A ‘Dangerous’ Group O Donor: Severe Hemolysis in All Recipients of Organs from a Donor with Multiple Red Cell Alloantibodies
Abstract
Alloimmune hemolysis is a recognized but infrequent complication of solid organ transplantation, particularly where there is incompatibility within the ABO blood group system. We describe severe hemolysis due to passenger lymphocyte syndrome (PLS) in all three recipients of organs from a single donor with multiple red cell (RC) alloantibodies. The first patient, a liver transplant recipient, required augmentation of immunosuppression to treat immune hemolysis due to anti-B, -D, -C and -Cellano (k). This is the first description of PLS caused by alloantibody to the high incidence RC antigen, k. The two single lung transplant recipients developed hemolysis due to anti-D. Both required escalation of immunosuppression and early transfusion support. Three months posttransplant, all three patients have ongoing evidence of compensated hemolysis. This series highlights the potential for severe non-ABO-mediated immune hemolysis following solid organ transplantation. A positive donor RC antibody screen should prompt careful monitoring of organ recipients for hemolysis.
Introduction
The passenger lymphocyte syndrome (PLS) refers to the clinical phenomenon of alloimmune hemolysis resulting from the adoptive transfer of viable lymphocytes from the donor during solid organ or hematopoietic stem cell (HSC) transplant (1,2). It has been most commonly reported following nonmyeloablative HSC transplant, particularly in the context of minor ABO mismatch (2,3). Rarely, the severity of hemolysis results in death despite transfusion support and supportive care (4). Hemolysis due to PLS appears to be less common following solid organ transplant (1,2), and the relative frequency of PLS appears to be related to the volume of transplanted lymphoid tissue. PLS usually results from antibodies active within the ABO and Rh systems (5–9). Rarely, it may occur due to non-ABO/Rh antibodies, particularly if the organ donor has been previously sensitized to other red cell (RC) antigens by transfusion or pregnancy (5,9,10). Where multiple organs are transplanted from a single donor, the development of PLS in two of four recipients has been reported (9). We describe series of cases where PLS developed in all three recipients of organs from a donor with multiple RC alloantibodies, including anti-k. The implications for management, including transfusion support, are discussed.
Case Series and Results
Donor
The donor was a 54-year-old male blood group O, Rh (D) negative (rr) with a positive antibody screen due to anti-D, -C and -k. The k antigen negative and RC alloantibody status were well characterized antemortem by the Australian Red Cross Blood Service (ARCBS) as he was a blood donor with this rare RC phenotype. The RC serology and human leucocyte antigen (HLA)-typing are summarized in Table 1. He had a distant history of nephrectomy, which was the likely reason for exposure and alloimmunization to homologous blood. The lungs and the liver were offered for organ donation following a cerebrovascular accident. No other organs were transplanted from this donor.
| Blood group | HLA typing | ||||||
|---|---|---|---|---|---|---|---|
| ABO | Rh (D) | K | k | −A | −B | −DRB1 | |
| Donor | O | − | + | − | 1, 3 | 7, 7 | 15 |
| Right lung recipient | O | + | − | + | 2, 29 | 8, 44 | 03, 07 |
| Left lung recipient | O | + | − | + | 1, 2 | 8, 44 | 03, 07 |
| Liver recipient | B | + | − | + | 0101, 3303 | 5701, 5801 | 0301, 1401 |
Recipient 1
A 61-year-old male with advanced emphysema and pulmonary fibrosis underwent right single lung transplant. He belonged to blood group O, Rh (D) positive [R2r], K–k+. His baseline full blood examination (FBE) was normal (hemoglobin [Hb] 150 g/L) and he had a negative RC antibody screen and direct antiglobulin test (DAT). No blood products were administered intraoperatively. Posttransplant immunosuppression included cyclosporine, mycophenolate and prednisolone.
From posttransplant day (PTD) 0 to 10, Hb fell from 126 to 69 g/L without bleeding (see Figure 1). Investigations revealed elevated hemolytic markers, a positive DAT due to IgG, and marked spherocytosis. The initial antibody screen was negative; however, anti-D (but not anti-C or −k) was detected in the RC eluate. There was no associated renal impairment or hemoglobinuria. Management included increasing the prednisolone dose to 100 mg daily, folate supplementation and transfusion of three RC units (O, Rh (D) negative [rr], k+), with a posttransfusion Hb increment to 113 g/L.
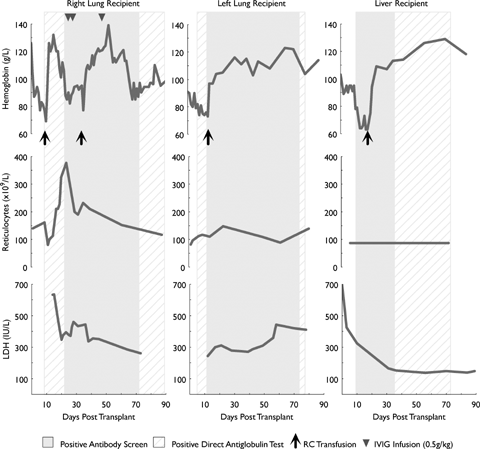
Course of hemolysis associated with passenger lymphocyte syndrome.
Ongoing immune hemolysis required a second transfusion episode at PTD 37. To facilitate tapering of corticosteroids, intravenous immunoglobulin (IVIG; 1.5 g/kg in divided doses) was administered. The patient subsequently stabilized from a hematological perspective, but required a protracted inpatient stay due to infective complications. Three months posttransplant, he is receiving 15 mg prednisolone, and has mild compensated hemolysis with a negative antibody screen, but persisting DAT positivity due to anti-D.
Recipient 2
A 59-year-old female with advanced emphysema underwent left single lung transplantation from the same donor. She belonged to blood group O, Rh (D) positive [R1r], K–k+. The preoperative FBE was normal (Hb 138 g/L) and the RC antibody screen and DAT negative. No blood products were administered intraoperatively. Baseline immunosuppression included cyclosporine, mycophenolate and prednisolone. In the absence of significant blood loss, there was a progressive fall in Hb from 91 g/L at PTD zero to 73 g/L at PTD 10 (Figure 1), necessitating transfusion with one RC unit (O, Rh (D) negative [rr], k+). Investigations were consistent with active hemolysis (including marked peripheral blood spherocytosis) and a positive DAT (IgG). The antibody screen and elution demonstrated anti-D. She received supplemental folate and an increased dose of prednisolone and made an otherwise uneventful recovery. Three months posttransplant, there is ongoing evidence of mild compensated hemolysis with persisting positive DAT positivity due to anti-D.
Recipient 3
A 45-year-old male with fulminant hepatic failure due to reactivation of hepatitis B (blood group B, Rh (D) positive [R1R1], K–k+) underwent liver transplantation from the same donor. Preoperative FBE was normal and the RC antibody screen and DAT were negative. Four units of B, Rh (D) positive RCs were administered perioperatively along with fresh frozen plasma and cryoprecipitate. Posttransplant immunosuppression consisted of tacrolimus, prednisolone and azathioprine. From PTD 0 to 13, Hb fell from 102 to 64 g/L (Figure 1) with marked spherocytosis, a positive DAT (IgG), elevated markers of hemolysis and no bleeding. The antibody screen was positive due to anti-D. A RC elution demonstrated the presence of anti-B, -C and -k in addition to anti-D. He was managed with an increase in corticosteroid dose and transfusion of two RC units (O, Rh (D) negative [rr], K+k–). Fresh k negative units were accessed through the ARCBS rare RC register. The patient subsequently stabilized, despite ongoing evidence of compensated hemolysis and seropositivity for anti-D (but not anti-k), three months posttransplant.
Discussion
PLS is a rare but recognized complication of solid organ transplantation which may result in significant hemolysis. Hemolysis can have a brisk onset and protracted natural history, despite escalation of immune suppression. In our case series, serological evidence of immune hemolysis was detectable as early as day 4 (in the liver transplant recipient) and persisted in all three organ recipients for at least 3 months. Fortunately, the period of transfusion support was short-lived, as all recipients mounted an adequate compensatory reticulocytosis.
Management strategies for PLS are anecdotal, as described in case reports and small series (1,2). Therapeutic interventions include escalation of immunosuppression, immune-modulation (e.g. by IVIG) and specifically targeting B-lymphocytes using monoclonal antibodies such as rituximab (8). Decompensated anemia usually requires provision of antigen negative RC units for transfusion. Antigen negative RC units are usually readily available when PLS is due to ABO or Rh antibodies. However, where multiple RC alloantibodies are implicated in hemolysis, antigen negative units are potentially more difficult to source, further confounded, as in this series, by the presence of antibodies to high-incidence RC antigens (e.g. Cellano [k] is expressed by 99.6% of the Australian blood donor population) (11). The possibility of multiple organ recipients requiring transfusion support with such a rare RC phenotype places considerable demand on blood services in these cases (although only one of our three organ recipients ultimately needed k-negative units).
Our series confirms the capacity for PLS to arise due to antibodies within the Rh system in the context of single lung transplantation. It is also the first known report implicating anti-k in PLS. Anti-k is a potentially clinically significant antibody which is known to cause delayed hemolytic transfusion reactions and hemolytic disease of the newborn. The contribution of anti-k antibody to hemolysis in this case series is uncertain, particularly as it was only detected in the liver recipient in the context of multiple other antibody specificities.
Although PLS occurring in half of the recipients of organs from the same donor has been described (9), in our series every organ recipient developed significant hemolysis. However, the severity of hemolysis and RC alloantibody status varied substantially between our individual patients. This clinical and laboratory heterogeneity underscores a complex biological situation. Engraftment of viable lymphocytes is facilitated by the dose of lymphoid tissue within the graft and degree of recipient immunosuppression (1,2). The importance of donor-derived memory B-lymphocytes within the transplanted organ is highlighted by this case series, as we observed hemolysis due to a variety of antibody specificities in all solid organ recipients from a single donor. Although HLA-sharing promotes lymphocyte engraftment in transfusion-associated graft versus host disease (12) and transfusion-associated microchimerism (13), it was not necessary to facilitate PLS in our transplant recipients (Table 1). Thus, PLS occurs despite the presence of HLA-disparity between donor and recipients.
We conclude that the detection of a positive RC antibody screen in a donor should prompt careful monitoring of organ recipients for the development of hemolysis. Preemptive adjuncts to immunosuppression (such as IVIG or rituximab) could be considered where an organ recipient has the potential to develop PLS against a high incidence RC antigen. In such cases, early notification of blood services may facilitate call-up of phenotyped rare RC donors and provision of compatible units.
Acknowledgment
The expertise and assistance of the transfusion scientists at the Alfred Hospital and Flinders Medical Centre is greatly appreciated.




