BKV in Simultaneous Pancreas-Kidney Transplant Recipients: A Leading Cause of Renal Graft Loss in First 2 Years Post-Transplant
Abstract
With the introduction of more potent immunosuppressive agents, rejection has decreased in simultaneous pancreas/kidney transplant (SPK) recipients. However, as a consequence, opportunistic infections have increased. The purpose of this report is to outline the course of SPK patients who developed polyomavirus-associated nephropathy (PVAN). A retrospective review of 146 consecutive SPK recipients from January 1, 1996 to December 31, 2002 was performed. Immunosuppression, rejection and development of PVAN were reviewed. Nine patients were identified. All received induction with either OKT3 or thymoglobulin. Immunosuppression included tacrolimus/cyclosporine, MMF/azathioprine and sirolimus/prednisone. Two patients were treated for kidney rejection prior to the diagnosis of PVAN. Time to diagnosis was an average of 359.3 days post-transplantation. Immunosuppression was decreased but five ultimately lost function. However, none developed pancreatic abnormalities as demonstrated by normal glucose and amylase. Two underwent renal retransplantation after PVAN diagnosis and both have normal kidney function. PVAN was the leading cause of renal loss in SPK patients in the first 2 years after transplantation and is a serious concern for SPK recipients. The pancreas, however, is spared from evidence of infection, and no pancreatic rejection occurred when immunosuppression was decreased.
Introduction
Polyomaviruses are common in the adult population with more than 80% of adults demonstrating serologic evidence of past exposure (1). Asymptomatic infection is acquired during childhood by primary infection through the respiratory or oral route (2). In healthy individuals, latent polyomavirus infection (PVI) does not appear to result in any health consequences (3) as the virus can establish latency in the uroepithelium, oligodendrocytes and mononuclear cells (4). Urinary shedding of virus has been detected in 0.5–20% of asymptomatic people (5,6).
Polyomavirus-associated nephropathy (PVAN), also known as BK virus (BKV) nephropathy, is becoming recognized as an increasingly important cause of allograft dysfunction and loss in renal transplant recipients (7–10). The clinical manifestations of PVI in renal allograft recipients include asymptomatic infection, ureteral strictures, transient impairment of function or irreversible allograft failure. Histological evidence of BKV nephropathy is reported to range from 1% to 5% in biopsy specimens (9,11,12). However, once PVAN is diagnosed, graft loss has been reported to be as high as 45% at 6 months (13). Little is known about the clinical course of the disease in simultaneous pancreas and kidney (SPK) transplant recipients. It is not thought that the pancreas allograft is vulnerable to PVI directly; however, this has not been clearly established and the treatment of such infections in the renal allograft, typically with reduction of imunosuppression, places the pancreas allograft at risk for rejection.
The purpose of the present study is to report the incidence of PVAN in SPK transplant recipients at a single center. In addition, we report their clinical characteristics and an analysis of their immunosuppression, rejection episodes, outcome and results after re-transplantation.
Methods
Patients and data collection
This study was approved by the Committee for Human Research at the University of California, San Francisco. A retrospective review of all pancreas transplant operations and patients at the University of California, San Francisco from January 1, 1996 to December 31, 2002 was performed to collect recipient, transplant and post-transplant data. Pancreas after kidney- and pancreas-transplant-alone patients were excluded from further study.
Kidney and pancreas transplantation procedures
Donor pancreata and kidneys were procured from young deceased donors with no evidence of pancreatic or renal dysfunction. Pancreata were placed in the recipient's right iliac fossa through a midline intra-peritoneal approach with systemic drainage of the endocrine pancreas with anastomoses to the iliac vasculature. Exocrine drainage was performed by anastomosis of the duodenal segment to either the recipient ileum or bladder. The kidneys were placed in the recipient's left iliac fossa through the same midline incision with vascular anastomoses to the iliac vessels. Extravesicular ureteroneocystotomy was performed without a stent. Furosemide and mannitol were given intravenously before kidney reperfusion.
After discharge, patients were routinely followed in the clinic on a weekly basis for the first month, obtaining laboratory analysis two times per week. Routine laboratory analysis was changed to weekly from weeks 5 to 8, once every 2 weeks to week 24, and monthly thereafter. After the first month, they were seen at weeks 6, 12, 24, 52 and yearly thereafter, unless an abnormal laboratory value prompted an earlier visit. No routine screening was performed for PVI.
Immunosuppression
Two eras of immunosuppression protocols were followed during the time period of this study. Era I (1/1996 to 10/2000) consisted of induction with OKT3 or thymoglobulin and solumedrol, with maintenance immunosuppression consisting of prednisone, cyclosporine (CsA) or tacrolimus (FK), and azathioprine or mycophenolate mofetil (MMF) (Figure 1). Era II (11/2000 to 12/2002) was a steroid withdrawal regimen consisting of induction with thymoglobulin and solumedrol, and maintenance with tacrolimus, MMF and sirolimus (Figure 2) (14). Initiation of a calcineurin inhibitor was delayed if there was delayed kidney allograft function. Calcineurin inhibitors were dose adjusted. Tacrolimus levels were maintained at 10–15 ng/mL for the first 6 months post-transplant, then 10 ng/mL for months 7–12 and at 5–8 ng/mL thereafter. CsA levels were maintained at 200–300 ng/mL for the first year and 100–200 ng/mL thereafter.
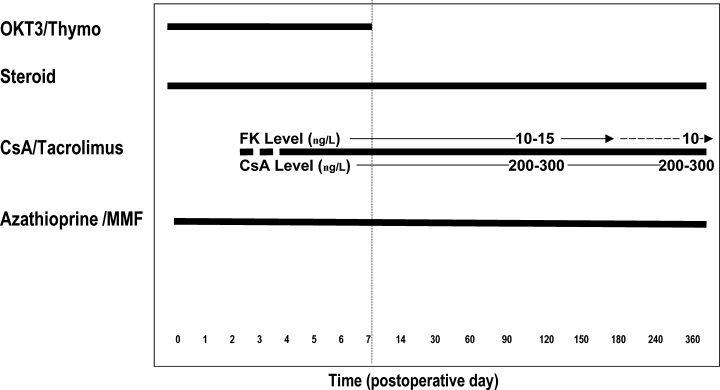
Era I immunosuppression. Induction immunosuppression consisted of methylprednisolone, and either OKT3 or thymoglobulin. Maintenance immunosuppression consisted of prednisone, a calcineurin inhibitor and an antiproliferative agent. If patients received FK, dose adjustment was made to maintain levels of 10 ng/mL.
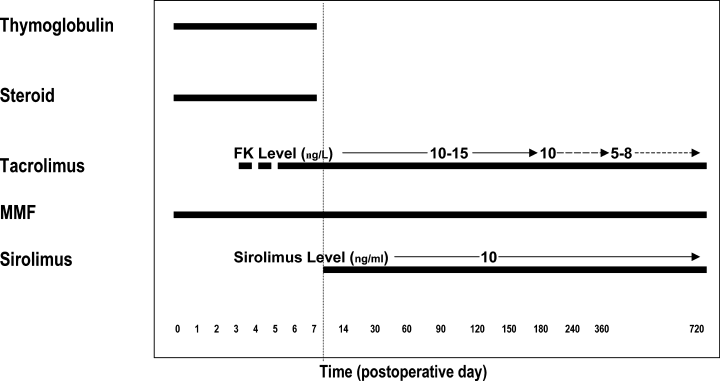
Era II immunosuppression is a steroid-sparing regimen. Patients were induced with thymoglobulin and methylprednisolone and FK and MMF were started immediately after transplantation; initiation of FK was delayed in cases of slow or delayed kidney allograft function. After receiving ≥6mg/kg of thymoglobulin, thymoglobulin and methylprenisolone were discontinued and sirolimus was begun. Both sirolimus and FK were dose adjusted to target levels of 10 μg/L and 10–15 ng/mL, respectively, in the first 6 months.
Diagnosis and treatment of PVAN
Elevated serum creatinine from baseline detected on routine laboratory analysis prompted hospital admission. Ultrasound and Doppler examination of the kidney allograft and biopsy was performed. Routine staining of kidney transplant biopsies demonstrated tubulointerstitial nephritis and intra-nuclear inclusions with cytopathic change on light microscopy consistent with polyoma virus infection. Positive staining for SV40 T antigen on immunohistochemistry was performed to confirm the diagnosis of PVI. When it became available, quantitative PCR of blood and urine for copies of BKV was performed (Viracor, Lee's Summit, MO). Immunosuppression was reduced; most patients receiving FK were changed to microemulsion CsA, while those receiving microemulsion CsA had doses reduced (target level 150 ng/mL). MMF was either discontinued or the dose was reduced. Some patients (n = 4) were treated with intravenous Cidofovir (dose of 0.25 mg/kg without Probenecid). Kidney graft loss was defined as a return to dialysis or a creatinine ≥5.0 mg/dL, suggesting a creatinine clearance of <20 mL/min.
Statistical analysis
We identified patients with biopsy-proven polyoma virus infection and analyzed clinical characteristics of patients and immunosuppression protocols, rejection rates and pancreatic function. Polyoma and non-polyoma groups were compared for demographic and other characteristics using the T-test for continuous variables and chi-squared test for non-continuous variables. The odds ratio for development of kidney allograft failure when the diagnosis of polyomavirus was made was determined by Cox regression analysis. Pancreas and kidney graft survival were determined by Kaplan–Meier analysis and compared by the log-rank test. All statistical analyses were performed utilizing SPSS 11.5 for Windows, a statistical software package (Chicago, IL).
Results
Recipient and transplant characteristics
Between January 1, 1996 and December 31, 2002, the University of California Kidney and Pancreas Transplant Service performed 146 consecutive SPKs. Pancreas graft losses that occurred in the first 30 days after transplantation (n = 9) were removed from further study.
We identified nine cases of biopsy-proven PVAN (6.6%) among the SPK recipients; five patients (5.8%) were identified receiving Era I immunosuppression while four patients (9.5%) were identified receiving Era II immunosuppression. Comparison of the incidence of PVAN between the two eras of immunosuppression was not statistically significant (p = 0.866). Mean follow-up for the cohort of patients was 35 ± 25 months. Patients that developed PVAN (n = 9) were of similar age at transplant compared to patients that did not develop PVAN (n = 128) (38.3 ± 6.4 years vs. 37.8 ± 6.3 years; p = NS) (Table 1). Eight of the nine patients who developed PVAN were male (89%) (p < 0.05); patients who did not develop PVAN were evenly divided among gender (51% male, 49% female). Eight of the nine patients (89%) who developed PVAN were Caucasian, the remaining patient being Hispanic. Patients who did not develop PVAN were of many racial and ethnic groups (13.2% Hispanic, 2.9% Asian, 76.5% Caucasian, 6.6% Black, 0.7% other); there was no statistically significant difference between racial groups and the development of PVAN.
| BKV nephropathy | No-BKV nephropathy | p-value | |
|---|---|---|---|
| N | 9 | 128 | |
| Mean age (years) at transplant | 38.3 ± 6.4 | 37.8 ± 6.3 | 0.796 |
| Gender: male n (%) | 8 (89) | 70 (51) | 0.028 |
| Race (%) | 0.89 | ||
| Caucasian | 88.9 | 76.5 | |
| Hispanic | 11.1 | 13.2 | |
| Black | 0.0 | 6.6 | |
| Asian | 0.0 | 2.9 | |
| Other | 0.0 | 0.7 | |
| Polyoma | |||
| Mean time: Tx to polyoma diagnosis (days) | 359.3 | ||
| Range | 136–836 | ||
| Time: polyoma diagnosis to Cr > 5.0 (days) | 72 | ||
- Tx = transplant; Cr = creatinine.
Kidney and pancreatic graft function after diagnosis of PVAN
On average, the time from transplantation to the diagnosis of PVAN was 359.3 days in this cohort of patients; the range from transplant to diagnosis was 136–836 days (Table 1). In addition, the average time from diagnosis to a creatinine ≥5.0 mg/dL was 72 days. Five of the nine patients progressed to a creatinine >5.0 mg/dL and four required hemodialysis (Table 2). The relative risk of kidney failure after development of PVI in the kidney allograft was 16.9 (95% confidence interval 5.3–53.8) (Figure 3).
| Patient | Exocrine drainage | Method of diagnosis | Viruria at Dx (copies/mL) | Immunosuppression before PVAN Dx | Immunosuppression after PVAN Diagnosis | Treatment cidofovir | Dialysis (after PVAN Dx) | Retransplant | Creatinine (mg/dl) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | at PVAN Dx | Latest | |||||||||
| 1 | Bladder | Biopsy | CsA 250 b.i.d., MMF 500 b.i.d., prednisone 15 q d | CsA 175 b.i.d., prednisone 25 q day | No | Yes | 1.6 | 2.1 | 8.0 | ||
| 2 | Enteric | Biopsy | FK 4/5, MMF 1000 b.i.d., prednisone 10 q d | FK 2 b.i.d., prednisone 5 q d | No | No | 1.2 | 2.2 | 1.9 | ||
| 3 | Bladder | Biopsy | FK 3 b.i.d., MMF 250 b.i.d., prednisone 10 q d | CsA 125 b.i.d., MMF 250 b.i.d., prednisone 10 q d | No | Yes→No | Yes | 2.0 | 2.9 | 1.5 | |
| 4 | Bladder | Biopsy | FK 6/5, MMF 1000 b.i.d., prednisone 30 q d | CsA 275 b.i.d., MMF 750 b.i.d., prednisone 25 q d | No | Yes→No | Yes | 2.1 | 3.7 | 1.4 | |
| 5 | Enteric | Biopsy | FK 3 b.i.d., MMF 750 b.i.d., prednisone 5 q d | CsA 300 b.i.d., MMF 500 b.i.d., prednisone 5 q d | No | Yes | 1.1 | 3.1 | 7.8 | ||
| 6 | Enteric | Biopsy/qPCR | 6.2 × 10e8 | FK 4 b.i.d., MMF 250 q d, sirolimus 4 q d | CsA 100 b.i.d., sirolimus 2 q d | Yes | Yes | 1.1 | 1.7 | 7.0 | |
| 7 | Enteric | Biopsy/qPCR | >1.25 × 10e9 | FK 2 b.i.d., MMF 250 b.i.d., sirolimus 3 q d | CsA 100 b.i.d., sirolimus alternating 2/1 | Yes | No | 1.3 | 1.7 | 3.2 | |
| 8 | Enteric | Biopsy/qPCR | 4.6 × 10e8 | FK 2 b.i.d., MMF 500 b.i.d., sirolimus 2 q d | CsA 100 b.i.d., MMF 250 b.i.d. | Yes | No | 1.2 | 2.1 | 4.0 | |
| 9 | Enteric | Biopsy/qPCR | >1.25 × 10e9 | FK 2 b.i.d., MMF 250 b.i.d., sirolimus 2 q d | CsA 100 b.i.d., sirolimus 1 q d | Yes | No | 2.0 | 3.3 | 4.8 | |
- Note: immunosuppressant doses are in milligrams. CsA = microemulsion cyclosporine; FK = tacrolimus; MMF = mycophenolate mofetil.
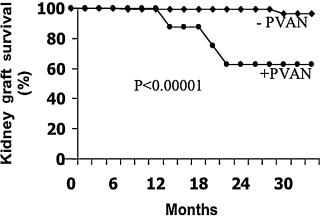
Renal allograft survival is severely affected by polyomavirus-associated nephropathy. The relative risk of kidney failure after development of polyomavirus infection in the kidney allograft was 16.9 (95% confidence interval 5.3–53.8).
There was no correlation between the use of either induction OKT3 or thymoglobulin and the development of PVAN (p = NS) (Table 3). In addition, there was no statistically significant association or trend between any single immunosuppression agent and the development of PVAN (Table 3). Despite a lowering of immunosuppression, there was no difference between PVAN patients and uninfected patients in their most recent serum glucose level (94 ± 13 vs. 96 ± 20; p = NS) (mg/dL), amylase (90 ± 34 vs. 70 ± 27; p = NS) (U/L) or pancreas graft survival (Table 4 and Figure 4); post-transplant hemoglobin A1c levels were not available. No patients suffered acute pancreas allograft rejection, nor did any suffer from acute kidney allograft rejection, after PVAN was diagnosed or with subsequent immunosuppression reduction. Histopathological examination of kidney explants from the two patients who underwent subsequent living-related renal transplant, though, did show chronic allograft rejection. Rates of overall kidney allograft rejection, prior to the development of PVAN, and subsequent treatment (OKT3 or thymoglobulin) between BKV nephropathy patients (22.2%) and patients who did not develop BKV nephropathy (19.0%) were not statistically significant (p = NS) (Table 3). The rate of kidney or pancreas allograft rejection with the Era II immunosuppression regimen has been previously reported as 2.5% at 1 and 3 months post-transplantation (14).
| BKV nephropathy (n = 9) | No-BKV nephropathy (n = 128) | p-value | |
|---|---|---|---|
| Induction immunosuppression | 0.412 | ||
| OKT3 (mg) | 52.5 | 48.5 | |
| Thymoglobulin (mg/kg) | 6.0 | 7.8 | |
| Maintenance immunosuppression (% of patients) | |||
| Tacrolimus | 66.7 | 83.2 | 0.209 |
| Cyclosporine | 33.3 | 15.3 | 0.158 |
| Azathioprine | 22.2 | 26.5 | 0.779 |
| MMF | 77.8 | 72.3 | 0.719 |
| Prednisone | 56.6 | 69.3 | 0.388 |
| Prednisone withdrawal | 44.4 | 30.7 | 0.388 |
| Percentage of patients with kidney rejection | 22.2 | 19.0 | 0.811 |
| BKV nephropahty (n = 9) | No-BKV nephropathy (n = 128) | p-value | |
|---|---|---|---|
| Kidney function | |||
| Creatinine (mg/dL) | 3.5 ± 1.2 | 1.4 ± 0.8 | 0.04 |
| Pancreas function | |||
| Amylase (U/L) | 90 ± 34 | 70 ± 27 | 0.12 |
| Glucose (mg/dL) | 94 ± 13 | 96 ± 20 | 0.71 |
- While the development of PVAN and treatment (with a reduction in immunosuppression) ultimately failed and led to elevated serum creatinine levels and renal allograft failure, no effect was seen in pancreatic function as serum glucose and amylase demonstrated no difference from patients without PVAN. Values are from most recent follow-up data. Creatinine values from both groups excludes patients that have returned to dialysis.
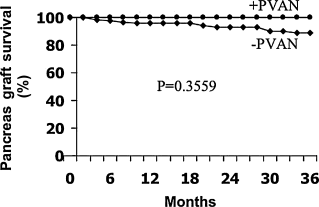
Pancreas allograft survival is not affected by polyomavirus. There is no difference in the pancreatic allograft survival rate between patients with PVAN and those without.
Other causes of kidney allograft loss in any 2-year period were reviewed. These included rejection, post-transplant lymphoproliferative disorder, recipient death and unknown as reasons for allograft loss in the first 24 months after transplant (data not shown). Polyomavirus-related allograft failure was the other cause of kidney allograft loss, representing more than 50% of renal allograft failures.
Kidney retransplantation after allograft loss from PVAN
To reduce the possibility of retained virus-infected tissue, transplant ureteronephrectomy and reduction of immunosuppression (MMF and prednisone doses were decreased) were performed 3–4 months prior to living-related renal transplantation in two patients that had progressed to complete renal failure. Pancreatic function was monitored by following serum glucose and amylase during this time. Histopathological examination of the transplant nephrectomy specimens revealed no active evidence of PVI but did demonstrate chronic allograft nephropathy in both. Follow-up at 4 and 5 years in these two patients showed excellent graft function (average creatinine 1.5 mg/dL) and no evidence of rejection or PVI in the second transplanted kidney allograft (Figure 5 and Table 2).
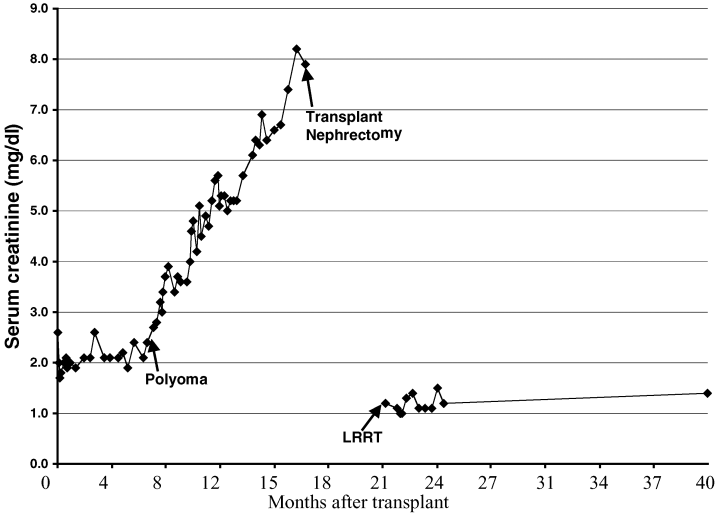
Retransplantation is possible after kidney allograft loss from PVAN. After ureteronephrectomy and resolution of viremia and viruria, SPK patients who have had renal allograft failure from PVAN can be retransplanted. This graph of one patient retransplated demonstrates stable renal function 4 years after receiving a living-related renal transplant (LRRT).
Discussion
There are limitations to this study including that it is retrospective, involves only a single center and that there are only nine cases of PVAN; this small number of cases makes the analysis of risk factors difficult to interpret. However, this study is the first to demonstrate the clinical course of the pancreas allograft in SPK patients with PVI and when immunosuppression is decreased subsequent to the diagnosis. The rate of PVAN in SPK patients at our institution is 6.6%; the single other report in the literature determined their institutional rate at 7.5% (11,15).
The rate of PVAN in our kidney-alone recipients is 2.0%; we identified 36 patients who developed PVAN (of 1788 transplanted) during the time period of this study. The mean time from transplantation to the diagnosis of PVAN was 359.3 days in the SPK cohort of patients; in 18 kidney-alone transplant recipients at our institution who we have reviewed that developed PVAN, the mean time to diagnosis was 628.0 days. It is unclear why the rate and rapidity of diagnosis of PVAN in SPK patients at our institution is three times higher and nearly twice as early than in the kidney-alone population. In part this may be because all of the SPK recipients receive a course of antibody induction which is not the case with the kidney-alone population at our institution. However, in a prospective study, univariate analysis demonstrated that induction immunosuppressive therapy with antilymphocyte preparations was not significantly associated with decoy-cell shedding or nephropathy in kidney-alone recipients (16).
In addition, diabetes itself may be a possible risk factor for PVAN and in part explain the increased incidence of PVAN in SPK. At the University of Maryland, 11–12% of pancreas-transplant-alone patients were found to have evidence of polyomavirus by urine cytology. It is unclear the extent to which this results in PVAN but it does support the concept that diabetic patients may be at greater risk for PVAN (17). Reducing immunosuppression in an attempt to salvage the kidney allograft did not result in subsequent pancreas allograft rejection. In addition, there was no alteration in pancreatic endocrine (i.e. serum glucose and amylase) function before or after the diagnosis of PVAN suggesting that polyomavirus does not clinically affect the pancreas allograft.
Numerous reports have attempted to link specific immunosuppression agents with the development of PVAN. Some have reported that certain agents (i.e. FK or MMF) may place the patient at greater risk for PVAN (13,18,19). Others believe that the main risk factor for the development of PVI is the intensity of pharmacologic immunosuppression (20,21). SPK patients may be at an increased risk in general for the development of PVAN because of a greater risk of overimmunosuppression when compared to kidney allograft recipients alone (11). Immunosuppressive protocols have been designed around the greater risk of rejection presented by the pancreas allograft and these patients may have a greater rate of exposure to T-cell depleting antibodies and pulsed steroids when compared to kidney recipients alone.
In our case series and analysis, like other reports (15), patients received various combinations of immunosuppressants (both protocols included antibody induction and a corticosteroid, followed by an anti-proliferative, a calcineurin inhibitor or sirolimus). We did not find an association or trend between any single agent and PVAN, suggesting the development of the infection is due to a state of overimmunosuppression. However, this lack of statistical association may be due to inadequate statistical power because of the small number of cases of BKV nephropathy in our patients.
This study has demonstrated an association between males and the development of PVAN in SPK patients, confirming several other studies that have also shown a male predominance in PVAN infection in recipients of kidney allografts alone (15,22–24). It is unclear why males appear to have a higher rate of PVAN; this may have to do with seroprevalence, methods of exposure or different immune responses between males and females. Other reports have determined that previous treatment of rejection increases the risk of developing PVAN (16,18). Our analysis differs with this finding in that there was no statistically significant difference in overall rates of treated rejection between patients that developed PVAN and of those that did not; these findings, however, may reflect the small number of patients (n = 2) in the SPK group that did develop acute rejection of their kidney allografts. Both patients were treated with OKT3 after rejection was diagnosed by kidney biopsy. One patient was diagnosed with PVAN 95 days later; the other demonstrated biopsy evidence of polyoma virus 348 days later. Certainly the proximity of OKT3 administration to the diagnosis of PVAN 3 months later in one patient suggests the role that intense immunosuppression may play in the incidence of this process. No patients developed acute rejection of the pancreas or kidney after reduction of immunosuppression with PVAN diagnosis; kidney explant histology did demonstrate chronic rejection, though in patients who were retransplanted.
It is critical that PVAN be differentiated from acute rejection as the treatments are different and opposing. In addition, methods of detection of PVAN have also improved, probably in part responsible for the reported increase in incidence. Clinicians have proposed that in order to rescue kidney allografts that have demonstrated PVAN, immunosuppression must be reduced or withdrawn (11,25) and this is currently the mainstay of treatment; in some patients, this has been associated with the stabilization of renal function when instituted early (20). However, patients often continue to have a degree of allograft dysfunction and their long-term outcome is not clear and may be compromised (20). In this study, when patients were diagnosed with PVAN they had a relative risk of 16.9 of progressing to kidney failure when compared with the cohort of uninfected patients. However, the data demonstrate that they were not at increased risk of developing pancreatic allograft failure; none of the patients in our series experienced clinical pancreatic allograft dysfunction or rejection while having PVI or during reduction of immunosuppression.
While no controlled study has yet been published, antiviral therapy using Cidofovir (HPMPC) (26), in combination with lowering of immunosuppression, has been shown in some cases to decrease the urine viral load (26); however, at this time Cidofovir has not been shown to be clinically successful in decreasing the risk of graft loss secondary to PVAN (11). Four patients in our series did receive intravenous Cidofovir (0.25 mg/kg without probenecid) as a further attempt at salvage therapy. Unfortunately, there was no improvement in renal function even with resolution of viremia and viruria. Whether this is due to a lack of efficacy or having started treatment too late in the progression of PVAN is not clear.
This series also supports the concept that retransplantation is possible after a kidney allograft is lost to PVAN (15,24,27,28). Some believe that reducing the total load of infected residual uroepithelium to prevent recurrent infection is important before retransplantation can be considered (11,27). In addition, avoidance of excessive immunosuppression after retransplantation to prevent recurrent PVAN is probably the more important issue (11). The two patients who underwent retransplantation in our series underwent transplant nephroureterectomies 3–4 months prior to living donor kidney transplantation. Both patients have stable renal function 4 and 5 years after retransplantation without clinical evidence of recurrent PVI.
In this series of patients, we have seen a relatively high rate of polyomavirus-related nephropathy and relatively low rates of rejection. With Era II immunosuppression (thymoglobulin and steroid induction, maintenance with FK, MMF and sirolimus) in particular, we have had an increase in the incidence of PVI (9.5%) and very low rates of early rejection (2.5%). In the classic sense, this suggests that our patients are overimmunosuppressed and we are now discontinuing MMF at 6 months after transplantation. The development of PVAN does not appear to be linked to MMF (or any single immunosuppressant agent) but while reduction in immunosuppression is the mainstay of treatment, we believe avoiding overimmunosuppression may be the mainstay of prevention.
Most important, this series demonstrates that pancreatic function is preserved in the face of clinically significant polyoma virus infection, as the pancreas is not adversely affected by the virus or by careful immunosuppression reduction. Surveillance screening for decoy cells in urine or by PCR for viruria should be considered as PVI was a leading cause of kidney allograft loss in this series in the first 2 years after SPK.




