Donor HLA-C Genotype Has a Profound Impact on the Clinical Outcome Following Liver Transplantation
Abstract
Late allograft dysfunction is a significant problem following liver transplantation and its pathogenesis is uncertain. HLA-C is the major inhibitory ligand for killer immunoglobulin-like receptors (KIRs) that regulate the cytotoxic activity of natural killer (NK) cells. HLA-C alleles can be allocated into two groups, termed HLA-C1 and HLA-C2, based on their KIR specificity. HLA-C2 interactions are more inhibiting to NK cell activation. We studied the clinical importance of HLA-C genotype in a large liver transplant cohort and found that possession of at least one HLA-C2 allele by the donor allograft was associated with less histological evidence of chronic rejection and graft cirrhosis, a 16.2% reduction in graft loss (p = 0.003) (hazard ratio: 2.7, 95% CI 1.4–5.3) and a 13.6% improvement in patient survival (p = 0.01) (hazard ratio: 1.9, 95% CI 1.1–3.3) at 10 years. Transplantation of an HLA-C2 homozygous allograft led to a particularly striking 26.5% reduction in graft loss (p < 0.001) (hazard ratio: 7.2, 95% CI 2.2–23.0) at 10 years when compared to HLA-C1 homozygous allografts. Donor HLA-C genotype is therefore a major determinant of clinical outcome after liver transplantation and reveals the importance of NK cells in chronic rejection and graft cirrhosis. Modulation of HLA-C and KIR interactions represents an important novel approach to promote long-term graft and patient survival.
Introduction
Liver transplantation is now the accepted treatment for end-stage liver disease and major improvements in outcome have been seen over the last 20 years. However, despite improvements in 1-year survival rates the incidence of late graft loss has not altered and remains a significant cause of morbidity and mortality (1). In addition to disease recurrence, particularly with hepatitis C (2), the most common causes of late graft damage are chronic rejection, biliary/vascular complications, de novo disease and ‘idiopathic’ chronic hepatitis (3–5). Irrespective of the underlying etiology, most late graft complications are associated with inflammatory changes and often lead to cirrhosis.
T-cell recognition of allogeneic HLA-peptide complexes is considered to be central to the rejection of vascularized allografts (6) but the lack of association between HLA mismatch and clinical outcome indicates that this is not the dominant pathway for liver allograft loss (7,8). HLA molecules also act as ligands for other immune receptors including killer immunoglobulin-like receptors (KIRs). KIRs are present on natural killer (NK) cells and a subset of T cells and modulate cytotoxic function (9–12). NK cells play an important role in eliminating virally infected or malignant cells that might otherwise evade immune recognition through downregulation of HLA-Class I (13). Thus a primary biological role of inhibitory KIRs is to allow effector cells to sense the presence of ‘self’ major histocompatibility complex (MHC) at the cell surface in order to prevent lysis of healthy cells (14).
KIRs are encoded within the leucocyte receptor complex on chromosome 19 and are inherited independently from HLA genes on chromosome 6 (15). In common with HLA genes they show considerable genetic variation with up to 14 different KIR types being expressed within the population (16). In terms of specificity there are two main groups of KIRs classified by the number of extracellular domains (D). 3D KIRs recognize certain HLA-A and B alleles: KIR3DL2 recognizes HLA-A3 and A11 whereas KIR3DL1 recognition is limited to HLA proteins carrying a Bw4 motif (17,18). In contrast, HLA-C alleles are recognized by one of the two subtypes of 2D KIRs. KIR2DL2/3 proteins bind HLA-C alleles carrying an asparagine at position 80 while KIR2DL1 recognizes HLA-C alleles with a lysine at this position. These two HLA-C types are termed group 1 (HLA-C1) and group 2 (HLA-C2), respectively (19), and all HLA-C allotypes (there are over 250) fall into one of these two groups. At a functional level, KIR ligation can be either inhibitory or activatory for NK cell function depending on whether the cytoplasmic tail is long (L, inhibitory) or short (S, activating).
The factors that have driven the development of polymorphism within KIR genes are unknown but interesting functional differences are emerging in the biological activity of the KIR2DL proteins. It is now apparent that KIR2DLl delivers a more potent inhibitory signal following interaction with HLA-C in comparison with the KIR2DL2/3 proteins (20,21), and this is likely to influence the cytotoxic threshold of the NK cell. It is therefore not surprising that KIR genotype is emerging as a major determinant of susceptibility to a variety of autoimmune (22,23), neoplastic (24) and infectious diseases (20), as well as outcome following hematopoietic stem cell transplantation (25).
To date, there is little published work on the effect of KIR genotype on the outcome of solid organ transplantation. A study of 2757 renal transplants showed no correlation between KIR ligand mismatch and graft survival (26); whereas for liver transplants an association with acute rejection has been observed (27,28).
The differential degree of NK cell inhibition that occurs following KIR interaction with HLA-C1 or C2 ligands led us to hypothesize that the nature of the donor HLA-C genotype might influence transplant outcome (29). To test this we carried out a retrospective analysis in which donor HLA-C genotype and recipient KIR status were correlated with acute rejection, chronic allograft injury, long-term graft survival and overall patient survival following liver transplantation.
Materials and Methods
Patient group
A total of 595 liver transplant recipients treated at the Queen Elizabeth Hospital, Birmingham between 1992 and 1999 had DNA archived, and in 459 cases donor DNA was also available. Forty-three cases of early graft loss (<30 days) were excluded and were the result of primary graft dysfunction secondary to primary failure or hepatic artery thrombosis without evidence of acute rejection (n = 15) or acute postoperative mortality resulting from hemorrhage, cardiac failure, respiratory failure or septicemia (n = 28). The final study population thus comprised 416 transplant pairs all with complete long-term follow-up. Approval for this study was granted by the South Birmingham Research Ethics Committee. All outcome data and samples were anonymized before testing and analysis.
Study design
The identification of an association between polymorphic genes and clinical outcome may be complicated by multiple testing that requires statistical correction for the number of comparisons made. In order to optimize the statistical analysis, we initially analyzed the transplants as two serial cohorts termed A and B. Any potential associations that emerged between KIR ligand genotype and clinical outcome in cohort A could then be addressed specifically in cohort B. The presence of the three major KIR ligand groups, HLA-C1, HLA-C2 and HLA-Bw4, was determined in both cohorts.
Cohort A consisted of 198 transplants in which routine HLA typing had been completed prospectively for both donor and recipient using DNA allotyping for HLA-C (30). KIR ligand HLA-C1 and C2 were deduced on the basis of lysine or asparagine at position 80 of HLA-C using reference sequences (31). This cohort had a mean graft survival time of 11.9 years (range 0.1–14.3) and mean patient survival time of 11.3 years (range 0.1–14.3).
Cohort B comprised 218 cases and KIR ligand groups were assigned directly by typing of the codon corresponding to amino acid 80 for HLA-C and codons 80–83 for HLA-Bw4 using specific oligonucleotide primers (32). KIR gene typing (33) of donors and recipients was also undertaken in this cohort. Mean graft survival time in cohort B was 11.7 years (range 0.1–13.2) with a mean patient survival time of 11.1 years (range 0.1–13.2).
Clinical and histological data
Clinical data comprised indication for transplantation, occurrence and severity of acute rejection, cause of graft/patient loss and graft/patient survival. Indications for liver transplantation were subdivided into four main groups: autoimmune, acute hepatic failure, viral infection and an additional group classified as ‘others’. The distribution of primary diseases was comparable in the two study cohorts (Table 1). Standard immunosuppression included a combination of calcineurin inhibitors (either cyclosporine or tacrolimus), azathioprine and prednisolone. The incidence of acute rejection was defined by standard histological and biochemical criteria.
| Original liver disease | Cohort A (n = 198) | Cohort B (n = 218) |
|---|---|---|
| Group I: Autoimmune etiology | 109 (55%) | 79 (36%) |
| Primary biliary cirrhosis | 67 | 47 |
| Sclerosing cholangitis | 25 | 22 |
| Autoimmune hepatitis | 17 | 10 |
| Group II: Acute hepatic failure | 6 (3%) | 22 (10%) |
| Non A non B | 3 | 17 |
| Subacute necrosis | 3 | 5 |
| Group III: Viral hepatitis | 22 (11%) | 21 (10%) |
| Hepatitis C | 15 | 15 |
| Hepatitis B | 7 | 6 |
| Group IV: Others | 61 (31%) | 96 (44%) |
| Alcohol liver disease | 32 | 18 |
| Drug-induced failure | 10 | 4 |
| Cryptogenic cirrhosis | 6 | 20 |
| Hemochromatosis | 3 | 1 |
| Alpha-1 antitrypsin deficiency | 2 | 9 |
| Wilsons disease | 2 | 3 |
| Hepatocellular carcinoma | 2 | 0 |
| Cystic fibrosis | 1 | 2 |
| Fulminant hepatitis A | 1 | 2 |
| Epitheloid tumor | 1 | 0 |
| Alagilles syndrome | 1 | 0 |
| Biliary atresia | 0 | 22 |
| Primary amyloid | 0 | 2 |
| Carolis disease | 0 | 2 |
| Congenital hepatic fibrosis | 0 | 2 |
| Hemochromatosis | 0 | 2 |
| Secondary liver tumor | 0 | 1 |
| Polycystic liver disease | 0 | 1 |
| Hepatoblastoma | 0 | 1 |
| Neonatal hepatitis | 0 | 1 |
| Schistosomiasis | 0 | 1 |
| Hemolytic anemia and giant cell hepatitis | 0 | 1 |
| Chronic Budd-Chiari | 0 | 1 |
Histological findings were reviewed for posttransplant biopsies obtained >30 days posttransplant and in hepatectomy specimens obtained at retransplantation. For each patient, the main histological diagnosis in the latest posttransplant specimen was recorded in the following categories (34): normal, mild nonspecific changes, chronic rejection, biliary complications, vascular complications, recurrent disease, de-novo disease, ‘idiopathic’ chronic hepatitis and miscellaneous other findings. For biopsies obtained >12 months posttransplant (excluding cases of rejection), inflammatory grade and fibrosis stage were scored on a scale of 0–3 (0 = absent, 1 = mild, 2 = moderate, 3 = severe) according to previously published criteria (4).
Statistical analysis
Actuarial data were analyzed using the Kaplan–Meier method and the log-rank test. Cox regression models for multivariable analysis were used to identify independent factors contributing to long-term graft and patient loss. Causes of patient loss and graft loss are listed in Tables 2 and 3. All correlations for variables and comparison of means were performed using Kendall's tau-b and analysis of variance (ANOVA) tests, respectively. Probability (p) values of less than 0.05 were considered significant for our analysis. All statistics were performed using SPSS.
| Patient loss | ||
|---|---|---|
| Cohort A | Cohort B | |
| Hepatic artery thrombosis | 1 | 0 |
| Chronic rejection | 2 | 2 |
| Cerebrovascular events | 4 | 3 |
| Cardiovascular events | 6 | 3 |
| Multiorgan failure | 4 | 6 |
| Adult respiratory distress syndrome | 0 | 2 |
| De novo tumors: | ||
| 1. Lung carcinoma | 3 | 1 |
| 2. Stomach cancer | 1 | 0 |
| 3. Oropharyngeal adenocarcinoma | 1 | 0 |
| 4. Cholangiocarcinoma | 1 | 2 |
| 5. Pancreatic carcinoma | 1 | 0 |
| 6. Facial squamous cell carcinoma | 1 | 0 |
| 7. Lymphoma | 0 | 1 |
| 8. Acute myeloid leukemia | 1 | 0 |
| 9. Breast carcinoma | 1 | 1 |
| 10. Lung adenocarcinoma | 1 | 2 |
| 11. Colorectal carcinoma | 1 | 1 |
| 12. Insulinoma | 0 | 1 |
| Major GI hemorrhage | 1 | 2 |
| Splenic artery aneurysm hemorrhage | 1 | 0 |
| Pneumonia | 4 | 1 |
| Sepsis: | ||
| 1. Bacterial | 8 | 4 |
| 2. Fungal | 1 | 2 |
| 3. CMV | 1 | 0 |
| Pulmonary embolism | 1 | 0 |
| Recurrent diseases: | ||
| 1. PBC | 1 | 0 |
| 2. PSC | 0 | 1 |
| 3. AIH | 0 | 1 |
| 4. Hepatitis B | 3 | 1 |
| 5. Hepatitis C | 1 | 2 |
| 6. ALD | 3 | 0 |
| 7. Hemolytic anemia & giant cell hepatitis | 0 | 1 |
| 8. Hepatocellular carcinoma | 2 | 0 |
| 9. Hepatoblastoma | 0 | 1 |
| Renal failure | 2 | 2 |
| Unknown | 4 | 4 |
| Total | 62 | 47 |
- Patient loss was defined as patient death from all causes. GI = gastrointestinal; CMV = cytomegalovirus; PBC = primary biliary cirrhosis; PSC = primary sclerosing cholangitis; AIH = autoimmune hepatitis; ALD = alcoholic liver disease.
| Graft loss | ||
|---|---|---|
| Cohort A | Cohort B | |
| Hepatic artery thrombosis | 5 | 5 |
| Chronic rejection | 12 | 7 |
| Ischemic graft damage | 1 | 0 |
| Recurrent diseases: | ||
| 1. PBC | 2 | 0 |
| 2. PSC | 4 | 1 |
| 3. AIH | 4 | 2 |
| 4. Hepatitis B | 2 | 1 |
| 5. Hepatitis C | 1 | 3 |
| 6. ALD | 3 | 0 |
| 7. Hemolytic anemia & giant cell hepatitis | 0 | 1 |
| Idiopathic graft cirrhosis | 0 | 2 |
| Multiorgan failure | 3 | 5 |
| Patient loss with significant graft disease: | ||
| 1. Septicemia with: | ||
| • Ischemic graft damage | 2 | 1 |
| • Recurrent hepatitis C and cirrhosis | 1 | 1 |
| • Recurrent AIH and cirrhosis | 1 | 0 |
| 2. Pneumonia with chronic rejection | 1 | 0 |
| 3. CMV viremia with severe graft cholestasis | 1 | 0 |
| 4. Renal failure with recurrent ALD and cirrhosis | 1 | 0 |
| 5. Renal failure with chronic rejection | 0 | 1 |
| 6. Splenic artery aneurysm hemorrhage with recurrent AIH and severe graft inflammation | 1 | 0 |
| 7. Major GI bleed with severe graft cholestasis (hemophagocytic syndrome) | 0 | 1 |
| Total | 45 | 31 |
- Graft loss included both graft failure requiring retransplantation and patient loss with significant graft disease. GI = gastrointestinal; CMV = cytomegalovirus; PBC = primary biliary cirrhosis; PSC = primary sclerosing cholangitis; AIH = autoimmune hepatitis; ALD = alcoholic liver disease.
Results
Donor HLA-C group genotype has a major influence on graft survival and patient survival
We initially studied the influence of donor HLA-C genotype on the rate and incidence of allograft loss. HLA-C alleles were assigned into HLA-C1 or HLA-C2 groups, as defined by KIR specificity. Graft loss was defined as either graft failure requiring retransplantation or patient death with significant graft disease. Patients who died with functioning grafts were excluded. Tables 2 and 3 list the causes of patient and graft loss in the two cohorts.
Graft survival within cohort A was increased by 15.6% at 10 years in those cases where the donor allograft carried at least one HLA-C2 allele (p = 0.004) (hazard ratio: 2.4, 95% CI 1.3–4.4). Patient survival for cases transplanted with an HLA-C2 allele was also improved by 12.5% at 10 years (p = 0.01) (hazard ratio: 1.9, 95% CI 1.1–3.1) (Figure 1A, B). These associations were then examined in cohort B and remained strongly significant with a 10-year graft and patient survival benefit of 16.7% and 14.7%, respectively, for donor allografts with an HLA-C2 allele (p = 0.002 and p = 0.01) (hazard ratio: 3.0, 95% CI 1.5–6.2 and hazard ratio: 1.9, 95% CI 1.1–3.5) (Figure 1C, D). In contrast, donor expression of HLA-C1 or HLA Bw4, the other major KIR ligand groups, did not improve clinical outcome in either cohort.
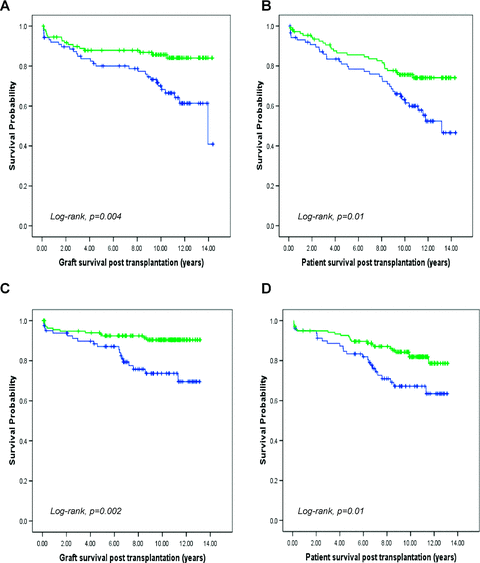
Kaplan–Meier survival curve showing allograft and patient survival following liver transplantation based on the presence or absence of a group HLA-C2 allele in the donor. The presence of an HLA-C2 allele in the donor is associated with improved graft and patient survival in both cohorts A (A and B) and B (C and D). Green line represents donor HLA-C2 allele positive (cohort A, n = 110; cohort B, n = 137) (10-year survival: A 85.7%, B 75.6%, C 90.4% and D 81.9%) and blue line represents donor HLA-C2 allele negative (cohort A, n = 88; cohort B, n = 81) (10-year survival: A 70.1%, B 63.1%, C 73.7% and D 67.2%).
Multivariable analysis confirmed the association of an HLA-C2 allele with improved graft and patient outcomes for both cohorts (Tables 4 and 5). In this analysis, recipient age also influences graft survival. For cohort B only, transplantation for hepatitis C virus infection and donor age had an adverse impact on graft survival. HLA-C genotype has been shown to influence clinical outcome following hepatitis C infection (20) and as this is the most common recurrent disease leading to graft failure (2), we reanalyzed the data excluding patients with hepatitis C as their primary diagnosis. The survival benefit seen with the possession of HLA-C2 allele remains unaltered by this exclusion (data not shown). Consistent with published literature (7), in a preliminary analysis (data not included) for these patient cohorts, we identified no long-term graft or patient survival benefit with HLA matching (locus A, B and DR).
| Demographics Study cohort A | Donor C1 homozygous n = 88 (44%) | Donor C1C2 heterozygous n = 82 (41%) | Donor C2 homozygous n = 28 (14%) | p-Value |
|---|---|---|---|---|
| Recipient age | 46.5 (CI 43.7–49.3) | 48.8(CI 46.1–51.5) | 49.6(CI 44.8–54.4) | 0.371 |
| Donor age | 38.7(CI 35.7–41.7) | 38.3(CI 35.3–41.3) | 37.1 (CI 30.8–43.5) | 0.881 |
| Recipient sex M:F | 41:47 | 42:40 | 16:12 | 0.342 |
| Seropositive CMV in recipient | 52 | 52 | 21 | 0.182 |
| Seropositive CMV in donor | 49 | 46 | 17 | 0.732 |
| Diagnosis group I | 52 | 43 | 14 | – |
| Diagnosis group II | 2 | 4 | 0 | – |
| Diagnosis group III | 8 | 9 | 5 | – |
| Diagnosis group IV | 26 | 26 | 9 | – |
| Demographics Study cohort B | Donor C1 homozygous n = 81 (37%) | Donor C1C2 heterozygous n = 105 (48%) | Donor C2 homozygous n = 32 (15%) | p-Value |
|---|---|---|---|---|
| Recipient age | 33.2(CI 28.1–38.3) | 44.8(CI 41.4–48.2) | 43.7(CI 36.8–50.6) | 0.0011 |
| Donor age | 31.0(CI 27.2–34.7) | 36.9(CI 33.9–40.1) | 35.0(CI 28.7–41.3) | 0.061 |
| Recipient sex M:F | 44:37 | 50:55 | 13:19 | 0.182 |
| Seropositive CMV in recipient | 41 | 67 | 17 | 0.332 |
| Seropositive CMV in donor | 36 | 58 | 20 | 0.062 |
| Diagnosis group I | 22 | 40 | 17 | – |
| Diagnosis group II | 7 | 13 | 2 | – |
| Diagnosis group III | 6 | 14 | 1 | – |
| Diagnosis group IV | 46 | 38 | 12 | – |
- CI is 95% confidence interval.
- 1Indicates significance by ANOVA test; 2indicates significance by Kendall's tau-b test; en dash indicates statistics not done.
| Cox regression multivariable analysis | ||||||
|---|---|---|---|---|---|---|
| Study cohort A for graft survival | Study cohort B for graft survival | |||||
| Significance | Hazard ratio | 95% CI | Significance | Hazard ratio | 95% CI | |
| Recipient age | 0.005 | 0.96 | 0.94–0.99 | 0.03 | 0.97 | 0.95–0.99 |
| Donor age | 0.29 | 0.99 | 0.97–1.01 | 0.005 | 1.04 | 1.01–1.07 |
| Recipient sex | 0.81 | 1.09 | 0.55–2.13 | 0.69 | 1.16 | 0.55–2.46 |
| Seropositive CMV in recipient | 0.07 | 0.51 | 0.25–1.05 | 0.20 | 0.56 | 0.23–1.36 |
| Seropositive CMV in donor | 0.22 | 0.70 | 0.35–1.28 | 0.10 | 1.96 | 0.88–4.35 |
| Diagnosis group I1 | 0.58 | 1.25 | 0.57–2.72 | 0.77 | 0.85 | 0.28–2.57 |
| Diagnosis group II1 | 0.68 | 0.64 | 0.08–5.21 | 0.91 | 1.08 | 0.29–4.00 |
| Diagnosis group III1 | 0.55 | 1.37 | 0.49–3.82 | 0.02 | 4.23 | 1.26–14.22 |
| Donor HLA-Bw4 ligand | 0.26 | 1.45 | 0.76–2.75 | 0.21 | 1.78 | 0.72–4.38 |
| Donor HLA-C2 ligand | 0.04 | 2.03 | 1.05–3.93 | 0.03 | 2.43 | 1.08–5.47 |
| Study cohort A for patient survival | Study cohort B for patient survival | |||||
| Recipient age | 0.10 | 1.02 | 1.00–1.05 | 0.22 | 1.01 | 0.99–1.03 |
| Donor age | 0.37 | 0.99 | 0.97–1.01 | 0.19 | 1.02 | 0.99–1.04 |
| Recipient sex | 0.73 | 1.11 | 0.62–2.00 | 0.81 | 1.08 | 0.58–2.02 |
| Seropositive CMV in recipient | 0.18 | 0.66 | 0.35–1.22 | 0.84 | 0.93 | 0.47–1.86 |
| Seropositive CMV in donor | 0.80 | 0.93 | 0.55–1.60 | 0.24 | 1.46 | 0.78–2.74 |
| Diagnosis group I1 | 0.05 | 0.52 | 0.27–1.00 | 0.16 | 0.56 | 0.25–1.26 |
| Diagnosis group II1 | 0.83 | 0.85 | 0.19–3.80 | 0.81 | 0.87 | 0.29–2.63 |
| Diagnosis group III1 | 0.91 | 1.05 | 0.47–2.36 | 0.10 | 2.17 | 0.86–5.50 |
| Donor HLA-Bw4 ligand | 0.20 | 1.45 | 0.83–2.54 | 0.89 | 1.05 | 0.54–2.02 |
| Donor HLA-C2 ligand | 0.02 | 1.90 | 1.10–3.31 | 0.02 | 2.13 | 1.13–4.02 |
- Statistically significant associations are indicated in bold. Donor C2 ligand is the only factor that remains significant for both graft and patient survival across both cohorts.
- 1Comparison of each group were made to Diagnosis Group IV.
The association of donor HLA-C group with clinical outcome was thus equivalent for both cohorts and all cases were combined into a single group for further analysis.
HLA-C group 2 alleles demonstrate a gene dosage effect on graft survival
HLA-C1 and C2 are allelic and donors who type solely as C2 are homozygous for group C2 alleles (genotype C2/C2) whereas individuals who type as C1 alone are homozygous for group C1 alleles (genotype C1/C1). The remainders are C1/C2 heterozygotes. For transplants with HLA-C2 homozygous donors, graft survival is 26.5% better at 10 years compared with transplants where the donor was homozygous for HLA-C1 alleles (p < 0.001) (hazard ratio: 7.2, 95% CI 2.2–23.0) (Figure 2A). Heterozygote donors impart an intermediate survival risk with a 14.3% reduction in graft survival at 10 years compared with HLA-C2 homozygotes (p = 0.02) (hazard ratio: 3.3, 95% CI 1.1–10.9) (Figure 2A).
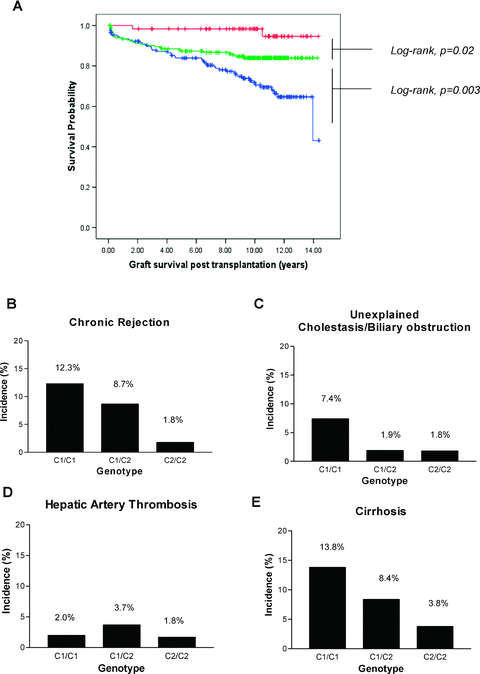
Both cohorts were combined to investigate the impact of HLA-C2 gene dosage on both long-term graft survival and histological features of chronic injury. (A) Kaplan–Meier survival curves showing significantly better graft survival for both HLA-C2 homozygotes (red line: 98.3% at 10-year survival, n = 60) and heterozygotes (green line: 84% at 10-year survival, n = 187) when compared to HLA-C1 homozygotes (blue line: 71.8% at 10-year survival, n = 169) (overall log-rank, p < 0.001). Possession of two HLA-C2 alleles (HLA-C2 homozygotes) has advantage over a single HLA-C2 allele providing proof for a gene dose effect (log-rank, p = 0.02). Histological analysis was performed for cases to correlate HLA-C group genotype with features of chronic injury including chronic rejection, unexplained cholestasis/biliary obstruction, hepatic artery thrombosis and cirrhosis (B–E). A significant protective effect is offered by the possession of HLA-C2 allele in a dose-dependent fashion against chronic rejection (C1/C1, n = 18/148; C1/C2, n = 14/161; C2/C2, n = 1/55) unexplained cholestasis/biliary obstruction (C1/C1, n = 11/148; C1/C2, n = 3/161; C2/C2, n = 1/55) and graft cirrhosis (C1/C1, n = 18/130; C1/C2, n = 12/143; C2/C2, n = 2/53) (Kendall's tau-b, p < 0.05, value –0.12). Possession of HLA-C2 allele does not protect against hepatic artery thrombosis (C1/C1, n = 3/148; C1/C2, n = 6/161; C2/C2, n = 1/55). Mean times at which biopsies were taken posttransplantation were similar between groups (B–D: C1/C1 5.7 years, C1/C2 6.1 years, C2/C2 6.3 years, ANOVA p = 0.57; E: C1/C1 6.4 years, C1/C2 6.6 years, C2/C2 6.3 years, ANOVA p = 0.80).
HLA-C group 2 alleles protect against late graft injury
Having established that donor HLA-C group genotype determines clinical outcome, we sought to investigate whether the underlying cause is due to a relationship between chronic allograft injury and donor HLA-C group genotype (Figure 2B–E). The main histological diagnoses made in specimens obtained >30 days posttransplant (n = 364) are listed in Table 6. A significant dose-dependent protective effect of donor HLA-C2 genotype was seen against the histological development of chronic rejection (Figure 2B). Chronic rejection is diagnosed through a characteristic combination of histological features including absence of bile ducts and fibrosis (Figure 3). Chronic rejection was observed in 1.8% of HLA-C2 homozygote and in 8.7% of HLA-C2 heterozygote patients compared to 12.3% in the HLA-C1 homozygous group (p < 0.05). HLA-C2 heterozygote and homozygote donors also had a lower incidence of biliary complications (Figure 2C) but no effect was seen on the development of hepatic artery thrombosis (Figure 2D). Analysis of biopsy specimens obtained >12 months posttransplant (n = 326) revealed that donor HLA-C2 genotype was associated with a dose-dependent reduction in the incidence of cirrhosis, which was observed in 3.8%, 8.4% and 13.8% of HLA-C2 homozygous, HLA-C1/2 heterozygous and HLA-C1 homozygous patients, respectively (p < 0.05)(Figure 2E).
| Histological diagnosis | Number of cases |
|---|---|
| Normal (minor abnormality) | 64 |
| Rejection | |
| 1. Acute rejection | 8 |
| 2. Chronic rejection | |
| • Early | 7 |
| • Late | 26 |
| Unexplained cholestasis/biliary obstruction | 15 |
| Vascular complications | |
| 1. Hepatic artery thrombosis | 10 |
| 2. Ischemic necrosis | 2 |
| Recurrent disease | |
| 1. PBC | 33 |
| 2. PSC | 14 |
| 3. AIH | 9 |
| 4. Hepatitis B | 5 |
| 5. Hepatitis C | 22 |
| 6. ALD | 10 |
| 7. NAFLD | 1 |
| De novo disease | |
| 1. AIH | 9 |
| 2. FLD | 11 |
| Chronic hepatitis (inflammation grade) | |
| 1. None | 113 |
| 2. Mild | 162 |
| 3. Moderate | 42 |
| 4. Severe | 9 |
| Fibrosis (stage) | |
| 1. None | 121 |
| 2. Mild | 123 |
| 3. Moderate | 50 |
| 4. Severe (cirrhosis) | 32 |
- Graft inflammation and fibrosis were determined on biopsies taken after 1-year posttransplantation (n = 326). Remaining cases of acute rejection were diagnosed on biopsies taken before day 30.
- PBC = primary biliary cirrhosis; PSC = primary sclerosing cholangitis; AIH = autoimmune hepatitis; ALD = alcoholic liver disease; NAFLD = nonalcoholic fatty liver disease; FLD = fatty liver disease.
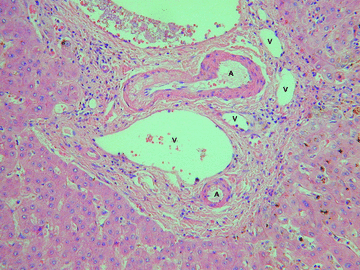
Chronic liver allograft rejection in a hepatectomy specimen obtained at retransplantation 4 years after an initial transplant operation for primary sclerosing cholangitis. This portal tract shows ductopenia (absence of bile ducts), which was evident in more than 50% of portal tracts examined. Hematoxylin and eosin. A = artery; V = vein.
HLA-C group genotype matching does not influence graft survival
The combined cohorts were then analyzed in terms of the ‘missing self’ model (35): transplants where the donor lacked a recipient KIR ligand were compared with those where the donor carried at least the same KIR ligands as the recipient. In these analyses no significant associations were found between HLA-C group genotype mismatch and long-term graft survival (p = 0.46, data not shown).
HLA-C and KIR genotypes do not influence the incidence of acute rejection
The analysis for acute rejection was performed on cohort B with complete data available for 204 cases. The incidence of acute rejection did not correlate with individual recipient KIR genotype; donor HLA-C/Bw4 genotype or HLA-C group ligand matching (Table 7). The incidence and severity of acute rejection also had no impact on long-term graft survival (Figure 4).
| Incidence of acute rejection | p-Value | |
|---|---|---|
| Recipient KIR receptor genotype | ||
| 2DL1 | ||
| Negative (n = 28) | 68% | 1.00 |
| Positive (n = 176) | 66% | |
| 2DL2 | ||
| Negative (n = 139) | 71% | 0.05 |
| Positive (n = 65) | 56% | |
| 2DL3 | ||
| Negative (n = 17) | 65% | 1.00 |
| Positive (n = 187) | 67% | |
| 3DL1 | ||
| Negative (n = 26) | 72% | 0.65 |
| Positive (n = 178) | 66% | |
| 2DS1 | ||
| Negative (n = 138) | 66% | 0.87 |
| Positive (n = 66) | 68% | |
| 2DS2 | ||
| Negative (n = 114) | 71% | 0.14 |
| Positive (n = 90) | 61% | |
| 2DS3 | ||
| Negative (n = 145) | 68% | 0.41 |
| Positive (n = 59) | 62% | |
| 2DS4 | ||
| Negative (n = 30) | 70% | 0.83 |
| Positive (n = 174) | 66% | |
| 2DS4v | ||
| Negative (n = 95) | 69% | 0.46 |
| Positive (n = 109) | 64% | |
| 2DS5 | ||
| Negative (n = 143) | 64% | 0.20 |
| Positive (n = 61) | 73% | |
| 3DS1 | ||
| Negative (n = 137) | 66% | 0.75 |
| Positive (n = 67) | 68% | |
| Recipient KIR Haplotype B | ||
| Negative (n = 70) | 67% | 0.88 |
| Positive (n = 134) | 66% | |
| Donor KIR ligand genotype | ||
| C1C1 (n = 76) | 67% | |
| C1C2 (n = 97) | 80% | 0.11 |
| C2C2 (n = 31) | 70% | |
| Bw4 negative (n = 117) | 68% | 0.55 |
| Bw4 positive (n = 87) | 64% | |
| HLA-C genotype matching | ||
| Match (n = 124) | 70% | 0.45 |
| Mismatch (n = 80) | 73% | |
- Statistics performed using Fisher's exact test for all analysis except donor KIR ligand HLA-C group genotype, which was performed using Kendall's tau-b test.
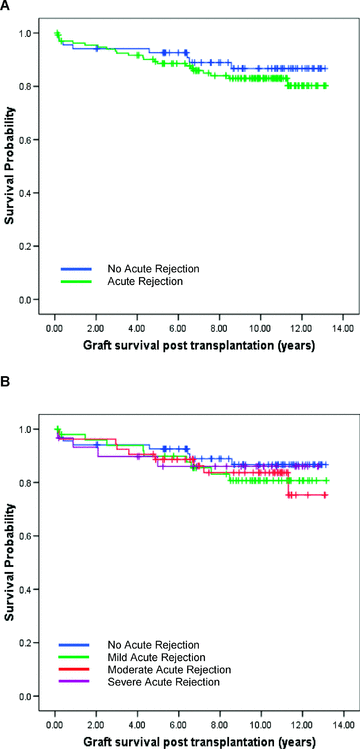
(A) The presence or absence of acute rejection following liver transplantation has no impact on long-term graft survival (log-rank, p = 0.41) (green line: grafts with AR, n = 135; blue line: grafts without AR, n = 69). (B) Varying grades of acute rejection postliver transplantation (mild, moderate or severe) has no impact on the long-term graft survival (log-rank, p = 0.84) (blue: no AR, n = 69; green: mild AR, n = 52; brown: moderate AR, n = 53; purple: severe AR, n = 30).
Discussion
We have shown that HLA-C is a critical determinant of transplant outcome: possession of at least one HLA-C group 2 allele by the donor allograft significantly improves long-term graft survival and patient survival following liver transplantation. A single HLA-C2 allele imparts a 16.2% improvement in graft survival at 10 years whereas HLA-C2 homozygous allografts have a striking 26.5% graft survival benefit compared with HLA-C1 homozygous allografts. Histological analysis showed that HLA-C2 alleles were associated with strong protection from chronic rejection and graft cirrhosis. This is the first description of a protective effect of HLA-C group against allograft injury in any form of solid organ transplantation and contrasts with the absence of any benefit from HLA matching in liver transplantation.
A number of factors are relevant for elucidating the potential mechanism for our observations. It is important to emphasize that the biological effects noted in our cohort appear to be due solely to the intrinsic properties of HLA-C2 allele expression by the allograft and are not the result of ‘missing self’ interactions as a result of allogeneic transplantation (36,37). As such, our findings add to the growing body of evidence that the two major subsets of HLA-C alleles exhibit significant differences in biological activity. This is likely to be related to the relative strength of NK cell inhibition that results from KIR binding to the different HLA-C alleles. The HLA-C2–KIR2DL1 interaction has been demonstrated to provide a stronger inhibitory signal to NK cells than HLA-C1–KIR2DL2/3 binding. This mechanism is believed to explain the clear association between host HLA-C genotype and outcome following hepatitis C virus infection (20,21). The improved rate of viral clearance observed in HLA-C1 homozygous patients compared with HLA-C2 homozygotes is thought to be a consequence of less inhibition of immune effector cells. Hiby and colleagues have also demonstrated an important role of HLA-C group alleles in reproductive success (38). Fetal expression of HLA-C2 is associated with increased risk of pre-eclampsia that is believed to reflect a strong inhibitory signaling between trophoblast and decidual NK cells serving to limit trophoblast invasion and subsequent placental vascularization. In vitro studies by Ahlenstiel and colleagues (39) also provide evidence for a functional difference in NK cell-mediated cytotoxicity based on HLA-C ligand status. In their model, T-cell-depleted peripheral blood mononuclear cells (PBMCs) were treated with influenza A virus and then exposed to autologous NK cells. They demonstrated that NK cells from HLA-C1 homozygous individuals produced significantly more IFN-γ and CD107a expression than those from HLA-C2 homozygous individuals revealing that HLA-C2 ligands were more inhibitory for NK cell-mediated cytotoxicity. We propose that expression of an HLA-C2 allele by the allograft is associated with more potent inhibition of host NK function and a consequent reduction in graft injury. As the frequency of KIR2DL1 exceeds 90% within the population, most patients transplanted with an HLA-C2 allograft should have this significant transplant survival advantage.
We found that donor HLA-Bw4 alleles, which act as ligands for the inhibitory protein KIR3DL1, do not influence transplant outcome despite their association with other disease models such as HIV (40). In addition, neither the number of activating KIR genes nor the presence of individual KIR genes was associated with altered clinical outcome in liver transplantation. HLA-C2 ligands also bind weakly with activating receptor KIR 2DS1 (41,42). In our analysis, survival benefit seen for recipients with HLA-C2 positive allografts was not influenced by the presence or absence of KIR 2DS1 in the recipient (data not shown). The lack of an effect here may relate to receptor ligand binding kinetics.
Bishara and colleagues demonstrated increased acute rejection in a study involving 66 liver transplant recipients where there was a HLA-C epitope mismatch (27). This is in contrast to our findings and may represent a type I statistical error relating to small study size. In fact, we demonstrate no relationship between recipient KIR genotypes, recipient KIR haplotype, donor HLA-C/Bw4 ligands and HLA-C genotype mismatch on acute rejection rates in liver transplantation. Furthermore, there appears to be no relationship between acute rejection rates and long-term graft survival after liver transplantation. A large study by Moya-Quiles and colleagues, involving 446 liver transplant recipients, focused primarily on the effect of a specific recipient HLA-C epitope, HLA-Cw * 07 and its influence on acute rejection rates. They demonstrated that possession of HLA-Cw * 07 by the recipient was protective against acute rejection but did not comment on the influence of donor HLA-C epitopes (28).
Our findings support a role for NK cells in long-term graft injury and outcome after liver transplantation. NK cell function recovers within an year following transplantation and is relatively resistant to conventional immunosuppression (43–45). It is noteworthy that NK cells comprise over 40% of lymphoid cells in the liver compared to around 13% in peripheral blood (46), and they have been associated with liver injury in chronic viral hepatitis (47). These observations are consistent with an important role for NK cells in allograft damage in the setting of pharmacological suppression of more classical T-cell-mediated mechanisms.
In conclusion, we have shown that KIR ligand-dependent mechanisms play a major role in long-term allograft survival after liver transplantation, and propose that NK cells play a critical role in determining the degree of chronic rejection and subsequent fibrosis in the allograft. Our observations have important implications for clinical transplantation including potential allocation of organs. A beneficial effect was observed for both heterozygous and homozygous HLA-C2 allografts, which together represent approximately 60% of transplanted organs. It might therefore be appropriate to allocate HLA-C2 homozygous allografts to high-risk recipients, such as those receiving a second transplant, in order to maximize the chance of long-term graft function. The remaining 40% of allografts will possess the HLA-C1/C1 genotype associated with impaired graft survival and this should be taken into account when planning future immunosuppressive regimens. Furthermore, our findings point the way toward novel approaches to improve long-term clinical outcome after liver transplantation through suppression of NK cell activity or pharmaceutical manipulation of HLA-C-KIR interactions.
Acknowledgments
We gratefully acknowledge support from the Department of Histocompatibility and Immunogenetics at the National Blood Service Centre, The Medical School, University of Birmingham and the University Hospital Birmingham, Birmingham, UK. Partial funding for this study was obtained from Howard Ostin Research Funds. Funding body had no role in the design or conduct of this study; the collection, analysis and interpretation of the data; or the preparation or approval of the manuscript. The authors report no conflict of interest.