirm the significance for this finding. These factors have been identified in other studies as being associated with persistence of DSA following transplantation (5) and the development of transplant glomerulopathy (28,29), a feature associated with chronic AMR.
The current study has a number of limitations. Due to sample size and cross-sectional study design, we were neither able to study prospectively the independent association of clinical predictors with subclinical rejection nor use robust analytical techniques controlling for known confounders. It is, however, among the largest series of surveillance biopsies studied among +XM recipients with follow-up at 1 year. Furthermore, we studied clinical factors that are already known to influence rejection to determine if we could determine who was at highest risk. Without a control group consisting of sensitized patients who do not have a positive crossmatch, no conclusions can be made concerning the applicability of these observations to patients with third-party sensitization or sensitization to non-HLA-antigens. Further studies will need to study this question prospectively.
The identification of a high prevalence of subclinical cellular- and antibody-mediated rejection during the first year following +XM transplantation is important, since subclinical cellular rejection during the first year for non-high immunologic risk patients has been linked to a poorer long-term outcome (13–18), and there is evidence that intervention could ameliorate this poor outcome (9,30). Several desensitization protocols that have been developed have proven very effective at overcoming early graft losses due to antibody-mediated injury. What remains uncertain is whether these high immunologic risk patients will have a higher incidence of premature graft loss from progressive interstitial fibrosis and tubular atrophy, vasculopathy and transplant glomerulopathy. In this cohort, subclinical rejection triggered interventions with pulse steroids ± T-cell depletional therapy for CMR and additional plasmapheresis ± anti-CD20 for AMR. This treatment protocol could be responsible for similar kidney function reflected by serum creatinine at 1 year for patients with and without subclinical rejection. Without a control group of patients where subclinical rejection was not treated, efficacy of such interventions cannot be determined. The use of surveillance biopsies to reveal occult processes such as subclinical cellular- and antibody-mediated rejection allows the development of individualized intervention strategies that could prove effective in preventing progression of chronic changes. In all cases where subclinical AMR was present so was DSA but in some cases DSA was detectable in the absence of biopsy-evident AMR. Our data show very convincingly that DSA monitoring is a very reliable tool for assessing risk of AMR inasmuch as the absence of DSA is associated with a very low risk of subclinical AMR. Currently, protocol surveillance biopsies combined with immune monitoring remain the basis for improving protocols of care designed to optimize long-term outcomes for patients requiring desensitization therapy for transplantation.
Acknowledgments
We are indebted to the patients and staff of the Johns Hopkins Incompatible Kidney Transplant program. Dr. Parekh was supported by 1UO1-DK-57304-01 and 1 R01 DK070657-01.




