αGal Ligation of Pig Endothelial Cells Induces Protection from Complement and Apoptosis Independently of NF-κB and Inflammatory Changes
Abstract
Cytoprotection of endothelial cells (EC) is important in EC biology and pathophysiology, including graft rejection. Using porcine aortic EC and human complement as an in vitro model of xenotransplantation, we have reported that ligation of EC Galα(1–3)Gal epitopes (αGal) with antibodies or lectins BS-I and IB4 induces EC resistance to injury by complement. However, before the protective response is observed, αGal ligation induces an early, proinflammatory response. Using a similar model, we now investigated whether the early inflammatory response, as well as NF-κB activation, is required for induction of cytoprotection. Despite up-regulation of EC mRNA for many inflammatory cytokines rapidly after BS-I stimulation, recombinant cytokines or conditioned media from EC incubated with BS-I failed to induce protection when used to stimulate EC. While the lectin-induced inflammatory response was markedly reduced by inhibition of NF-κB, the protection from complement and apoptosis was unaffected. The lectins caused up-regulation of mRNA for protective genes A20, porcine inhibitor of apoptosis protein and hemoxygenase-1, which was not modified by NF-κB inhibition. These findings suggest that induction of cytoprotection in porcine EC by αGal ligation results from activation of pathways that are largely independent of those that elicit NF-κB activation and the inflammatory response.
Introduction
As part of tissue homeostasis, several mechanisms have evolved that contribute to promotion of apoptosis or activation of the complement system. Apoptosis and complement activation are controlled by inhibitory proteins intrinsic to their respective pathways that contribute to cell protection from unwanted injury. Additionally, other substances, such as cytokines, may facilitate or impede apoptosis or complement-mediated injury. Often, however, regulation may be inappropriate and play an important pathophysiologic role in disease states. In certain cases, it may be beneficial to promote selected protective mechanisms within cells or tissues to enhance their survival in a hostile environment. For example, ischemia-reperfusion injury that occurs after regional ischemia or solid organ transplantation has been ameliorated by activating the target tissues to express a protective phenotype (1,2). In certain settings of organ transplantation, induction of protective genes within cells of an organ graft may be central to the survival of that organ under conditions that would otherwise lead to its rejection, a process known as accommodation (3,4). Accommodation can often take place in ABO-incompatible renal allografts (5) and has been readily achieved in rodent models of xenotransplantation (4,6); however, it has been difficult to achieve in porcine organs transplanted into primates (7). As the pig is considered the donor of choice for xenotransplantation in humans, it is important to understand the mechanisms that may result in cytoprotection in donor tissues, as cytoprotection may possibly facilitate the development of accommodation following transplantation.
One approach to study cytoprotection that may be relevant to pig-to-human transplantation consists of the analysis of the response of cultured porcine endothelial cells (EC) to stimulation with various agents (8–11). Using this approach, we have reported that ligation of Galα(1–3)Gal epitopes (abbreviated αGal) on porcine EC with either Abs or the αGal-binding lectins BS-I or IB4 induces resistance of the EC to injury by the membrane attack complex of human complement (8,11). However, before the protective phenotype was observed several hours after stimulation, αGal ligation rapidly induced a proinflammatory response. This initial proinflammatory response consists of reorganization of actin filaments, cell retraction, formation of intracellular gaps, activation of p42/44 MAP kinase and NF-κB, up-regulation of genes for adhesion molecules and IL-8 (11–13) and loss of adhesion molecules CD31 and VE-cadherin from cell junctions (14). Thus, activation of porcine EC by αGal ligation results in a rapid proinflammatory response, which is followed by a delayed protective response. The inflammatory response lasted less than 24 h and thereafter, when the cells exhibited the highest degree of protection, the morphological appearance, actin organization and E-selectin expression reverted to those of controls (11).
We previously reported that these two cellular responses, proinflammatory and protective, may overlap for several hours (11). Their exact relationship, however, has yet to be defined. Therefore, we investigated whether products generated in the early proinflammatory response participated in the induction of the later protective response in EC stimulated with αGal-binding lectins. We first established the time course of mRNA expression for cytokine and chemokine genes in EC treated with αGal-binding lectins. We then tested whether some of the expressed inflammatory cytokines or conditioned medium from EC cultures stimulated with αGal-binding lectins would induce cytoprotection in naïve cells. Finally, since the proinflammatory response is largely NF-κB-dependent (13), we investigated whether EC would develop resistance to killing by human complement and to apoptosis induced with TNF-α plus cycloheximide (CHX) when stimulated with lectins in the presence of NF-κB inhibition. Our studies demonstrate that protection from complement and apoptosis induced in porcine EC by αGal ligation develops independently of the inflammatory response and of NF-κB activation.
Materials and Methods
Reagents
DMEM, RPMI-1640, L-glutamine, FBS, TRIzol and antibiotics were purchased from Life Technologies (Invitrogen, Grand Island, NY). B. simplicifolia lectin I (BS-I) and B. simplicifolia I isolectin B4 (IB4) were obtained from Vector Labs (Burlingame, CA). Porcine rTNF-α and IL-8 were purchased from Biosource International (Camarillo, CA), IL-1α and IL-1β were from R&D Systems (Minneapolis, MN) and IL-6 was from Endogen (Woburn, MA). SN50 and ISN50 were purchased from Calbiochem (LaJolla, CA). A mouse mAb against porcine E-selectin was kindly provided by Martyn Robinson (Celltech, Berks, UK). Affinity-isolated, HRP-conjugated, goat anti-mouse IgG and Fluoromount-G were from Southern Biotechnology Associates (Birmingham, AL), and 3,3,3′,3′-tetramethylbenzidine was from Pierce (Rockford, IL). Neutral red, phalloidin-tetramethylrhodamine isothiocyanate (TRITC), LPS (Escherichia coli O127) and general chemicals were from Sigma (St. Louis, MO).
Endothelial cells
EC were explanted from pig aortae, cultured and identified as previously described (11). The cells were maintained in DMEM containing L-glutamine, gentamicin, penicillin/streptomycin and 10% FBS. Experiments were performed with cells at passages 4–9 that were 1–2 days post-confluent, in gelatin-coated tissue culture plates, as follows: 96-well flat-bottomed plates for ELISA, 48-well plates for cytotoxicity and 24-well plates for apoptosis (Costar, Cambridge, MA), 150 mM dishes for RNA isolation and 8-well chamber slides for cellular morphology and confocal microscopy studies (Becton Dickinson, Franklin Lakes, NJ). All incubations were carried out at 37°C in a 5% CO2 atmosphere.
Treatment of EC with lectins and cytokines
EC were preincubated with lectins or cytokines in DMEM containing 1% FBS (100 μL/well for ELISA, 250 μL/well for complement-mediated cytotoxicity, 500 μL/well for apoptosis, 25 mL/dish for RNA isolation, or 250 μL/well for microscopic studies) for different time periods, as indicated. After this preincubation, the lectin or cytokine solution was removed and the cells were washed twice with DMEM–1% FBS. In some experiments, the same volume of medium as used in the previous step was added and incubation continued for various time periods. The supernatants were then removed, the EC were prepared for treatment with human serum or various analyses, and cell viability was assessed by neutral red uptake (15), as validated before by microscopic cell count following lectin treatment (11); the number of viable EC was within ±6.0% of EC treated with medium alone.
Complement-mediated cytotoxicity and apoptosis
Complement cytotoxicity was assessed in pretreated EC after two washes with RPMI-1640. The EC were incubated for 2 h with 150 μL of a human serum pool (8) diluted in RPMI-1640 as a source of anti-pig Abs and complement. EC cytotoxicity was measured with a vital dye assay, as described previously (11). Percent specific cytotoxicity was calculated after correction for the OD of solubilized cells not treated with the dye solution as follows: % cytotoxicity ={1 − (test OD/control OD)}× 100. Apoptosis was induced in pretreated, washed EC by incubation with porcine TNF-α (20 ng/mL) and CHX (3 μg/mL) during 3 h, and measured by flow cytometry to analyze the population distribution of EC DNA content, as previously described (16). Values are given as mean ± SE of triplicate samples.
Assessment of mRNA expression by Northern blotting and RNase protection assay
Total RNA was isolated from EC cultured in 150 mM dishes at various times following incubation with 25 mL of 1% FBS–DMEM with or without 50 μg/mL IB4 or 100 μg/mL BS-I. RNA isolation and Northern blot analysis were performed as described earlier, using 10 μg RNA (11). The blots were hybridized as previously described (17) using gel-purified porcine cDNA inserts. The amounts of RNA in loaded samples were normalized by hybridization with a β-actin cDNA insert (18). The amount of probed mRNA and β-actin signal was determined from densitometry using NIH Image. EC cytokine and chemokine expression was examined using RiboQuant multiprobe porcine RNase protection assay kits (pCK, BD-PharMingen, San Diego, CA). These kits include probes for the housekeeping genes L32 and GAPDH to serve as internal controls. Probes were labeled using [α-32P] UTP (Amersham, Piscataway, NJ), resulting in an average specific activity of 1 × 106 cpm/μg. The RNase protection assay was carried out according to the manufacturer's protocol using 20 μg total RNA. Normal porcine spleen and liver and yeast RNA were used as controls.
Expression of E-selectin
Endothelial cells were preincubated with 1% FBS–DMEM or 1% FBS-DMEM containing 50 μg/mL SN50 or ISN50 during 1 h. Following preincubation, 50 μg/mL IB4, 100 μg/mL BS-I or 100 ng/mL LPS, with the corresponding pretreatment reagent, was added to respective wells and incubation continued for 5 h. The EC were then processed for ELISA as described earlier (11). Nonspecific binding of mouse IgG was assessed with purified mouse IgG1 myeloma protein (Harlan Bioproducts, Indianapolis, IN) and these values were subtracted from those with the anti-E-selectin mAb.
Immunofluorescence localization of actin filaments and NF-κB
Endothelial cells were preincubated with 1% FBS–DMEM, 50 μg/mL SN50 in 1% FBS–DMEM or 50 μg/mL ISN50 in 1% FBS–DMEM during 1 h using an 8-well chamber slide. Then the EC were incubated with 100 μg/mL BS-I in 1% FBS–DMEM or 1% FBS–DMEM alone. For actin filament visualization, the supernatants were removed at 4 h and the EC were processed, stained with TRITC and visualized as previously described (11). For NF-κB localization the supernatants were removed at 90 min and the EC were fixed, permeabilized, stained and visualized as previously described (19), using a rabbit polyclonal Aby against the p65 subunit of NF-κB (Chemicon, Temecula, CA), FITC-conjugated goat anti-rabbit IgG Aby (Sigma), and propidium iodide.
Transduction of porcine EC with dominant-negative adeno-IκB
IκBα adenovirus was purchased from Imgenix (San Diego, CA). Recombinant control adenoviruses lacked the IκB insert (Imgenix) or expressed β-galactosidase (LacZ) (BD-Bioscience, San Diego, CA). For some experiments, an adenovirus expressing IkBalphaM (Stratagene, La Jolla CA) was constructed by cloning the IkBalphaM cDNA insert into pAAV-CMV (Stratagene), then excising that expression cassette and inserting it into pAdTrack. This was followed by recombination with pAdEasy-1 in BJ5183-AD-1 (Stratagene) and selection for recombinants (20). Initial adenovirus stock was made by transfecting the PacI linearized plasmid pAdEasy-IkB into 293 cells ( ATCC, Manassus VA). Adenovirus was propagated on 293 cells, and used as unpurified culture lysates. Adenovirus titer was determined on 293 cells, using supernatant from 2Hx-2 hybridoma ( ATCC), followed by anti-rat second antibody. Endothelial cells at 70% confluence were transduced at 250 multiplicities of infection (MOI) in 10% FBS–DMEM for 16 h at 37°C, 5% CO2. The adenovirus was removed, fresh medium added and the EC incubated for 24 h to confluence before use. Transduction efficiency on EC was determined with LacZ adenovirus; 48 h following infection, the EC were fixed and stained with Xgal. Efficiency was approximately 80% at 100 MOI and >95% at 250 MOI.
Statistical analysis
Quantitative results are expressed as means ± standard error. Statistical analysis was performed using unpaired Student's t-test. p-Values of less than 0.05 were considered significant. Statistical analyses are shown for individual representative experiments and were performed on all experimental replicates, with results similar to those shown.
Results
Induction of cytoprotection of porcine EC by αGal ligation is not mediated by expression of soluble inflammatory cytokines or other soluble mediators
We investigated whether early proinflammatory changes caused by αgal-binding lectins were related to the subsequent protective phenotype. We first determined the time sequence of expression of several proinflammatory cytokine and chemokine genes following stimulation with BS-I, using RNase protection assays. Figure 1A demonstrates increased expression of several proinflammatory mRNA beginning at 1–2 h, reaching maximal expression at 2–4 h, and returning to the levels of nonstimulated EC controls by 20 h. Increased expression was found for alveolar macrophage chemotactic factor-2 (AMCF-2), GM-CSF, MCP-1, IL-8, IL-1α and IL-1β. RANTES and IL-18 mRNA were increased as earlier, but remained elevated by 20 h. There was minimal or no up-regulation of mRNA for G-CSF, IL-6, IL-10, IL-12 and IFN-γ(Figure 1A), and IL-2, IL-4, IFN-β, TNF-α, TNF-β and TGF-β2 (results not shown).
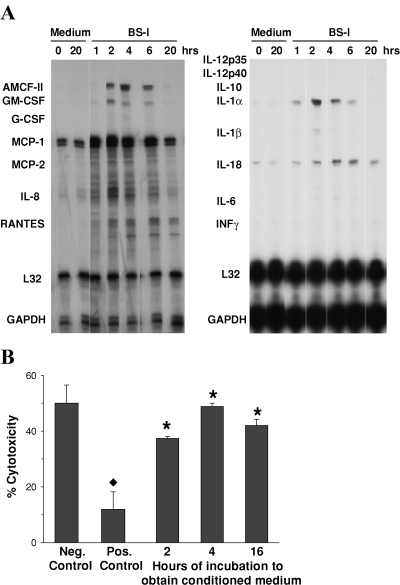
αGal ligation of porcine EC induces up-regulation of mRNA for inflammatory cytokines but released soluble mediators fail to induce protection against complement. (A) Time course of cytokine and chemokine mRNA expression by EC that were incubated with medium or 100 μg/mL BS-I. mRNA expression was assessed using a porcine RNase protection assay. Results are representative of two independent experiments. (B) Conditioned medium from EC stimulated with BS-I was obtained as follows: EC were incubated with 100 μg/mL BS-I for 4 h and then the supernatants were discarded. Fresh medium containing no BS-I was added for 2, 4 or 16 h to obtain the conditioned media. The recovered conditioned media were added to naive EC for 20 h, which were then tested for complement sensitivity. “Negative control” and “Positive control” consisted of EC incubated for 20 h with medium or 100 μg/mL BS-I, respectively. Not shown are results obtained with sham conditioned media prepared as follows: EC were incubated during 4 h with medium (instead of BS-I), washed, and then incubated with medium during 2, 4 or 16 h. The supernatants were recovered as sham conditioned media, which were then incubated with EC during 20 h. The sensitivity to complement of these EC was similar to that of “Neg. Control”. , p < 0.002 vs. Neg. control; *, p > 0.09 vs. Neg. control. Results are representative of three independent experiments.
We then investigated whether individual cytokines identified above were able to induce the late protective response when added to the EC. Endothelial cells that were incubated with IL-1α, IL-1β, IL-6 or IL-8 during 12–72 h at doses of 0.1–100 ng/mL were not protected from complement, whereas control EC incubated with BS-I were consistently protected (results not shown). Additionally, we investigated whether soluble mediators produced by EC following incubation with BS-I could induce protection in an autocrine fashion. Endothelial cells were first stimulated with BS-I for 2 or 4 h and then incubated with BS-I-free medium to produce conditioned media. These conditioned media were added to naive EC for 20 h, which were then tested for sensitivity to complement. We found that these cells were not protected, exhibiting a similar sensitivity to complement as control cells exposed to medium alone. Similar results were obtained with conditioned media from EC that were stimulated with BS-I during 2 (not shown) or 4 h (Figure 1B). These experiments indicate that it is unlikely that secreted products of EC activation, such as inflammatory cytokines, would be directly involved in the induction of EC protection.
αGal ligation of porcine EC in the presence of NF-κB inhibition results in reduced inflammatory changes and unimpaired induction of cytoprotection
The expression of many inflammatory genes is regulated by NF-κB, and NF-κB has also been shown to play a role in the inflammatory response induced in porcine EC by stimulation with BS-I (12). Moreover, NF-κB may also participate in cytoprotection. To delineate the relationship of NF-κB activation with the development of the early proinflammatory response and the later protective response of EC activated by αgal ligation, we used agents that inhibit NF-κB and abrogate expression of proinflammatory genes. We used SN50, which inhibits translocation of NF-κB to the nucleus, and its inactive analog ISN50 as a control (21). We first validated that SN50 inhibited NF-κB translocation in porcine EC stimulated with BS-I and LPS by immunofluorescence (results not shown). We then asked whether the inflammatory response of porcine EC to αgal-binding lectins was suppressed by NF-κB inhibition via SN50. We found that, while exposure of EC to BS-I during 4 h resulted in reorganization of actin filaments (from a finely spaced parallel arrangement to a more clumped and radial arrangement), cell retraction and formation of intercellular gaps, these changes were markedly reduced by the presence of SN50 (Figure 2A), but not by ISN50 (results not shown) during stimulation with BS-I. SN50 also partially inhibited the expression of IL-1α mRNA that is induced in EC stimulated with BS-I (Figure 2B) and prevented E-selectin expression in EC stimulated with IB4 (Figure 2C). Finally, we tested the response to IB4 lectin in EC that were transduced with an IκB mutant that is not susceptible to phosphorylation and thus retains NF-κB in the cytoplasm (22). We found that expression of E-selectin was suppressed in EC transduced with IκB mutant but not with adeno control (Figure 2D). Therefore, these studies suggest that the inflammatory changes induced in porcine EC by αgal ligation require the participation of NF-κB.
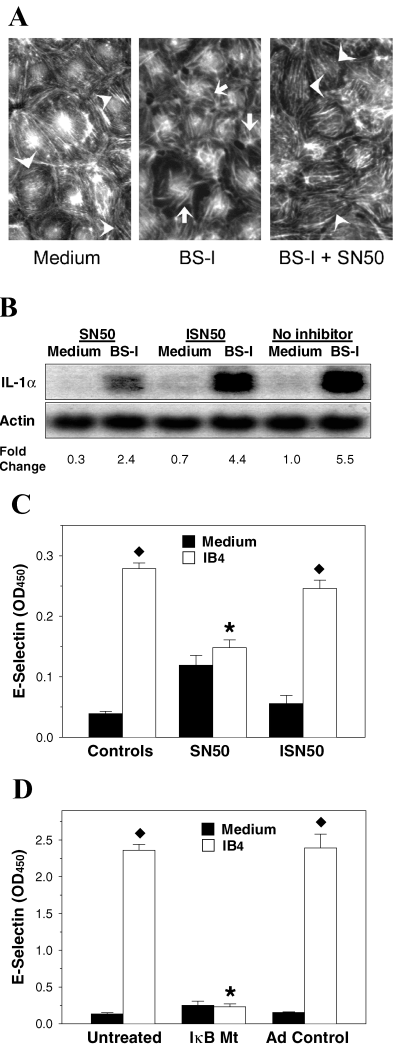
NF-κB inhibition reduces the early proinflammatory response of porcine EC to αGal ligation. (A) Inhibition of NF-κB with SN50 prevented reorganization of actin filaments and changes in cell shape in EC incubated with BS-I. Endothelial cells were incubated during 4 h with medium alone (Medium), with medium containing 100 μg/mL BS-I (BS-I) or with 50 μg/mL SN50 during 1 h followed by 4 h with 100 μg/mL BS-I plus 50 μg/mL SN50 (BS-I + SN50). The EC were then washed, fixed, permeabilized and stained with TRITC for actin visualization. Arrowheads indicate finely spaced parallel arrangement of actin in EC incubated with medium or BS-I plus SN50 and arrows indicate disorganized distribution of actin in EC incubated with BS-I. (B) Inhibition of NF-κB with SN50 reduced the up-regulation of IL-1α induced by BS-I. Endothelial cells were incubated during 1 h in medium containing SN50 (SN50) or the inactive analog ISN50 (ISN50), or in medium alone (No inhibitor). The EC were then washed and incubated during 4 h with medium or medium plus BS-I containing the respective pretreatment reagent. SN50 and ISN50 were used at 50 μg/mL and BS-I was used at 100 μg/mL. RNA was isolated and analyzed by Northern blotting. (C) Inhibition of NF-κB with SN50 prevented surface expression of E-selectin following stimulation with IB4 lectin. Endothelial cells were incubated during 1 h in medium alone (Controls), in medium containing SN50 (SN50) or in medium containing ISN50 (ISN50). The EC were then washed and incubated during 5 h with medium or medium plus IB4 containing the respective pretreatment reagent. SN50, ISN50 and IB4 were used at 50 μg/mL. The EC were washed and fixed, and expression of E-selectin was determined by ELISA. , p < 0.001; *, p > 0.3. (D) Porcine EC transduced with IkB mutant did not exhibit surface expression of E-selectin following stimulation with BS-I. Endothelial cells were left untreated or transduced with IkB mutant (IkB Mt) or adeno control, as indicated in Materials and Methods. The EC were then washed and incubated during 4 h with medium or medium plus 100 μg/mL BS-I. After cell washing and fixation, expression of E-selectin was determined by ELISA. , p < 0.01; *, p > 0.7 vs. result with medium. Results in panels A–D are representative of two to three independent experiments.
We then used NF-κB inhibitors to investigate whether cytoprotection induced in EC by αGal ligation required the participation of NF-κB-dependent mechanisms. Endothelial cells that were stimulated with BS-I or IB4 in the presence of SN50 were found to be as resistant to complement (Figure 3A) and to TNF-α-induced apoptosis (Figure 3B) as EC that were incubated with BS-I or IB4 alone or together with ISN50. Lastly, we assessed the protective response in EC that were transduced with the IκB mutant and found that these cells, similar to the EC transduced with adeno control, were fully capable of developing protection from complement following stimulation with BS-I (Figure 3C). These results demonstrate that αGal ligation in porcine EC causes cytoprotection by a mechanism independent of the inflammatory response and participation of NF-κB.
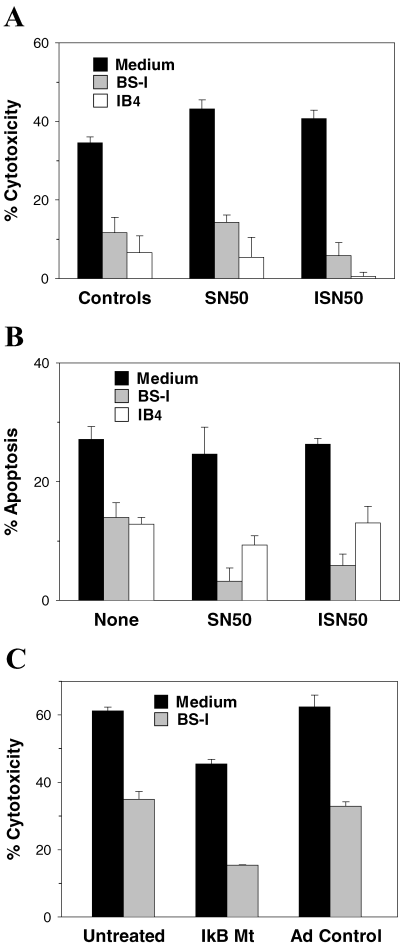
NF-κB inhibition does not impair the late protective response in porcine EC stimulated with αGal-binding lectins. Inhibition of NF-κB with SN50 did not prevent induction of protection from complement (A) or apoptosis (B) in EC stimulated with BS-I or IB4 lectin. Endothelial cells were incubated during 1 h in medium alone (Controls), in medium containing SN50 (SN50) or in medium containing ISN50 (ISN50). The EC were then washed and incubated during 20 h with medium, or with medium plus BS-I or IB4 and the respective pretreatment reagent. SN50, ISN50 and IB4 were used at 50 μg/mL and BS-I was used at 100 μg/mL. The EC were then washed and tested for susceptibility to complement and apoptosis. (C) Porcine EC transduced with IkB mutant developed an intact protective response following stimulation with BS-I. Endothelial cells were left untreated or transduced with IkB mutant (IkB Mt) or adeno control, as indicated in Materials and Methods. After washing, the EC were incubated during 20 h with medium or with medium plus 100 μg/mL BS-I. The EC were then washed and tested for susceptibility to complement. In panels A–C, p < 0.01 for all experiments with BS-I or IB4 vs. respective results with Medium. Results are representative of three independent experiments.
Stimulation of porcine EC with αGal-binding lectins increases mRNA for protective genes A20, HO-1 and porcine inhibitor of apoptosis protein, which is not reduced by NF-κB inhibition
We also investigated the effect of NF-κB inhibition on the expression of cytoprotective genes induced by αGal ligation. Endothelial cells that were incubated with BS-I or medium alone during various time periods were analyzed for mRNA expression. While there was up-regulation of A20, HO-1 and porcine inhibitor of apoptosis protein (PIAP) mRNA expression, there was little or no change in Bcl-XL expression (Figure 4A). Increased mRNA expression was unaffected when EC were incubated with IB4 lectin (Figure 4B) or BS-I (results not shown) during 6 h in the presence of SN50.
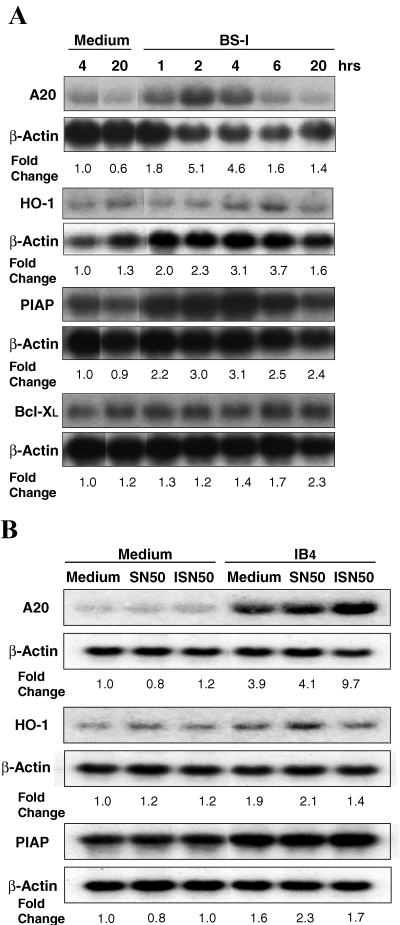
Stimulation of porcine EC with αGal-binding lectins causes up-regulation of protective genes, which is not reduced by NF-κB inhibition. (A) Northern blot analysis of A20, HO-1, PIAP and Bcl-XL mRNA from EC stimulated with BS-I during various time periods. Total RNA was isolated from EC incubated with medium alone or medium containing 100 μg/mL BS-I. Results are representative of two independent experiments. (B) Northern blot analysis of A20, HO-1 and PIAP mRNA from EC stimulated with IB4 in the presence of SN50. Endothelial cells were incubated during 1 h in medium alone (Controls), medium containing SN50 (SN50) or medium containing ISN50 (ISN50). The EC were then washed and incubated during 6 h with medium or medium plus IB4 containing the respective pretreatment reagent. SN50, ISN50 and IB4 were used 50 μg/mL. The cells were then washed and total RNA was isolated for analysis.
Discussion
In the xenogeneic environment of a primate, the initial injury sustained by an immediately vascularized porcine organ graft results from the binding of preformed anti-pig Abs, especially anti-αGal, to the vascular endothelium of the graft and complement activation, which causes hyperacute rejection of the graft (3,23). If hyperacute rejection is prevented, the life of the graft is extended a few days until the graft is lost owing to acute vascular rejection (24). One approach to prevent acute vascular rejection is the induction of accommodation (3,25), but accommodation has been difficult to induce and maintain in porcine organs transplanted into primate recipients (7). Therefore, to facilitate the induction and maintenance of accommodation, it is important to understand cytoprotection in simple models of pig-to-primate transplantation. Given the role that critical concentrations of anti-A or anti-B Abs are thought to play in accommodation of ABO-incompatible kidney allografts in humans (5,25), we have previously studied cytoprotection induced in porcine EC by ligation of αGal epitopes with Abs or αGal-binding lectins BS-I and IB4. After several hours, these cells express a protected phenotype consisting of resistance against the effects of the membrane attack complex of human complement (8,11), apoptosis induced by TNF-α/CHX (as reported here), and oxidative injury induced by H2O2 (unpublished results). This protective response, which is not simply a general perturbation of cell metabolism (11), is of interest because of its broad nature, comprising apoptosis, complement, and oxidative damage, which are implicated in graft rejection, ischemia/reperfusion and vascular diseases. However, the induction of the protective response by αGal ligation is preceded by an early response, which is proinflammatory.
In the current studies, we first investigated whether there is a relationship between the proinflammatory response and the protective response elicited in porcine EC by αGal ligation. Endothelial cells incubated with BS-I exhibited a rapid increase in expression of mRNA for several proinflammatory cytokines and chemokines, but incubation of EC with cytokines that showed increased mRNA expression did not result in protection from complement. Moreover, protection could not be induced with conditioned medium obtained from EC that were pretreated with BS-I and should contain soluble factors released from EC while undergoing stimulation to induce protection. These experiments strongly suggest that protection occurs independently of the proinflammatory response.
To further define a possible relationship of the proinflammatory response with development of protection, we investigated whether NF-κB activation is required for the induction of protection by αGal ligation. Previous studies have shown that NF-κB is activated when αGal epitopes are ligated by BS-I or IB4 (12), which was anticipated since proinflammatory responses are generally dependent on NF-κB activation. However, NF-κB-mediated mechanisms have also been implicated in the regulation of apoptosis and cytoprotection in response to a variety of stimuli (26). Therefore, we examined the role of NF-κB in the expression of both phases of the EC response to stimulation with αGal ligation using the NF-κB inhibitor SN50 and EC that were transduced with a mutant IκB gene that generates IκB unable to undergo phosphorylation and, thus, retains NF-κB in the cytoplasm following agonist stimulation (22). We found that the inhibition of NF-κB during incubation of EC with αGal-binding lectins resulted in reduction or elimination of proinflammatory changes but, in contrast, had no effect on protection from complement or apoptosis.
In our studies, we found that stimulation of EC with BS-I caused increased expression of mRNA levels for genes that may be associated with cytoprotection—A20, PIAP and HO-1—from 2 to 6 h after beginning of stimulation. Despite the short duration of this mRNA up-regulation, our unpublished observations indicate that HO-1 protein expression continues to increase at least during 20 h after BS-I stimulation, being several fold higher at 20 h than at 1 and 4 h. Up-regulation of one or more of these genes is likely involved in the induction of protection in our model system. This increased expression was not prevented by NF-κB inhibition with SN50, suggesting that expression of these genes in porcine EC activated with αGal-binding lectins is largely independent of NF-κB activation. It is known that induction of anti-apoptotic signals often depends on NF-κB activation, but protection may also develop without participation of NF-κB (26,27). HO-1 up-regulation in porcine EC stimulated with αGal-binding lectins has been reported previously (4) and protection from complement in EC stimulated with monkey immune serum was found to be abrogated by inhibition of HO-1 activity (28), which suggests an important role of HO-1 in protection. Our initial studies showing that EC stimulated with BS-I also exhibited increased expression of the complement inhibitor CD59 suggested that CD59 might be a factor that contributed to protection from complement (11). However, in additional studies, we found that up-regulated expression of CD59 contributed to protection only during the first hours after lectin stimulation, but not at days 2 and 3 when protection was strongest (29).
In conclusion, our results suggest that the protective response that is elicited in vitro in porcine EC by stimulation with αGal-binding lectins develops independently of the inflammatory changes, without a causative relationship between the products of the inflammatory response and the subsequent protective response. Our results indicate that NF-κB activation is required for induction of the early effects of EC cell stimulation but not for induction of protection from complement and apoptosis. The occurrence of strong inflammatory changes as protection develops has been a major obstacle to investigate the mechanisms of protection in EC activated by αGal ligation. Therefore, our findings should facilitate the delineation of mechanisms involved in the induction of a broad protective phenotype, which is of interest in studies of graft rejection and other forms of vascular injury.
Acknowledgments
We acknowledge the excellent technical assistance of Robert Konz. This work was supported by a grant from the National Institutes of Health (HL62195 to A.P.D.). J.F.G. was supported by National Research Service Award 1F32DK10006 and training grant T32HL07934 from the National Institutes of Health and a Richard L. Vanco Surgical Research Fellowship Award.




