Identification of a New Member of the CD20/FcεRIβ Family Overexpressed in Tolerated Allografts
Abstract
We identified a novel rat gene specifically overexpressed in tolerated heart allografts in a model of tolerance induced by donor-specific blood transfusion (DST). We named this gene TORID, for tolerance-related and induced transcript. We show that TORID expression can be attributed to non-T cells infiltrating tolerated grafts. Interestingly, TORID overexpression was also observed in long-term grafts from a different model of tolerance in which chronic rejection does not occur. Comparison of the predicted amino acid sequence of TORID and of its human counterpart LR8 showed an homology with the four-transmembrane CD20/FcεRIβ family proteins. We investigated TORID expression in naive rat immune cells and lymphoid tissues. TORID was found to be preferentially expressed in cells of the myeloid lineage such as macrophages and dendritic cells (DCs). Its expression dramatically decreased following activation/maturation. Similar results were obtained in human monocyte-derived DCs. Interestingly, TORID overexpression in bone marrow-derived DCs alters expression of MHC II and CD86 and production of IL12p40 following activation. These results suggest that TORID may be involved in the control of DC maturation and may, therefore, play a role in the induction or maintenance of allograft tolerance.
Introduction
Tolerance is defined as the capacity of the immune system to avoid mounting a tissue-destructive response against a specific antigen, while maintaining its responsiveness to other antigens. Long-term morbidity and mortality in organ transplant recipients could be circumvented by tolerance induction (1). Considerable progress has been made in the experimental induction of tolerance to allogenic grafts since Billingham, Brent and Medawar's first attempts in 1953 (2). Our recent understanding of active regulation of the induction of transplantation tolerance mediated by tolerogenic dendritic cells (DCs) and regulatory T cells will hopefully give rise to significant advances (3–5). The molecular mechanisms underlying induction of tolerance, however, remain poorly understood. As cell differentiation and activation are regulated by specific differential gene expression, identification of novel genes coding for signaling proteins, transcription factors or cell surface antigens may help reveal these mechanisms.
In order to get new insights into the active regulatory mechanisms mediating allograft tolerance, we looked for differentially expressed genes in a model of tolerance induced by donor-specific blood transfusion (DST) (6,7). We identified a number of genes specifically overexpressed in allografts during induction of tolerance (8,9). We report the identification of a novel rat cDNA transcript that we termed TORID, which stands for tolerance-related and induced transcript, which corresponds to the human LR8 gene, whose function is still unknown. An extensive search for sequence homology revealed that TORID/LR8 has yet undescribed sequence and structural similarities with the CD20/FcεRIβ family proteins. Members of this newly discovered family termed MS4A (for membrane-spanning 4-domains subfamily A), are mainly expressed in cells of the hematopoietic lineage (10–12). In this study, we aimed at characterizing TORID expression and regulation. We show here that TORID overexpression is maintained in long-term tolerated allografts and is found in a different model of allograft tolerance but not in other models exhibiting chronic rejection (CR). Our results suggest that TORID expressed in immature DCs or macrophages may play a role in the induction of allograft tolerance.
Materials and Methods
Animals and transplantations
Six to 10-week-old Lewis and Sprague-Dawley rats were obtained from the Centre d'Elevage Janvier (Le Genest-Saint-Isle, France). They were housed in our animal facility under standard conditions in accordance to our institution's regulations on animal care.
LEW.1W (RT1.u) or LEW.1A (RT1.a) rats were used as blood or heart donors, and LEW.1A rats, as allograft recipients. In order to induce tolerance, allograft recipients were transfused with 1 mL of fresh donor blood 14 and 7 days prior to transplantation, as previously described (13). Heterotopic heart transplantations were performed using the Ono and Lindsey technique (14). Graft function was monitored daily by palpation through the abdominal wall and rejection was defined as the complete cessation of heart beat and confirmed by histology.
Long-term allografts from different models of tolerance induction were obtained as described (15–17). Briefly, for gene transfer into heart, recombinant adenoviruses (Ad.CD40Ig +/− Ad.RANKIg, 1–5 × 1010 IP in 150 μL) were slowly injected into the apex and ventricular walls of the heart at four different locations, at the time of transplantation. Alternatively, allograft tolerance was induced with a 20-day treatment with LF15-0195, a deoxyspergualine analogue.
Suppressive subtractive hybridization
Subtractive hybridization and differential screening were performed as previously described (9) with mRNA from tolerated (DST model) and rejected heart grafts harvested at day 5 post-transplantation, using the PCR-Select Subtraction Kit (Clontech, Palo Alto, CA).
PCR cloning of rat TORID
The rat full-length coding sequence of TORID transcript was obtained by PCR using a 5′ primer derived from the mouse sequence AB031386, including the ATG start codon ( TGCACGGCAGGAAAGATG), and a 3′ primer derived from the (rat) 2H11 clone sequence downstream of the stop codon ( GATCAGTGCCGTTCTCCCTG). PCR amplifications were carried out using the High Fidelity Platinum Taq DNA polymerase according to the manufacturer's instructions (Invitrogen, Cergy Pontoise, France), with cDNA preparations from tolerated heart allografts as template.
Rat cells
Rat cells were isolated and stimulated as previously described (18–20) and as reported in supplemental data. Purity was routinely ≥95%.
Generation and maturation of human DCs
Immature monocyte-derived DCs were generated, as previously described (21). Leukapheresis products of HLA-A2+ healthy donors were obtained through the Etablissement Français du Sang (Nantes) after obtaining the donor's informed consent. Maturation was induced by a 48-h treatment with a combination of 20 ng/mL TNFα (R&D Systems, Abingdon, U.K.) and 50 μg/mL polyinosinic-polycytidylic acid (polyI:C) (Sigma-Aldrich, St-Quentin-Fallavier, France).
Real-time quantitative RT-PCR
Total RNA from tissues or cells was prepared using the TRIzol extraction kit (Invitrogen). Real-time quantitative RT-PCR was performed as previously described (9) using a GenAmp 7700 Sequence Detection System and SYBR Green PCR Core Reagents (Applied Biosystems, Applera, Courtaboeuf, France). Oligonucleotides used in this study are reported in Table 1. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) or 18S ribosomal RNA were used as endogenous control genes to normalize for variations in the starting amount of RNA. Absolute expression, i.e. the number of copies of the TORID and HPRT RNA transcripts, was assessed by comparison with the measured fluorescence of known concentrations of purified target DNA (standards). Alternatively, relative expression was calculated using the 2−ΔΔCt method (9,22), and expressed in arbitrary units.
| Gene | Primer | Sequence |
|---|---|---|
| Rat TORID | forward | CGGAACTCCCAGGTCTTGAA |
| reverse | GATCAGTGCCGTTCTCCCTG | |
| Universal 18S | forward | AGTTCCGACCATAAACGATGC |
| reverse | CCCTTCCGTCAATTCCTTTAA | |
| Rat HPRT | forward | CCTTGGTCAAGCAGTACAGCC |
| reverse | TTCGCTGATGACACAAACATGA | |
| Human TORID/LR8 | forward | CGGAACTCCCAGGTCTTGAA |
| reverse | GATCAGTGCCGTTCTCCCTG | |
| Human IL12p35 | forward | TACCAGGTGGAGTTCAAGACCA |
| reverse | TGGCACAGTCTCACTGTTGAAA | |
| Human HPRT | forward | ATTGACACTGGCAAAACAATGCA |
| reverse | TCCAACACTTCGTGGGGTCC |
Expression of rat TORID and production of antiserum
Rat TORID cDNA obtained by RT-PCR was subcloned into the pIVEX 2.3 plasmid (NdeI-XhoI). TORID was thus expressed as a His-tagged protein using the Rapid Translation System (Roche, Meylan, France), and purified on a Ni-NTA column. A New Zealand rabbit was immunized by injection of 50 μg of recombinant TORID in the presence of complete Freund's adjuvant (Sigma-Aldrich). Booster immunizations were induced by subclavicular injection of the same amount of recombinant TORID in incomplete Freund's adjuvant.
Immunohistology Studies
Antibodies and reagents: MAbs were obtained from the European Collection of Cell Culture (Salisbury, U.K.). The anti-rat TCRαβ was purified from supernatants and coupled to Alexa 647 (AlexaFluor®647 protein labeling kit, Molecular Probes, Eugene, OR) in our laboratory. Control mouse IgG1 was purchased from Pharmingen (San Diego, CA). Alexa 568-conjugated anti-mouse antibody was purchased from Molecular Probes. The biotinylated anti-rabbit antibody and Streptavidine-DTAF was purchased from Jackson Immunoresearch (West Grove, PA).
Rat spleen sections were prepared as previously described (17). A first staining with the anti-TORID polyclonal antibody (or control rabbit antibody) was performed (step I), followed by staining with a biotinylated anti-rabbit antibody (step II), and a streptavidine-DTAF conjugate (step III). For double stainings, slides were concomitantly incubated at step II with an anti-rat leukocyte antigens MAb (or control mouse MAb), and then with an Alexa 568-conjugated anti-mouse antibody (step III). For triple staining with anti-TCRαβ, sections were incubated with control mouse IgG (step IV), and then with an Alexa 647-conjugated anti-TCRαβ MAb (step V). Dilution buffer contained 1% BSA and 10% heat-inactivated rat serum in PBS. Sections were mounted in ProLong antifade mounting medium (Molecular Probes). Fluorescence was observed by confocal microscopy (Leica TCS-SP1) as previously described (17).
Recombinant adenovirus and gene transfer into BMDCs
An adenovirus coding for TORID was constructed using the pAdEasy and pAdTrack-CMV system in 293 cells (23). The cDNA sequences for GFP and TORID were each placed under the control of the human CMV promoter. The control adenovirus consisted of GFP alone. Recombinant adenoviruses were purified and titered (infectious particles, IP) as previously described (16).
Adherent cells from cultured BMDCs were harvested on day 8 and infected with GFP or TORID/GFP adenoviral vectors at a multiplicity of infection of 500 IP/cell at 37°C for 1 h in 1% FCS medium. Cells were then washed and cultured at 1 million/mL in 6-well plates for 48 h and nonadherent cells were harvested and plated at 1 million/mL in 96-well plates (NUNC, Merck Eurolab, Strasbourg, France) with or without LPS (1 μg/mL) for an additional 48 h. Transduced cells (GFP+ cells, ≥50%) were then analyzed by FACS (FACSCalibur cytofluorometer, BD Biosciences, Mountain View, CA) for surface expression of MHC II and CD86 (B7-2). The levels of IL12p40 in the supernatants were assayed by ELISA according to the manufacturer's instructions (Pharmingen).
Sequence analysis
Amino acid sequences were aligned using the ClustalW sequence alignment program (24), and edited using the BioEdit freeware (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Phylogenetic analysis was performed using the WebPhylip program (25). Occurrence of transmembrane helices in amino acid sequences was estimated using the PHDhtm algorithm (26), available on the NPS@ server(27). Kyte-Doolittle hydropathy plots were generated (28).
Statistical analysis
Statistical analyses of gene transcript expression were performed using the nonparametric Mann-Whitney test. p-values <0.05 were considered significant.
Results
Cloning of the rat TORID cDNA and identification of orthologs
A novel partial 0.5 kb rat cDNA (clone 2H11) was isolated after screening of the subtraction library of the tolerated allograft. A sequence analysis was performed using the BLAST program (29). Although no corresponding rat cDNA was found, striking homologies with the 3′ ends of two identical mouse transcripts, LR8 and Clast1, and a human transcript known as LR8 were found. We cloned the rat full-length coding sequence by PCR and termed it TORID, which stands for tolerance-related and induced transcript (GenBank accession number: AF370882, reference for NM_134390). Amino acid sequence alignment of the predicted sequence of the rat TORID (263 aa), the mouse LR8/Clast1 (263 aa) and the human LR8 (270 aa) proteins revealed an overall identity of ∼50%, whereas 78% identity was found between rat TORID and mouse LR8/Clast1 (supplemental data Figure S1). This strongly suggests that TORID and LR8 are orthologs. BLAST analysis on genome databases revealed that human, rat and mouse TORID/LR8 genes are located on chromosomes 7q36.1, 4q24 and 6B2.3, respectively (Table 2). LR8 mRNA was found to be differentially expressed in a human lung fibroblast subpopulation expressing the receptor for the collagen domain of the C1q molecule (cC1q-R) (30), but sequence homology and function had never been investigated before.
| Human | Rat | Mouse | ||||
|---|---|---|---|---|---|---|
| Locus | Gene name | Locus | Gene name | Locus | Gene name | |
| TORID / LR8 | LR8 | TORID | 1810009M01Rik | |||
| 7q36.1 | 4q24 | 6B2.3 | ||||
| HCA112 | HCA112 | LOC297077 | 0610011I04Rik | |||
| MS4A family | 11q12 | 1q43 | 19A | |||
Expression of TORID mRNA in grafts
Real-time quantitative PCR analysis revealed that large amounts of TORID mRNA progressively accumulate from day 1 to day 5 in tolerated allografts but not in rejected allografts or syngeneic grafts (Figure 1A). TORID was still highly expressed in tolerated allografts 40 days after transplantation. This overexpression was also observed in graft-infiltrating cells (GICs) of DST-treated compared to untreated recipients (Figure 1B). Interestingly, T-cell-depleted GICs from tolerated grafts maintained high TORID expression (Figure 1B). This suggests that TORID is expressed in non-T cells that specifically infiltrate tolerated grafts during the induction phase of tolerance.
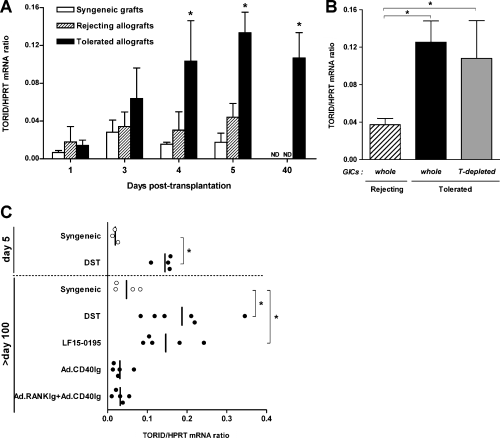
TORID mRNA expression in heart grafts. TORID mRNA expression was assessed by real-time quantitative PCR and results are expressed as TORID to HPRT mRNA copy ratios. (A) TORID mRNA expression in syngeneic and allogeneic rejecting and tolerated grafts harvested at different time points after transplantation. Allogeneic grafts were rejected within 6.8 ± 1.6 days. Tolerance is induced by donor-specific blood transfusion (DST). Results represent the mean ± SD of 3–5 animals in each group and time point (*p < 0.05, tolerance vs. rejection group). (B) TORID mRNA expression in purified GICs from rejected (n = 5) and tolerated (n = 4) heart allografts, and in T-depleted GICs from tolerated allografts (n = 4) on day 5 after transplantation (*p < 0.05). (C) TORID mRNA expression in long-term (days 100–120) syngeneic grafts (open circles) or allografts (closed circles) from recipients treated with DST, LF15-0195 or CD40Ig encoding recombinant adenovirus either alone or combined with RANKIg adenovirus (*p < 0.05).
Long-term allografts (>day 100) still express high levels of TORID mRNA (Figure 1C). However, in this model, allografts display some signs of CR (K. Renaudin, R. Josien, unpublished data). We therefore assessed TORID expression in different models of partial or true tolerance (allografts with no signs of CR). In a model of true tolerance induced by a short term treatment with a deoxyspergualine analogue, LF15-0195 (15,31), we observed high TORID mRNA expression in long-term allografts. On the contrary, very low amounts of TORID were detected in costimulatory blockade models displaying strong to moderate signs of CR (16,17). These results show that TORID expression is not associated with CR and suggest that it could be involved in tolerance processes common to distinct models.
TORID/LR8 and HCA112 belong to the CD20/FcεRI βfamily
We performed a protein sequence analysis using the PSI-BLAST program (32), with the human TORID/LR8 as the query sequence. A great similarity (28.5% identity) was found with a protein termed HCA112 (hepatocellular carcinoma-associated antigen 112). HCA112 has been cloned and identified as a human hepatocellular carcinoma-associated antigen (33). It has also been identified with immunity-related genes in mouse kidney proximal tubules exposed to proteinuria, and was referred to as GS188 (34). The HCA112 gene is located in the immediate vicinity of LR8 in the human genome, and so are its rat and mouse orthologs (Table 2). Furthermore, both genes have a similar intron/exon organization. TORID/LR8 and HCA112 may therefore be paralogs due intragenome gene duplication.
Further analyses with PSI-Blast (>2 iterations) revealed previously undescribed significant similarities with members of the MS4A family (CD20/FcεRIβ family). In human, rat and mouse genomes, TORID/LR8 and HCA112 genes are located at a different locus than that of the MS4A family cluster (Table 2). Sequence analysis of the TORID/LR8 protein and its putative human homologs was conducted. Comparison of the amino acid sequences of TORID/LR8 and HCA112 with the 12 known MS4A family members showed that they were similar in size and structure, with four predicted transmembrane domains, and had 10–20% identity (Figures 2A, 3C, D). A phylogenetic tree (Figure 2B) illustrates amino acid sequence conservation. As described with MSA4 proteins (10–12), the highest degree of identity was observed in the first three transmembrane domains (Figure 2A). It is important to mention that TORID/LR8, HCA112 and MSA4 family members have a similar intron/exon organization (Figure 2A). Despite the fact that the chromosomal location of these genes is different, the aforementioned similarities suggest that TORID/LR8, HCA112 and MSA4 family members evolved from a common ancestor; they should therefore be considered homologs.
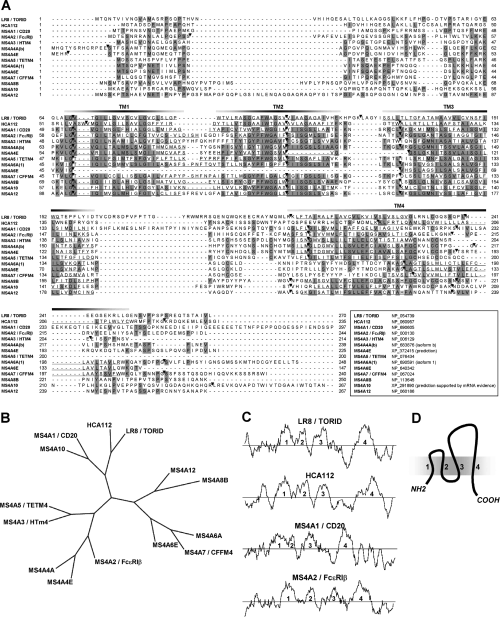
Comparison of the amino acid sequence of TORID/LR8 and HCA112 with that of MS4A family members. (A) Amino acid sequence alignment of the human TORID/LR8, the HCA112 and the 12 known human MS4A family proteins. Accession number is provided for each sequence. Amino acids common to four or more proteins are shaded. The four predicted transmembrane regions (TM1-4) are underlined. Intron/exon junctions are indicated by black triangles. (B) Maximum-parsimony phylogenetic tree of the human TORID/LR8, HCA112 and MS4A family members. An unrooted cladogram was generated following multiple alignment. (C) Comparison of hydropathy profiles of the predicted human amino acid sequences for TORID/LR8, HCA112, CD20 and FcεRIβ, using the Kyte-Doolittle hydrophobicity analysis. A window of 10 residues was used. Numbers (1–4) indicate segments showing peak hydrophobicity which correspond to the four predicted transmembrane regions. (D) Schematic representation of TORID/LR8, HCA112 and MS4A family proteins with their four membrane spanning regions.
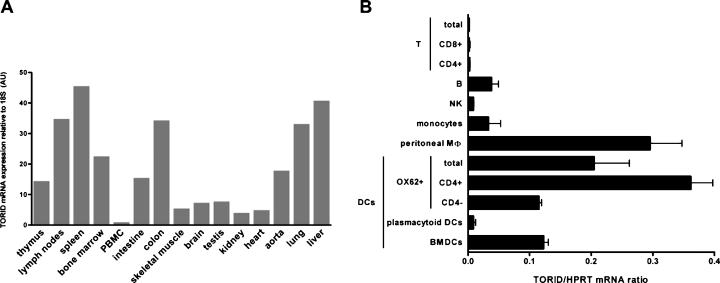
Expression of TORID mRNA in rat tissues and immune cells. (A) Expression of TORID mRNA in cDNAs generated from RNA extracts from of various rat tissues was assessed by real-time quantitative PCR. TORID mRNA levels were normalized with 18S rRNA and expressed in arbitrary units (AU). PBMC, peripheral blood mononuclear cells. (B) Expression of TORID mRNA in cDNAs generated from mRNA extracts from freshly isolated leukocytes, excluding bone-marrow derived DCs (BMDCs) was assessed by real-time quantitative PCR. Results are expressed as TORID to HPRT mRNA copy ratios and represent the mean ± SD of two independent experiments in each cell subset.
TORID mRNA distribution in naive rat tissues and leukocytes
Expression of TORID mRNA was analyzed in tissues from naive rats by real-time quantitative PCR (Figure 3A). The highest expression was detected in the spleen, lymph nodes, colon, lungs and liver, and to a lower extent, in the thymus, bone marrow, intestine and aorta. Relatively lower expression levels were observed in other tissues. TORID mRNA therefore appears to be preferentially expressed in lymphoid tissues.
We next analyzed expression of TORID mRNA in different populations of freshly isolated leukocytes (Figure 3B). The highest expression was found in DCs and peritoneal macrophages. Among the different DC subsets described in the rat (18,19,35), the one in which expression of TORID mRNA was the highest was the CD4+ subpopulation; expression of TORID mRNA in these cells was three times as high as that in CD4− DCs. Similar mRNA levels were found in BMDCs and CD4− DCs. Contrastingly, virtually no expression was detected in plasmacytoid DCs. Low levels of TORID mRNA were found in monocytes and B cells. Almost no expression could be detected in other cells of the lymphoid lineage, such as NK cells and different subsets of T cells. In summary, expression of TORID mRNA was predominantly observed in certain cells of myeloid origin, and the highest expression levels were found in CD4+ DCs and peritoneal macrophages.
Expression of the TORID protein in the spleen
We generated a rabbit anti-TORID polyclonal antibody raised against the recombinant TORID-His-tag fusion protein, produced in a cell-free translation system. We confirmed that this antibody could stain 293T cells transfected with TORID, but could not stain nontransfected 293T cells (data not shown). Detection of anti-TORID-stained cells by flow cytometry, however, failed. We investigated TORID distribution in vivo by immunostaining rat spleen sections. Staining arterioles was considered nonspecific (Figure 4A). Triple staining with anti-TCRαβ (blue) and anti-MHC II (red) revealed that TORID-positive cells (green) predominantly localized in the red pulp, and to a lesser extent, in the periarteriolar lymphoid sheath (PALS), in the marginal zones, and B-cell follicles in particular (Figure 4B–D). Positive cells exhibited a perinuclear ring-like staining pattern, which indicates a localization to the nuclear envelope, as confirmed by immunofluorescence analysis of isolated macrophages and BMDCs (supplemental data Figure S2). Virtually all T and B cells were negative for TORID. As they all expressed CD45, TORID+ cells were considered leukocytes (data not shown). In the red pulp, staining for TORID and MHC II appeared to be mainly mutually exclusive. Contrastingly, in B-cell follicles and marginal zones, coexpression, i.e. high expression of MHC II and intracellular localization of TORID, was seen in many cells (Figure 4D). Such cells appeared to be morphologically different from B lymphocytes. The rare TORID+ cells observed in areas rich in T cells were generally MHC II−, although a number of double-positive cells were also detected. The distribution of TORID+ cells was compared to that of SIRPα+ (CD172a, myelomonocytic cells) and CD68+ (macrophages and DCs) cells. Although most TORID+ cells were negative for both markers, some cells localized to the red pulp clearly coexpressed SIRPα and CD68 (Figure 4E, F). TORID and SIRPα colocalized in a limited number of cells (Figure 4E). Likewise, some TORID+ cells coexpressed OX62 (CD103), a rat DC subpopulation marker (data not shown). Very few TORID+ cells were observed in lymph nodes, compared to the spleen, and the latter were essentially localized in the subcapsular sinus (data not shown). The observed staining pattern indicated that TORID was preferentially expressed in myeloid cells, and while not at all in lymphoid cells. TORID expression was, however, not strictly linked to one of the antigens that we used, and therefore cannot be associated with any specific cell subset. These results therefore suggest that TORID may be expressed in a variety of myeloid cell subpopulations disseminated in the spleen at different stages of differentiation, which may include particular macrophages and DCs.
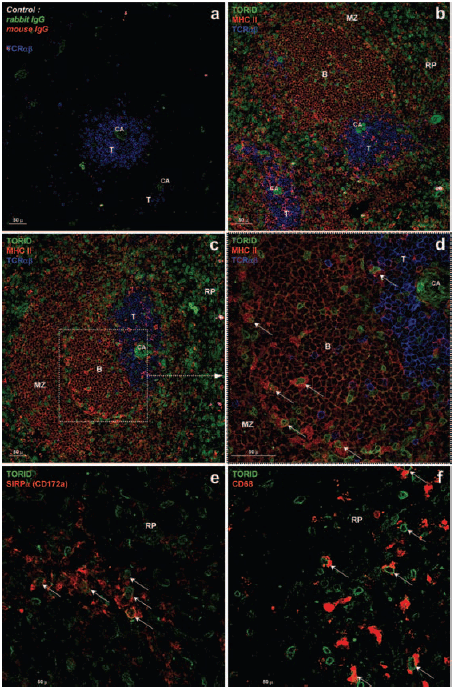
Immunohistological characterization of TORID expression in spleen tissue sections. Frozen rat spleen sections were sequentially stained with anti-TORID rabbit polyclonal antibody, biotinylated anti-rabbit antibody and streptavidine-DTAF conjugate (green). Triple staining (A–D) included staining with an anti-MHC II MAb and an Alexa 568-conjugated anti-mouse antibody (red), and a staining with an anti-TCRαβ MAb and an Alexa 647-conjugated anti-mouse antibody (blue). Fluorescence was analyzed by confocal microscopy. Central arteriole (CA) staining with anti-TORID is non specific as staining is also observed with the rabbit isotype-matched antibody (A). Roughly, areas rich in T cells (T), which appear in blue, are surrounded by B follicles (B) and marginal zones (MZ), in red (B–D). An enlarged view of the white pulp is shown in D. Most of the TORID-positive cells (green) are located in the red pulp (RP), although these cells with or without high expression of MHC II (in B follicles, arrows) are also found in the white pulp (D). Double staining was performed using an anti-SIRPα (E) or an anti-CD68 (F) and an Alexa 568-conjugated anti-mouse antibody (red). TORID-positive cells coexpressing SIRPα or CD68 are indicated (arrows).
Expression of TORID/LR8 mRNA is drastically downregulated during activation
In order to investigate the regulation of TORID expression during activation, rat peritoneal macrophages were stimulated with LPS in vitro. As shown in Figure 5A, expression of TORID mRNA dramatically decreased as early as 6 h after stimulation with LPS, and was still low at 24 h. In freshly isolated splenic DCs expression of TORID was likely downregulated 2 h after stimulation with CD40L, and was unchanged at 5 h and 24 h (Figure 5B). An identical decrease was observed in DCs stimulated with LPS or polyI:C (data not shown).
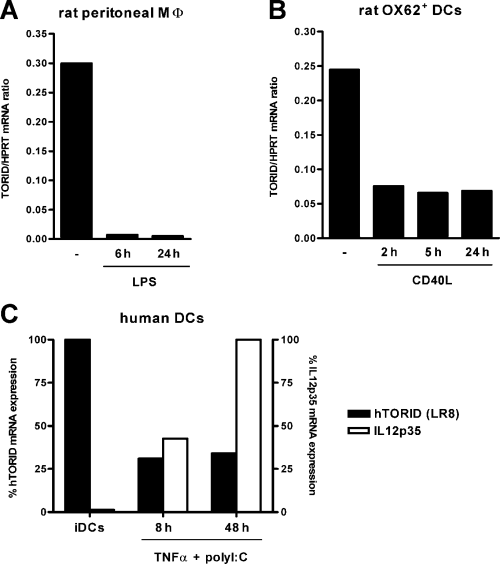
TORID regulation in rat and human cells. (A–B) Fresh rat peritoneal macrophages (A) or purified OX62+ DCs (B) were cultured in the presence of LPS or CD40L, respectively. Cells were harvested for RNA extraction after the indicated times of stimulation, and expression of TORID mRNA was assessed by real-time quantitative PCR. Results are expressed as TORID to HPRT mRNA expression ratios. (C) Human immature monocyte-derived DCs (iDC) were cultured in the presence of TNFα and polyI:C. Cells were harvested for RNA extraction after 8 h and 48 h. Relative expression of human TORID (LR8) and IL12p35 mRNA, normalized with HPRT, were assessed by real-time quantitative PCR (arbitrary units: 100% represents maximum expression for each gene). Each panel of the figure represents results from one of three experiments.
Interestingly, human monocyte-derived DCs also expressed the human TORID homolog (Figure 5C). In order to determine whether rat and human DCs exhibit the same regulation, human DCs were stimulated with TNFα and polyI:C. As shown in Figure 5C, while IL12p35 mRNA progressively accumulated during activation, hTORID was markedly downregulated as early as 8 h after stimulation. This downregulation could still be observed at 48 h.
Taken together, these results show that expression of TORID mRNA is inversely related to the activation or maturation of rat macrophages and DCs as well as human DCs.
TORID overexpression alters BMDCs maturation
To evaluate the effect of TORID overexpression on BMDC maturation, recombinant adenoviruses coding for TORID and GFP or, as a control, GFP alone, were used. BMDCs transduced with TORID were found to express lower levels of MHC II (88%, Mean Fluorescence Channel (MFC): 2052) and CD86 (45%, MFC: 881) than BMDCs transduced with the control adenovirus (98%, MFC: 2935 and 78%, MFC: 852, respectively) (Figure 6A). Stimulation with LPS had no effect on the immature phenotype of TORID-transduced BMDCs in contrast to control transduced BMDCs where MHC II and CD86 increased. In addition, stimulated BMDCs transduced with TORID produced less IL12p40 than those transduced with the control adenovirus (Figure 6B). TNFα and IL10 production was also decreased (data not shown). In this assay, cytokines were measured in the supernatants of whole cell cultures, which suggests that the decrease in cytokine production could have been more dramatic if it had been assayed in TORID transduced cells only.
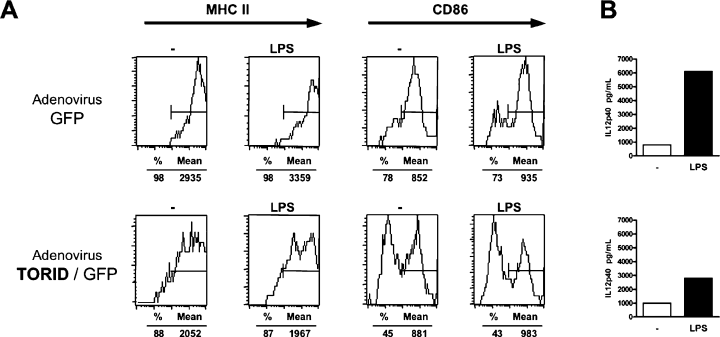
Effect of TORID overexpression in BMDCs. BMDCs were transduced with a control GFP adenovirus or a TORID/GFP adenovirus. Forty-eight hours after the transduction, nonadherent cells were replated and stimulated or not with LPS (1 μg/mL). Forty-eight hours later, (A) expression of MHC II and CD86 (B7-2) by transduced cells (based on expression of GFP) was assessed by flow cytometry and (B) IL12p40 levels in supernatants were determined by ELISA. This figure is representative of three independent experiments.
These results strongly suggest a role of TORID in the maturation of DCs.
Discussion
We cloned a novel rat cDNA overexpressed in tolerated heart allografts in DST-treated recipients, which we named TORID, for tolerance-related and induced transcript. In this model, tolerance is an active process characterized by the presence of large amounts of infiltrating leukocytes in the allograft. These leukocytes express very low levels of Th1- or Th2-related cytokines (36) and high levels of TGFβ, which plays a critical role in this tolerance induction process (7). We show that TORID is overexpressed in non-T cells infiltrating tolerated allografts during the induction phase of tolerance. TORID overexpression is maintained both in long-term allografts from DST-treated recipients and in a different model of tolerance in which CR does not occur, and is not detected in chronically rejected allografts (Figure 1C). In addition, we observed a significant increase of TORID expression in long-term kidney allografts with no signs of CR from recipients treated with either anti-MHC II or anti-CD28 antibodies (data not shown). These results strengthen the hypothesis that TORID plays a role in active tolerogenic mechanisms.
We show that TORID, which corresponds to the human LR8 gene, is related to members of the CD20/FcεRIβ family (membrane-spanning 4-domains subfamily A, MS4A). Common features of this family include a predicted size of ∼150–300 amino acids, four potential membrane spanning domains with the highest degree of identity in the first three domains and a similar intron/exon organization. The chromosomal location of TORID/LR8 and HCA112 genes, however, is different from that of the MS4A family genes. As genes of the MS4A family evolved from a common ancestor and are the result of a series of successive duplications, one duplicated segment may have likely translocated to another chromosome and produced the TORID/LR8 and HCA112 genes through an additional local duplication. The MS4A gene family first comprised the CD20 gene (MS4A1), the high-affinity IgE receptor β chain (FcεRIβ, MS4A2) and the HTm4 gene (MS4A3). Nine novel human genes of this family, and their mouse counterparts, were recently identified (10–12,37,38), although no functions have been reported so far for these proteins. Expression of the MS4A family members is found in various tissues however most of them are expressed in tissues rich in lymphoid cells, and especially in subpopulations of hemotopoietic cells. Despite their similar structure, notably, with regard to the transmembrane domains, MS4A family members, as well as TORID/LR8, may likely have different functions in hematopoietic cells.
We showed that TORID mRNA is predominantly expressed in immature/unactivated myeloid cells, including macrophages and DCs. Such a TORID expression pattern in naive cells is consistent with the high expression of TORID observed in the non-T GICs of tolerant recipients (Figure 1B), which are mostly monocytes/macrophages/DCs (39). However, TORID is not expressed at the same level in all myeloid cells, but rather in particular types of differentiated myeloid cells like CD4+ DCs. Immunohistological analysis of the spleen confirmed that TORID was not expressed in lymphoid cells such as T and B cells. Conversely, the fact that TORID is preferentially expressed in cells located in the red pulp and in CD45+ cells, and the fact that numerous TORID+ MHC II+ high cells are found in PALS, is consistent with the expression of TORID in cells of the myeloid lineage. Furthermore, we observed that several TORID+ cells coexpressed the myeloid markers CD68 or SIRPα. No precise phenotype was, however, defined as all cells were not stained. In areas rich in T cells, TORID+ cells likewise occasionally expressed MHC II. Interestingly, in primary B-cell follicles, the distribution of TORID was reminiscent of the follicular dendritic cell network or ‘tingible body’ macrophages (40,41). In marginal zones, TORID could be expressed by specialized macrophages, which are extremely potent phagocytic cells (42). Furthermore, in this region directly exposed to the circulating blood, TORID+ cells could also correspond to marginal zone DCs, which have been shown to actively internalize dying cells under steady-state conditions, and thus may participate in induction and maintenance of peripheral T-cell tolerance (43,44). Collectively, these results show that TORID is expressed by a variety of myeloid cell subpopulations disseminated in the spleen, and/or subsets of cells at different stages of differentiation, which could include particular macrophages and DCs with phagocytic and antigen presentation capacities.
We demonstrated that TORID expression strongly decreases following stimulation of rat macrophages or DCs and human DCs. Therefore, considering the fact that its downregulation occurs early after activation, we can hypothesize that TORID is more likely to be involved in functions such as regulation of antigen uptake or initiation of differentiation in immature antigen presenting cells, than in direct interactions of mature antigen presenting cells with T cells. In this regard, forced expression of TORID in BMDCs altered the expression of MHC II and CD86 and decreased IL12p40 production following stimulation. These results strongly suggest that TORID could control/maintain the immature state of BMDCs. The fact that it is mainly expressed in immature APCs, suggests that TORID most likely promotes tolerogenic presentation of antigens and does not induce an immune response, as described for certain tolerogenic DCs that induce self-tolerance or tolerance to alloantigens (4). Phenotypic analysis of TORID-expressing cells in tolerated grafts would have been very informative, however, we failed to obtain specific staining of heart sections with the anti-TORID polyclonal antibody.
In conclusion, we have identified a novel member of the MS4A family, which is preferentially expressed in DCs and macrophages, and appears to regulate their maturation. Further characterization of TORID-expressing cells in tolerated grafts will provide direct information about TORID's functions in allograft tolerance.
Acknowledgments
We are grateful to Marc Grégoire for providing human DCs, to Marina Anger and Claire Usal for excellent technical assistance, and to the Vector Core of the University Hospital of Nantes, supported by the Association Française contre les Myopathies (AFM), for producing adenovirus used in this study. We thank Claude Godin for his technical support for TORID production and immunization. We are indebted to Francois-Xavier Hubert, Benjamin Trinité, Cécile Voisine and Régis Josien for help with cell isolation. We thank Jeffrey Bluestone for his helpful comments, and Isabelle Brisson for her great help in editing the manuscript.
This work was supported by a grant from the ‘Association pour la recherche contre la mucovicidose’, and by the ‘Fondation PROGREFFE’. CL was supported by a fellowship from the ‘Ministère de l'Enseignement Superieur et de la Recherche’ and by the ‘Association pour la Recherche contre le Cancer’. EC was supported by the ‘Association pour la Recherche contre le Cancer’.