Laparoscopic Live Donor Nephrectomy: A Risk Factor for Delayed Function and Rejection in Pediatric Kidney Recipients? A UNOS Analysis
The data contained in this manuscript was presented, in part, at the American Transplant Congress 2004 (Fifth Joint Meeting of the American Society of Transplant Surgeons and the American Society of Transplantation), May 15–19, 2004, Boston, MA.
Abstract
The impact of laparoscopic (vs. open) donor nephrectomy on early graft function and survival in pediatric kidney recipients (≤18 years) is unknown.
We studied 995 pediatric live donor txs reported to UNOS from January 2000 to June 2002, in two recipient age groups: 0–5 years (n = 212, 44% laparoscopic donors [LapD]) and 6–18 years (n = 783, 50% LapD).
Delayed graft function (DGF) rates were higher for LapD versus open donor (OpD) txs (0–5 years, 12.8% vs. 2.5%[p = 0.004]; 6–18 years, 5.9% vs. 2.8%[p = 0.03]). Acute rejection incidence for LapD versus OpD txs was higher at 6 months for recipients 0–5 years (18.6% vs. 5.9%, p = 0.01) and 6–18 years (22.5% vs. 15.6%, p = 0.03), and 1 year for recipients 0–5 years (24.3% vs. 7.9%, p = 0.004). In multivariate analyses, significant independent risk factors for rejection at 6 months and 1 year were recipient age 6–18 years, pretx dialysis, LapD nephrectomy and DGF. Graft survival was similar for LapD versus OpD txs.
In this retrospective UNOS database analysis, LapD procurement was associated with increased DGF and an independent risk factor for rejection during the first year, particularly for recipients 0–5-years old. Future investigations must confirm these findings and identify strategies to optimize procurement and pediatric recipient outcome.
Introduction
Since its first description in 1995, laparoscopic nephrectomy has rapidly become the technique of choice for procurement of kidneys from live donors that are transplanted into adult recipients (1,2). Based on a few small single center series and anecdotal reports, a similar trend appears to have taken place in live donor transplantation of pediatric recipients (3–5). The impact of this changed surgical practice on pediatric recipients, however, has not been studied in detail.
The pneumoperitoneum that is created during laparoscopy is known to be associated with adverse renal hemodynamic effects and acutely decreased urine output of native kidneys (7–10). Hence, it is not surprising that some studies of adult recipients of live donor kidney grafts showed slower early graft function for laparoscopic (vs. open) donor kidneys (11–13). For pediatric recipients, the scarce available data on the potential impact of laparoscopic (vs. open) nephrectomy are controversial. A single-center report noted slightly higher early post-transplant creatinine levels for laparoscopic (vs. open) grafts, without, however, any effect on long-term outcome (3). In contrast, the only two other studies that compared directly laparoscopic versus open procurement did not observe significant differences in graft function or outcome at any time point (4,5). All three studies, however, included only a relatively limited number of pediatric recipients.
Thus, the safety and efficacy of the laparoscopic procurement technique have yet to be demonstrated in a larger pediatric recipient population. Simple extrapolation of adult data to pediatric recipients may not be appropriate, as pediatric renal transplants pose unique challenges, including operative-technical aspects, hemodynamics at the time of graft reperfusion and increased immune reactivity (compared with adults), resulting in higher rejection rates (14–19). We hypothesized that any intrinsic adverse functional or immunologic impact of laparoscopic procurement might, therefore, be exacerbated in pediatric renal transplant recipients.
In order to study our hypothesis, we compared functional and immunologic outcomes, including graft survival, of laparoscopic and open kidney grafts in pediatric recipients reported to the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) database. Since very small children pose a particular perioperative hemodynamic (and potentially immunologic) challenge, we studied the pediatric recipients in two separate age categories: 0–5 years and 6–18 years.
Patients and Methods
We reviewed the OPTN/UNOS database for all live donor kidney transplants in pediatric recipients (defined as recipient age at transplant 0–18 years) that were done from 1 January 2000 to 30 June 2002 with follow-up through 10 October 2003. For the purpose of this analysis, we defined as ‘open’ kidneys only those that had been removed via a flank approach.
We also studied the proportion of the laparoscopically procured live donor kidney grafts for pediatric versus adult (>18 years) recipients for the time period between 1 January 2000 and 31 December 2003.
Graft function analysis
We assessed early graft function as follows: >40 mL of urine production during the first 24 h (yes vs. no), serum creatinine level decline by greater than 25% on two separate serum samples taken within the first 24 h (yes vs. no), delayed graft function (DGF) (defined as need for dialysis within the first week post-transplant [yes vs. no]) and serum creatinine level (mg/dL) at the time of discharge. We also compared serum creatinine levels at 6-month- and 1-year post-transplant and causes of graft loss (overall and within the first 30-day post-transplant).
Immunologic outcome analysis
Acute rejection was defined as treatment for rejection as reported to UNOS and was analyzed at three different time points: before discharge, within the first 6 months and within the first year post-transplant.
Statistical analysis
Donor and recipient demographic and outcome variables were compared between the laparoscopic and open (flank approach) donor groups for recipients 0–5-years old and 6–18 years-old that were transplanted between 1 January 2000 and 30 June 2002. Categorical variables were analyzed using the Chi-square test. The means of continuous variables were analyzed using t-tests and Wilcoxon rank sum tests. Graft failure was defined as permanent return to dialysis or death with a functioning graft. Graft survival rates were calculated using the Kaplan-Meier method, employing the log-rank test to detect differences in the survival curves.
A multivariate Cox regression analysis was performed to determine donor and recipient covariates that were independent risk factors for acute kidney graft rejection at 6-month- and at 1-year post-transplant. We included the following covariates into our multivariate analysis: recipient risk factors (recipient age [0–5 years vs. 6–18 years], recipient ethnicity [non-white vs. white], pre-transplant dialysis [yes vs. no]); surgical risk factors (laparoscopic donor nephrectomy versus open donor nephrectomy); and transplant risk factors (DGF [yes vs. no], antibody induction therapy [yes vs. no]). For the purpose of this analysis, ‘antibody’ induction therapy was defined as the use of IL-2 receptor antibodies, or of mono- or polyclonal antilymphocyte antibodies, prior to discharge from the transplant admission.
For all statistical tests, a p-value of <0.05 was considered statistically significant.
Results
Nephrectomy technique over time
The proportion of live donor kidney grafts that were procured laparoscopically for transplantation into pediatric recipients increased from 37% in 2000 to 66% in 2003 (Figure 1). We observed a higher proportion of laparoscopically (vs. openly) procured grafts for adult versus pediatric recipients. The rate of increase of the proportion of laparoscopically procured grafts, however, was similar for adult and pediatric recipients (Figure 1).
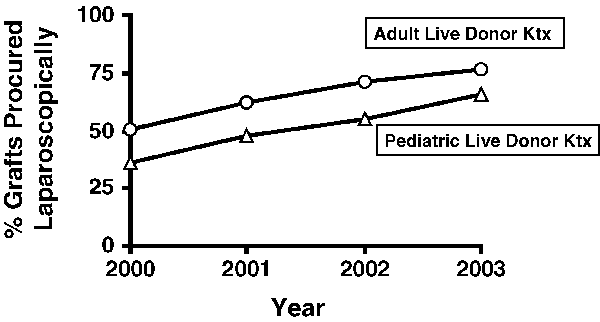
Proportion of live donor kidneys that were procured laparoscopically for transplantation into pediatric and adult recipients in the United States between 1 January 2000 and 31 December 2003.
Pediatric study population
From 1 January 2000 to 30 June 2002, for pediatric recipients, 512 live donor kidney transplants from open donors and 483 live donor transplants from laparoscopic donors (LapD) were reported to UNOS.
Live donor demographics
Gender distribution, mean age and height as well as all other studied demographic variables were not significantly different for open versus LapD in both pediatric recipient age categories (Table 1).
| Covariate | Recipient age 0–5 years | Recipient age 6–18 years | ||
|---|---|---|---|---|
| Open (n = 118) | Laparoscopic (n = 94) | Open (n = 394) | Laparoscopic (n = 389) | |
| Male/female (%) | 46/54 | 38/62 | 45/55 | 46/54 |
| Mean age ± SD (years) | 32.9 ± 7.9 | 33.0 ± 8.3 | 38.8 ± 8.3 | 37.9 ± 8.7 |
| Mean height ± SD (cm) | 171 ± 10 | 170 ± 9 | 169 ± 11 | 170 ± 11 |
| Mean weight ± SD (kg) | 77.5 ± 166 | 74.3 ± 15.9 | 79.3 ± 15.7 | 78.1 ± 17.6 |
| Ethnicity (%) | ||||
| Caucasian | 76 | 69 | 64 | 70 |
| African-American | 8 | 10 | 14 | 12 |
| Hispanic | 14 | 17 | 15 | 14 |
| Other | 2 | 4 | 7 | 4 |
| Relationship to recipient (%) | ||||
| Biologically related | 97 | 95 | 93 | 89 |
| Biologically unrelated | 3 | 5 | 7 | 11 |
- †p = n.s.
Pediatric recipient demographics
Recipient demographics are listed in Table 2. We observed no significant differences in both age groups for all studied variables, including preemptive transplant rates, immunologic risk factors, cold ischemia time and use/non-use of induction therapy. We were not able to analyze warm ischemia time because this data point was inconsistently reported (i.e. centers reported variably the warm ischemia time in donor, the warm ischemia time in the recipient or the combined [donor plus recipient] warm ischemia time).
| Covariate | Recipient age 0–5 years | Recipient age 6–18 years | ||
|---|---|---|---|---|
| Open (n = 118) | Laparoscopic (n = 94) | Open (n = 394) | Laparoscopic (n = 389) | |
| Male/female (%) | 63/37 | 71/29 | 59/41 | 58/42 |
| Mean age ± SD (years) | 2.2 ± 1.5 | 2.4 ± 1.4 | 13.7 ± 3.5 | 13.5 ± 3.5 |
| Mean height ± SD (cm) | 86 ± 18 | 85 ± 20 | 151 ± 21 | 149 ± 23 |
| Mean weight ± SD (kg) | 13.7 ± 6.7 | 14.3 ± 11.2 | 49.5 ± 20.4 | 49.0 ± 21.6 |
| Ethnicity (%) | ||||
| Caucasian | 78 | 68 | 64 | 69 |
| African-American | 7 | 10 | 14 | 13 |
| Hispanic | 12 | 18 | 16 | 14 |
| Other | 3 | 4 | 6 | 4 |
| Pre-transplant dialysis (%) | 69 | 76 | 65 | 60 |
| Primary renal disease (%) | ||||
| Congenital, familial and metabolic diseases | 39 | 36 | 27 | 26 |
| Glomerular diseases | 17 | 18 | 35 | 29 |
| Tubular and interstitial diseases | 15 | 10 | 12 | 12 |
| Other diseases | 29 | 36 | 26 | 33 |
| Re-transplant (%) | 3 | 2 | 6 | 8 |
| PRA at transplantation >20% (%) | 2 | 3 | 3 | 4 |
| Mean cold ischemia time ± SD (h) | 2.0 ± 1.4 | 2.5 ± 4.6 | 2.4 ± 4.9 | 2.3 ± 4.2 |
| Induction therapy (%) | ||||
| Anti-T-cell (poly- and monoclonal) | 17 | 15 | 10 | 12 |
| IL-2 receptor antibody | 41 | 31 | 47 | 38 |
| No induction therapy | 42 | 53 | 43 | 50 |
- †p = n.s.
Pediatric recipient outcomes and graft function
The post-operative length of stay was statistically not significantly different for recipients of laparoscopic versus open kidneys in both recipient age categories (Table 3). Early post-transplant urine production and serum creatinine decrease during the first 24 h post-transplant were similar for recipients of laparoscopic versus open kidney grafts in both recipient age categories (Table 3). A significantly higher proportion of laparoscopic kidney recipients in both groups, however, experienced DGF (open vs. laparoscopic, respectively: 0–5 years, 2.5% vs. 12.8%, p = 0.004; 6–18 years, 2.8% vs. 5.9%, p = 0.03) (Table 3). Mean serum creatinine levels at discharge, at 6 months and at 1 year were similar for open versus laparoscopic kidney recipients (Table 3).
| Covariate | Recipient age 0–5 years | Recipient age 6–18 years | ||||
|---|---|---|---|---|---|---|
| Open (n = 118) | Laparoscopic (n = 94) | p-value | Open (n = 394) | Laparoscopic (n = 389) | p-value | |
| Recipient length of stay | ||||||
| Mean post-operative length of stay ± SD (days) | 13.9 ± 10.6 | 16.6 ± 28.6 | 0.9 | 9.0 ± 8.3 | 8.5 ± 7.3 | 0.1 |
| Early graft function | ||||||
| >40 mL urine during first 24 h (n) | 104 (88.1%) | 83 (88.3%) | 0.8 | 366 (92.9%) | 359 (92.3%) | 0.8 |
| ≥25% decrease in creatinine during first 24 h (n) | 100 (84.7%) | 77 (81.9%) | 0.7 | 337 (85.5%) | 340 (87.4%) | 0.2 |
| DGF (n) | 3 (2.5%) | 12 (12.8%) | 0.004 | 11 (2.8%) | 23 (5.9%) | 0.03 |
| Mean recipient serum creatinine level | ||||||
| At discharge ± SD (mg/dL) | 0.4 ± 0.4 | 0.6 ± 1.0 | 0.055 | 1.3 ± 1.3 | 1.4 ± 1.6 | 0.7 |
| At 6 months ± SD (mg/dL) | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.6 | 1.2 ± 0.7 | 1.3 ± 0.7 | 0.98 |
| At 1 year ± SD (mg/dL) | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.3 | 1.3 ± 0.8 | 1.3 ± 0.7 | 0.7 |
| Acute rejection incidence | ||||||
| Rejection during transplant hospital admission (n) | 2 (2%) | 8 (11.4%) | 0.02 | 22 (6.8%) | 19 (6.1%) | 0.7 |
| Rejection at 6 months (n) | 6 (5.9%) | 13 (18.6%) | 0.01 | 50 (15.6%) | 70 (22.5%) | 0.03 |
| Rejection at 1 year (n) | 8 (7.9%) | 17 (24.3%) | 0.004 | 73 (22.7%) | 88 (28.1%) | 0.1 |
- *Total number of patients for individual variables may be less than overall (n[laparoscopic]+ n[open]) due to incomplete reporting and variable follow-up time.
Acute rejection
Acute rejection incidence at 6 months was significantly higher for recipients of laparoscopic donor kidneys in both age groups (open vs. laparoscopic, respectively: 0–5 years, 5.9% vs. 18.6%, p = 0.01; 6–18 years, 15.6% vs. 22.5%, p = 0.03) (Table 3). We also observed higher rejection incidences at 1 year for laparoscopic donor kidney recipients that were 0–5-years old: 7.9% (open) versus 24.3% (laparoscopic) (p = 0.004) (Table 3).
Graft failure cause analysis
Overall, significantly more laparoscopic (vs. open) grafts were lost in recipients that were 0–5-years old (Table 4). Approximately half of these graft losses occurred within the first 30 days post-transplant. However, we observed no significant differences in graft failure rates and graft loss causes for laparoscopic versus open recipients in either recipient age category (Table 5).
| Covariate | Recipient age 0–5 years | Recipient age 6–18 years | ||
|---|---|---|---|---|
| Open (n = 118) | Laparoscopic (n = 94) | Open (n = 394) | Laparoscopic (n = 389) | |
| Primary failure | 2 | 2 | 2 | |
| Graft thrombosis | 1 | 2 | 3 | 7 |
| Surgical complication | 1 | |||
| Rejection | ||||
| Hyperacute rejection | 1 | |||
| Acute rejection | 1 | 5 | 6 | |
| Chronic rejection | 6 | |||
| Recurrent disease | 1 | 4 | 5 | 6 |
| Non-compliance | 1 | 4 | ||
| Other causes | 2 | 1 | 1 | 3 |
| Total graft failures | 4 (3.4%)* | 10 (10.6%)* | 25 (6.3%) | 28 (7.2%) |
- *p = 0.049 (Fisher's Exact test).
| Covariate | Recipient age 0–5 years | Recipient age 6–18 years | ||
|---|---|---|---|---|
| Open (n = 118) | Laparoscopic (n = 94) | Open (n = 394) | Laparoscopic (n = 389) | |
| Primary failure | 2 | 2 | 2 | |
| Graft thrombosis | 1 | 2 | 2 | 7 |
| Surgical complication | 1 | |||
| Rejection | ||||
| Hyperacute rejection | 1 | |||
| Acute rejection | 1 | |||
| Recurrent disease | 1 | |||
| Other causes | 1 | 1 | 1 | 1 |
| Total graft failures | 2 (1.7%) | 6 (6.4%) | 7 (1.8%) | 11 (2.8%) |
- *p = n.s.
Multivariate analyses—risk factors for acute rejection
Recipient age 6–18 years, pre-transplant dialysis, laparoscopic procurement, and DGF were all independent risk factors for rejection at 6 months and at 1 year (Table 6).
| Logistic regression | Covariate | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|---|
| Rejection at 6 months | Recipient age (6–18 vs. 0–5 years) | 2.0 | 1.16–3.41 | 0.01 |
| Recipient ethnicity (non-white vs. white) | 1.2 | 0.81–1.83 | 0.34 | |
| Pre-transplant dialysis (yes vs. no) | 1.66 | 1.07–2.55 | 0.02 | |
| Laparoscopic vs. open donor nephrectomy | 1.74 | 1.18–2.55 | 0.005 | |
| DGF (yes vs. no) | 5.26 | 2.51–11.0 | <0.0001 | |
| Antibody induction therapy (yes vs. no) | 1.2 | 0.80–1.75 | 0.4 | |
| Rejection at 1 year | Recipient age (6–18 vs. 0–5 years) | 1.53 | 1.25–3.25 | 0.004 |
| Recipient ethnicity (non-white vs. white) | 1.09 | 0.76–1.58 | 0.6 | |
| Pre-transplant dialysis (yes vs. no) | 1.49 | 1.02–2.18 | 0.04 | |
| Laparoscopic vs. open donor nephrectomy | 1.53 | 1.08–2.15 | 0.02 | |
| DGF (yes vs. no) | 4.37 | 2.10–9.12 | <0.0001 | |
| Antibody induction therapy (yes vs. no) | 1.08 | 0.76–1.53 | 0.67 |
Graft survival
For recipients 0–5-years old, graft survival rates were not statistically significantly different for open and laparoscopic donor recipients: at 1 year, 97.4% versus 90.4%; at 2 years, 95.8% versus 90.4% (p = 0.12). Similarly, we observed no significant differences in graft survival for open and laparoscopic recipients 6–18-years old: at 1 year, 96.0% versus 94.9%; at 2 years, 92.1% versus 90.6% (p = 0.22).
Discussion
The recently observed increase in live kidney donations in the United States is thought to be, at least in part, the result of the introduction of laparoscopic donor nephrectomy, which was shown to result in a shorter length of hospital stay, less post-operative pain, improved cosmesis and more rapid return to work and to normal activities of daily life for the kidney donors (20–24). Hence, it is not surprising that the laparoscopic procurement mode has rapidly become the preferred approach to the live kidney donor for transplantation of adult recipients (2). In contrast, relatively little is known about the impact of this new procurement technique on the field of pediatric kidney transplantation. Analysis of laparoscopic donor nephrectomy in the context of pediatric transplantation is very relevant, because live donor transplantation is the treatment of choice for nearly all children with end-stage renal disease (16). Although the results of our study suggest that laparoscopic (vs. open) procurement for transplantation of pediatric recipients has started to gain ground later than for their adult counterparts, the current annual rate of increase of the proportion of laparoscopically procured grafts is similar to the one observed in adult live donor transplantation. As a consequence of this significant trend, the majority of all live donor kidneys for pediatric recipients are currently procured laparoscopically. In both adult and pediatric transplantation, this shift in surgical practice concerning the donor was not evidence based, but mostly driven by the donors' needs and expectations, and, to a lesser extent, the recipients' attitudes (12,13,25–28).
Initial concerns regarding the potential implications of the laparoscopic procurement mode for the recipient did center on two areas: technical-surgical complications and impaired early graft function. With respect to technical-surgical complications, these concerns have now been largely alleviated as a result of the learning curve and technical improvements pertaining to the procurement technique itself. If donor vessel length, particularly when procuring a right kidney, is adequately preserved, and the ureter is not devascularized, there is no available evidence that adult or pediatric recipients of laparoscopically procured kidneys experience higher technical complication rates (3–6,27–29). In contrast, several studies have suggested that adult recipients of laparoscopic donor kidneys may experience slower early graft function—without any impact on short- and intermediate-term graft outcome (11–13). For pediatric live donor kidney recipients, however, the impact of the laparoscopic procurement on functional and immunologic outcomes remains unclear. To date, there are only three published studies, each involving only a small number of pediatric recipients that compare the effects of laparoscopic versus open procurement (3–5). In one of these three studies, laparoscopic procurement was associated with slower early graft function (3), while there were no differences in the two other studies (4,5). This lack of definitive evidence and the conflicting data in the currently available literature prompted us to review the UNOS database and to compare outcomes for laparoscopic versus open live donor grafts in pediatric kidney recipients.
Why is the study of the effect of the procurement mode particularly important for pediatric recipients? Pediatric (vs. adult) kidney recipients are unique in several regards. Large adult kidneys, especially when transplanted into very small pediatric recipients, face unique hemodynamic challenges (17–19). Any additional injury that a graft may suffer during procurement may be exacerbated by the post-reperfusion hemodynamic challenges in pediatric recipients. It is now well established that early graft injury, manifested either by slow early function or DGF, is associated with increased acute and chronic rejection for both adult and pediatric recipients (30–33). Thus, even a modest degree of added initial non-specific injury may portend poorer long-term graft survival (34). Also, pediatric recipients are more immunoreactive than adults (14,35). Any increased graft immunogenicity as a result of a non-specific procurement injury may, therefore, potentiate the likelihood for adverse immunologic outcomes in pediatric recipients and lead to more graft losses from chronic rejection. Modifications of the procurement or transplant procedure that may increase graft injury and immunogenicity must therefore be closely scrutinized with respect to potential long-term consequences.
We analyzed our pediatric study population in two separate age categories because (i) glomerular filtration rates and serum creatinine levels are age-dependent (36); (ii) recipient age at transplantation was noted by previous investigators to be a significant independent predictor of graft survival (14,35); and (iii) hemodynamic and immunologic challenges for the kidney graft may be age dependent (17–19).
Importantly, all donor kidneys were from adults and had thus comparable nephron masses, minimizing the potential impact of an important variable that may affect long-term outcome. To further increase the meaningfulness of our analysis, we included only open kidneys that had been procured by an extraperitoneal flank approach. If we had included the few kidneys that were procured transabdominally, we may have introduced a bias because the more invasive transabdominal approach may be associated with more operative stress and fluid requirements; both could affect kidney perfusion during the procurement and quality of early graft function after transplantation.
Our open versus LapD were demographically comparable. The recipient study groups were also demographically homogeneous, particularly with respect to pre-transplant dialysis incidence, race and retransplantation status—all significant predictors of graft survival.
In our study, which involved a large number of pediatric patients, we observed significantly impaired early graft function, as evidenced by significantly higher DGF rates and higher discharge creatinine levels, for laparoscopic donor kidney recipients. These findings corroborate the observations of slower early laparoscopic kidney graft function that were made in prior studies with adult live kidney donor recipients. However, none of the prior studies did demonstrate such a significant (e.g. for recipients 0–5 years greater than four-fold) increase of the DGF rate associated with laparoscopic procurement. We observed similarly significant differences of acute rejection rates at 6 months for both age categories and at 1 year for recipients 0–5-years old, with significantly higher rejection rates in laparoscopic kidney recipients. To the best of our knowledge, no previous investigator has reported to date such a significant impact of the procurement mode on immunologic outcome. Our observed differences in early function quality and rejection rates are in concordance with the injury-inflammation-immune recognition triangle proposed by Halloran et al. (34). Any increased non-specific graft injury (such as the one presumably caused by the laparoscopic procurement) would engender increased tissue immunogenicity and a higher rate of adverse immunologic events (e.g. acute rejection, chronic rejection, immunologic graft losses) (30,34).
Our univariate analysis findings were confirmed by the results of our multivariate analyses, which demonstrated laparoscopic procurement to be an independent risk factor for acute rejection at 6 months and at 1 year. Our multivariate analyses showed also that higher pediatric recipient age (i.e. 6–18 years) was an independent risk factor for acute rejection at 6 months and 1 year, as well. Further studies will have to delineate the potential role of non-compliance, which may be encountered more frequently among adolescents. Our multivariate analyses results are also consistent with previous studies in adult and pediatric recipients that demonstrated pre-transplant dialysis and DGF to be independent risk factors for acute rejection (16,30,37).
Importantly, our study has several limitations. First, serum creatinine level is a suboptimal parameter for assessing graft function (36). Interpretation of any differences, or lack thereof, must take the insufficient discriminatory power of this variable, particularly in smaller recipients, into account (36). Nevertheless, DGF rates, a more objective parameter, were also substantially affected by procurement mode, supporting a true difference in the quality of early graft function.
Second, the length of stay of recipients of laparoscopic (vs. open) donor kidneys in the 0–5-years age group was slightly longer, albeit without statistical significance. Interpretation of differences in serum creatinine levels and rejection rates at hospital discharge is therefore difficult. At any rate, differences with regards to the quality of early function and immunologic outcomes were also observed when analyzing variables that are not affected by the recipients' length of stay (i.e. DGF rates and rejection at 6 months and 1 year, respectively).
Third, our follow-up times were fairly short, owing to (i) the only relatively recent introduction and increasing popularization of the laparoscopic technique for pediatric live donor transplants and (ii) the fact that information regarding the nephrectomy technique has only been reported to UNOS since 25 October 1999. It may therefore be premature to conclude that there is, in spite of the increased frequency of rejection episodes in the laparoscopic group, no detriment to long-term graft outcome. Clearly, studies with longer follow-up are necessary before a final conclusion on the impact of laparoscopic donor nephrectomy on long-term graft survival can be reached. Nevertheless, it is worrisome to note that previous studies have clearly shown that acute rejection episodes are a significant risk factor for poorer long-term graft outcome in pediatric recipients (16,32).
Fourth, a potential limitation of our retrospective study was that we were not able to control for center differences in management protocols and intensity of immunosuppression. In particular, differences in early calcineurin inhibitor dosing may have affected both short-term renal function (e.g. serum creatinine levels) and immunologic outcome (e.g. graft rejection). Theoretically, there may also be a time-treatment selection bias since the majority of open donor cases was done towards the beginning and the majority of the laparoscopic donor cases towards the end of the study period. This may potentially have led to differing levels of immunosuppression in the face of ever evolving immunosuppressive protocols and may have contributed to the observed differences in acute rejection. Such a bias as explanation for our findings seems unlikely, though, because the study period was relatively short, with presumably relatively few changes in immunosuppressive protocols over time, and because variables that are largely independent of immunosuppression (e.g. DGF rate) showed significant differences. Also, given the nature of our retrospective study, we disposed of only relatively few early data points, i.e. serum creatinine levels, because our analysis was limited by the type of data that was reported to UNOS.
Fifth, our study results may reflect center effects and may have been influenced by the laparoscopic learning curve. Distinction of ‘experienced’ and ‘inexperienced’ centers, however, would have been difficult within our registry database, in part due to the fact that the proportion of laparoscopic transplants increased so dramatically, even during our relatively short study period. Also, because the live donor nephrectomy technique has only been reported to UNOS since late 1999, distinction between ‘experienced’ and ‘inexperienced’ centers at the beginning of our study period (January 2000) was not possible based on the available data. Clearly, only a prospective repeat analysis in several years would allow to address more definitively the potentially confounding effect of the laparoscopic learning curve.
In conclusion, laparoscopic nephrectomy has also gained widespread acceptance for the procurement of live donor kidneys that are to be transplanted into pediatric recipients. As with adult recipients (11–13,38), laparoscopic procurement of live donor kidney grafts for pediatric recipients appears safe from a recipient perspective, resulting in similar short-term graft survival as for openly procured kidneys—even in very small recipients.
The advent of laparoscopic nephrectomy has undoubtedly contributed to the increase of available live donor kidney grafts, also for pediatric recipients (21–24). Hence, advocating for a return to a more widespread use of open nephrectomy based on the results of this study would only decrease the overall number of available kidney grafts for pediatric recipients—a patient group that benefits most from receiving a kidney transplant in a timely fashion (39). Rather, we believe that our results should serve as an impetus to (i) study the impact of the procurement mode on outcome in pediatric live donor kidney recipients in a prospective multicenter analysis, (ii) to further investigate whether our findings are the result of the laparoscopic learning curve (and could be expected to improve over time with more of the centers gaining experience with the use of this technique in the pediatric transplant setting) and (iii) to aggressively implement supportive hemodynamic measures that have been shown to be renoprotective in both the donor and the recipient. For donors, previous experimental and clinical studies have suggested that volume loading and administration of certain pharmacologic agents can improve intraoperative renal blood flow during the pneumoperitoneum phase (7,40). For recipients, hemodynamics must be optimized, including the provision of adequate pre-load and systemic blood pressure at the time of graft reperfusion, particularly in smaller recipients (18,19,41). Future studies are warranted to identify additional potential strategies to further optimize laparoscopic procurement outcomes.
Acknowledgment
The authors thank Deborah Hoang for preparation of the manuscript.




