HLA-Specific Antibodies are Risk Factors for Lymphocytic Bronchiolitis and Chronic Lung Allograft Dysfunction
Abstract
Bronchiolitis obliterans syndrome (BOS) represents a major limitation in lung transplantation. While acute rejection is widely considered the most important risk factor for BOS, the impact of HLA-specific antibodies is less understood. Of 51 lung recipients who were prospectively tested during a 4.2 ± 1.6-year period, 14 patients developed HLA-specific antibodies. A multi-factorial analysis was performed to correlate the prevalence of BOS with HLA antibodies, persistent-recurrent acute rejection (ACR-PR), lymphocytic bronchiolitis, and HLA-A, -B, and -DR mismatches. HLA-specific antibodies were associated with ACR-PR (10/14 vs. 11/37 with no antibodies, p < 0.05), lymphocytic bronchiolitis (8/14 vs. 10/37, p < 0.05), and BOS (10/14, vs. 9/37, p < 0.005). Other risk factors for BOS were: lymphocytic bronchiolitis (13/18 vs. 6/33 with no lymphocytic bronchiolitis, p < 0.0001), ACR-PR (12/21 vs. 7/30 with no ACR-PR, p < 0.05), and the number of HLA-DR mismatches (1.7 ± 0.48 in BOS vs. 1.2 ± 0.63 without BOS, p < 0.05). The presence of antibodies exhibited a cumulative effect on BOS when it was associated with either lymphocytic bronchiolitis or ACR-PR. The complex relationship between the development of HLA antibodies and acute and chronic lung allograft rejection determines the importance of post-transplant screening for HLA-specific antibodies as a prognostic element for lung allograft outcome.
Introduction
Bronchiolitis obliterans syndrome (BOS) is a major outcome in lung transplantation (LTX), due to its high prevalence, poor prognosis and contribution to shorter graft survivals in LTX compared to other solid-organ transplants (1,2), The 5-year graft and patient survival in LTX are 42 and 44%, respectively, while 65–69% in kidney, heart and liver transplantation (3,4). The prevention of BOS is highly desirable, since its response to augmented immunosuppressive therapy has limited success.
BOS is considered a multi-step injury-remodeling phenomenon, which has a self-evolving potential when a no-return point is reached (5). Various risk factors have been linked with BOS (6). Among them, perivascular acute rejection (ACR) is considered the most important (4,7). The number and severity of ACR episodes appear to have a cumulative effect (8–11). Other reported but still controversial risk factors can be categorized as immunologic (cytomegalovirus (CMV) disease, lymphocytic bronchitis/bronchiolitis [LBB], HLA and non-HLA antibodies and the number of HLA mismatches) or non-immunologic (donor and recipient's age, sex and race, the type of LTX, ischemia time, recipient disease and non-compliance to therapy and infections) (11–15).
Preformed or post-transplant developed HLA-specific antibodies were associated with BOS in several clinical studies (16–21). We have previously reported that HLA antibodies were associated with ACR of the lung allograft in the first 18 months after transplantation (22). The strength of the association was proportional with the number and severity of ACR episodes, especially high-grade and persistent-recurrent acute rejection (ACR-PR) (23). Since ACR is considered the most important risk factor for BOS, it seems that HLA antibodies could have an indirect effect on the development of BOS. To assess the direct impact of HLA-specific antibodies on the development of BOS after 3 additional years of follow-up, we have performed a multi-variate risk factor analysis, including HLA antibodies, ACR-PR and LBB. All data were collected prospectively, in a single-center longitudinal study.
Materials and Methods
Study subjects
Fifty-one consecutive patients who received a lung transplant at the University of Pittsburgh Medical Center between January 1999 and May 2001, and had at least 1 year of follow-up, were eligible for study. The protocol was approved by the Institutional Review Board of the University of Pittsburgh and informed consent was obtained from all patients. The systemic triple-immunosuppressive therapy was similar for the entire cohort, and included tacrolimus, azathioprine and steroids. The mean follow-up was 4.2 ± 1.6 (range: 2–4.5) years. During this time period, our program was conducting a prospective, randomized, double-blinded trial of aerosolized-cyclosporine versus aerosol-placebo. Of the patients examined in the present study, 27 received aerosolized-cyclosporine, and 24 received aerosol-placebo (24).
Detection of HLA-specific antibodies
Anti-coagulated blood samples were collected on the day of transplantation and at the time of transbronchial biopsies. A total of 518 serum/plasma specimens were prospectively tested by enzyme-linked immunosorbent assay (ELISA). We used commercial LAT-M™ and LAT-1288™ ELISA kits (One Lambda; Canoga Park, CA, USA), in accordance with the manufacturer's instructions to identify IgG anti-class I and/or class II HLA-specific antibodies (23).
Transbronchial biopsies
Fiberoptic bronchoscopy and fluoroscopically guided transbronchial lung biopsies were routinely performed approximately 2 weeks after transplantation and at 3-month intervals thereafter, to detect asymptomatic rejection or infection. Bronchoscopy was performed whenever changes in clinical or functional parameters, such as oxyhemoglobin desaturation, >10% decline in forced expiratory volume in 1 second (FEV1) from baseline or new infiltration by chest radiography occurred, as well as 4–6 weeks after pulse metylprednisolone and 6–8 weeks after anti-thymocyte globulin, to assess the response of the augmented immunosuppression.
Acute perivascular rejection was graded as described (25,26). Persistent acute rejection was defined as at least two consecutive episodes ≥A2, requiring treatment with anti-thymocyte globulin and/or pulsed steroids. Recurrent acute rejection was defined as two treated episodes of acute cellular rejection (≥A2), with a single no-rejection (A0–A1) biopsy in-between. LBB was diagnosed as described (27). At least moderate grades (ISHLT grade ≥ B) were considered. The diagnosis of BOS was established accordingly with ISHLT published criteria, while surveillance spirometry in accordance with American Thoracic Society criteria (28,29). For statistical analysis, we also used FEV1 at 12, 18, 24, 30 and 36 months post-LTX, and at the time of BOS diagnosis, when other causes for lower FEV1 (especially infections and bronchial stenosis) were excluded. The values of FEV1 were compared to the best airflow of every patient in the first 100 days post-LTX, and expressed as a percentage.
Statistical analysis
Exploratory analysis determined the relation of independent variables (sex, donor and recipient age, type of lung transplant—single/double, number of HLA-A, -B, and -DR matches, acute cellular rejection, HLA antibodies, lymphocytic bronchiolitis and CMV pneumonitis) with BOS or FEV1 as dependent variables. For nominal variables, we made use of contingency tables and the χ2 test (p-values expressed as Fisher's exact test), while Student's t-test and analysis of variance (ANOVA) were utilized for continuous outcomes. After exploratory analysis, several models of logistic regression were used. Rejection-free survival analysis also assessed the relation of risk factors with the onset of BOS, considering p-values calculated by log-rank statistics and a significance level of 0.05. A Kaplan-Meier estimator was used to calculate the BOS-free survival curve. Statistical analysis was done by STATISTICA software from StatSoft™ (30).
Results
The pattern of HLA-specific antibodies
All 51 patients had a negative lymphocytotoxic cross-match before LTX, and were tested by ELISA for the presence of HLA-specific antibodies both pre-transplant, and at 1, 2, 3, 6, 9 and 12 months post-LTX. After the first year, all samples available at the time of transbronchial biopsies were also tested.
During a mean follow-up of 4.2 ± 1.6 years after LTX, 14 patients (27%) had ELISA-detectable HLA antibodies, while 37 remained ELISA negative. A total of 149 samples were analyzed for the ELISA-positive group (10.7 samples/patient), and 369 samples were available for the ELISA-negative group (9.98 samples/patient, no statistical difference). The pattern of HLA-specific antibodies is summarized in Table 1.
| Patient | Initial detection (month post-LTX) | HLA class | Donor-specific HLA | ACR-PR | LBB | BOS | Grade 2–3 BOS |
|---|---|---|---|---|---|---|---|
| 1 | 1 | I | B51 | Yes | Yes | Yes | No |
| 2 | 1 | II | DQ2 | Yes | No | No | No |
| 3 | 2 | I | B7, 67 | No | Yes | No | No |
| 4 | 1 | I | No* | Yes | Yes | Yes | Yes |
| 5 | Pre-LTX | II | DR52 | Yes | Yes | No | No |
| 6 | 1 | I | No* | Yes | No | Yes | No |
| 7 | 1 | II | No | Yes | Yes | Yes | Yes |
| 8 | Pre-LTX | I + II | No | Yes | Yes | Yes | Yes |
| 9 | 2 | I | B7 | Yes | No | Yes | Yes |
| 10 | 2 | I | No | Yes | Yes | Yes | No |
| 11 | 3 | I | No* | No | No | Yes | Yes |
| 12 | 13 | I + II | A2 | No | No | No | No |
| 13 | 22 | II | DR4 | Yes | No | Yes | No |
| 14 | 35 | I + II | No* | No | Yes | Yes | Yes |
- LTX, lung transplantation; ACR-PR, persistent-recurrent acute rejection; LBB, lymphocytic bronchiolitis/bronchitis; BOS, bronchiolitis obliterans syndrome.
- *HLA-specific antibodies against public epitopes of the donor.
Two patients had HLA antibodies before LTX, while 12 patients developed antibodies only after transplant. The majority of de novo HLA antibodies (9/12) were detected in the first post-operative year, while three patients developed de novo antibodies in the second through fourth post-operative year. The HLA class distribution was as follows: 7 patients had anti-class I, 4 patients anti-class II, and 3 patients both anti-class I and anti-class II HLA antibodies. Donor specificity was documented in seven patients. Another four patients exhibited reactivity to public antigens cross-reactive with the donor HLA types.
Risk factors for BOS
BOS was diagnosed in 19 patients (37%), with grade 2–3 BOS present in 10 cases (20%). The variables that reached significant statistic associations with BOS were: HLA antibodies, lymphocytic bronchiolitis, ACR-PR (Table 2) and the number of HLA-DR mismatches.
| Risk Factor | Outcome | |||||
|---|---|---|---|---|---|---|
| BOS | p* < RR = | BOS 2–3 | p* < RR = | FEV1 (%)** | p*** < | |
| Ab (N = 14) | 10 | 0.005 | 6 | 0.05 | 41 | 0.0005 |
| No Ab (N = 37) | 9 | 3 | 4 | 4 | 63 | |
| De novo Ab (N = 12) | 9 | 0.005 | 5 | 0.05 | 45 | 0.005 |
| No Ab (N = 37) | 9 | 3 | 4 | 4 | 63 | |
| LBB (N = 18) | 13 | 0.0001 | 8 | 0.001 | 49 | 0.05 |
| No LBB (N = 33) | 6 | 3 | 2 | 8.8 | 61 | |
| ACR-PR (N = 21) | 12 | 0.05 | 7 | 0.05 | 48 | 0.005 |
| No ACR-PR (N = 30) | 7 | 2.5 | 3 | 3.3 | 64 | |
- BOS, bronchiolitis obliterans syndrome; RR, relative risk; FEV1, forced expiratory volume in 1 second; Ab, ELISA-detected HLA-specific antibodies; LBB, lymphocytic bronchiolitis; ACR-PR, persistent-recurrent acute rejection; N, number of patients.
- *Fisher's exact p.
- **FEV1 at 2.5 years post-transplantation.
- ***ANOVA.
HLA-specific antibodies: HLA-specific antibodies were associated with a higher prevalence of BOS (71%) and grade 2–3 BOS (43%), when compared to ELISA-negative patients (24 and 11%, respectively), with a relative risk of 3 for BOS, and 4 for grade 2–3 BOS (Tables 1 and 2). Patients with de novo HLA antibodies developed BOS in 75% of cases, while patients with donor-specific and donor cross-reactive antibodies developed BOS in 64% of cases, significantly higher than ELISA-negative patients.
The detection of antibodies took place before the diagnosis of BOS in nine patients, and after the onset of BOS in one patient (no. 14). The average detection of antibodies was in the 177th ± 331 post-operative day, while the average period of BOS diagnosis in ELISA-positive group was in the 566th ± 259 post-operative day. These results indicated that the detection of HLA antibodies preceded the onset of BOS by approximately 1.1 years.
The cumulative proportion of BOS-free survival at 4 years after LTX was 72% in the group of patients without antibodies, versus 23% in patients with ELISA-detected HLA-specific antibodies (Cox's p < 0.001, Figure 1).

BOS-free cumulative survival was significantly lower in patients with HLA-specific antibodies (Ab) versus no HLA-specific antibodies (no Ab); Cox's p < 0.001.
Persistent-recurrent acute rejection: In this cohort, 12 out of 21 patients with ACR-PR developed BOS, compared to 7 out of 30 patients without ACR-PR (p = 0.02, Table 2). Furthermore, the odds ratio test (log alpha = 1.477, 95% CI =[0.459, 2.495]) indicated a high degree of association between ACR-PR and BOS. The frequency of ACR-PR episodes/patient was also higher in patients with BOS (0.84 ± 0.85) when compared to no BOS patients (0.37 ± 0.65, p < 0.05)
The prevalence of ACR-PR was higher in patients with antibodies (10/14 ELISA-positive vs. 11/37 ELISA-negative, p = 0.01). The frequency of ACR-PR episodes/patient was also significantly higher in patients with antibodies (1.07 ± 0.91) relative to ELISA-negative patients (0.35 ± 0.58, p = 0.001). In all cases except patient 13 (Table 1), HLA antibodies were detected before or at the time of biopsy-proven ACR-PR. Furthermore, the antibodies and ACR-PR had a cumulative effect on the development of BOS: patients in whom both risk factors occurred exhibited a higher incidence of BOS than patients with ACR-PR, but no HLA antibodies (p = 0.04, Figure 2).
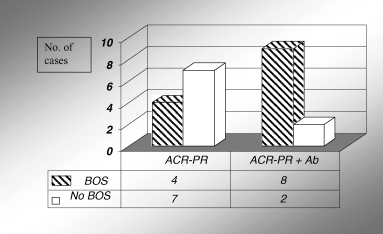
HLA-specific antibodies (Ab) and ACR-PR have cumulative effect on the prevalence of BOS; p < 0.05.
Lymphocytic bronchiolitis/bronchitis: LBB was diagnosed in 18 patients (35%). The prevalence of BOS in patients with LBB was 69%, compared to 18.7% in patients without LBB (relative risk = 3.7, Table 2). Furthermore, the prevalence of grade 2 and grade 3 BOS was significantly higher in LBB-positive patients (8/18) versus LBB-negative (2/33, relative risk = 8.8).
HLA antibodies were associated with a higher prevalence of LBB (8/14 ELISA-positive patients had LBB, compared to 10/37 ELISA-negative patients, p = 0.04). Furthermore, there were more LBB episodes/patient in ELISA-positive group compared to ELISA-negative (1.07 ± 1.38 vs. 0.37 ± 0.79, p = 0.029).
The prevalence of BOS in the group of LTX recipients without LBB (N = 33) was as follows: 4 out of 6 patients with HLA antibodies developed BOS, compared to only 2 out of 27 in ELISA-negative group (χ2= 11.59, p = 0.001). These findings indicate a direct association of HLA-specific antibodies and BOS, in the absence of LBB. Conversely, in the group of patients without HLA-specific antibodies (N = 37), 7 out of 10 LBB-positive developed BOS, while only 2 out of 27 LBB-negative developed BOS (χ2= 15.53, p = 0.0001), demonstrating an association between LBB and BOS not influenced by antibodies. The highest prevalence of BOS was in the subgroup with LBB and HLA antibodies (6/8 patients). The cumulative effect of LBB and antibodies on airflow decrease is depicted in Figure 3, showing that the presence of both risk factors was associated with significantly lower FEV1 than when only one (or none) risk factor was involved.
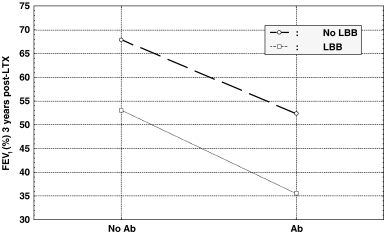
The cumulative effect of HLA-specific antibodies (Ab) and lymphocytic bronchiolitis (LBB) on airflow (FEV1 measured at 36 months post-transplantation, y-axis).
HLA-DR mismatches: HLA-DR mismatches were associated with high-grade BOS (3 patients with BOS 2–3 out of 29 patients with 0–1 HLA-DR mismatches, vs. 7 out of 22 with two HLA-DR mismatches, p = 0.05). There were no significant associations between the number of HLA-A and/or HLA-B mismatches and the prevalence of BOS.
The complex relationship of multiple risk factors with BOS is shown in Figure 4. The first 6 months after LTX were characterized by a higher prevalence of ACR-PR and de novo HLA antibodies, while most LBB episodes were diagnosed after 6 months.

HLA-specific antibodies and the relationship with other immunologic risk factors for BOS. All arrows indicate significant statistic associations.
Discussion
The impact of HLA-specific antibodies
In this longitudinal study, patients with HLA antibodies had increased prevalence of ACR-PR, LBB and BOS. The relative risk for BOS and high-grade BOS (between 3 and 4) was in the same range as reported by Palmer et al. (18).
Several authors have also shown that pre- (17,31) or post-transplant (18,21,32) humoral sensitization in lung recipients was associated with increased incidence of BOS. In contrast, others found no significant difference in chronic rejection in patients with HLA antibodies that were detected by complement-dependent cytotoxicity (33). However, the lower sensitivity of the complement-dependent cytotoxicity assay as compared to the ELISA could have underestimated the level of humoral sensitization in these cohorts.
Reznik et al. indicated that HLA antibodies binding to HLA class I molecules were associated with BOS (20), while Palmer et al. showed the importance of anti-class II HLA antibodies (18). Our data and of others (31) demonstrated a significant association of both class I and class II HLA antibodies with BOS. In particular, our prospective study also found an early association of HLA-specific antibodies with ACR-PR, and a late association with LBB.
We could demonstrate donor specificity in the half of sensitized patients, and most of those in whom we failed to show donor specificity had de novo HLA-specific antibodies cross-reactive with donor public antigens. Furthermore, de novo HLA antibodies exhibited the strongest association with BOS. Other authors also showed that de novo HLA antibodies had a significant association with BOS, but were often not donor specific, presumably because the pathogenic (highest affinity) antibodies were bound to the graft (19,34–36). According to the humoral theory of transplantation, IgG antibodies can cause chronic allograft rejection in all solid allografts, and they may appear in circulation before evidence of graft dysfunction (37). When rejection was observed, IgG and C5a levels were significantly increased in the transplanted lungs (38). We have also shown that the presence of HLA-specific antibodies was associated with increased soluble C4d in bronchoalveolar lavage (39). Furthermore, the inhibition of complement activation has been reported to attenuate the development of BOS (40). Infiltration of B lymphocytes in the lung allograft during rejection episodes was a very good marker to predict refractoriness to augmented immunossupression (41). Similarly, in kidney allografts, a cluster of B-cell markers analyzed by micro-array technique was associated with steroid-resistant rejection and graft loss (42). These findings indicate that cellular and humoral immune responses may concomitantly occur during rejection, and contribute to the severity of graft dysfunction.
In this 5-year prospective study, we observed that the majority (75%) of lung recipients with de novo humoral sensitization developed HLA-specific antibodies during the first year post-transplantation. Similarly, a high rate of sensitization in the first year post-transplantation was reported in lung and other solid-organ transplants (31,43). Furthermore, we also detected the antibodies before the diagnosis of ACR-PR and/or LBB, while we and others (32) have shown that HLA antibodies preceded the diagnosis of BOS by more than 1 year.
Although the use of solid-phase assays such as flow cytometry or ELISA has improved the sensitivity of antibody detection, the level of humoral sensitization in LTX might still be underestimated. Possible limitations could be inadequate sampling timing, the ‘sponge’ effect of the graft, and the involvement of non-HLA antibodies (15,44).
Persistent-recurrent acute rejection and BOS
Increased frequency and severity of acute rejection episodes are strongly associated with the development of BOS, and late ACR episodes augment the risk of advanced disease (2,5,10,11). Our data confirmed the high degree of association between ACR-PR and BOS, with a relative risk of 2.5.
An important risk factor for ACR-PR was the detection of HLA antibodies (22,23). Furthermore, patients with ACR-PR and antibodies had increased prevalence of BOS when compared to patients with ACR-PR alone (cumulative effect). In multi-variate analysis, the association of antibodies with BOS was stronger than the association of ACR-PR with the same outcome. These findings indicated a distinct, direct association of HLA-specific antibodies with BOS, apart from the association of antibodies with ACR-PR.
Other authors reported the co-existence of cellular and humoral immune response, as well (45–47).
Lymphocytic bronchiolitis and BOS
The association of HLA-specific antibodies with LBB is first reported in this study. In addition, patients who exhibited both antibodies and LBB had the highest prevalence of BOS (75%) and suffered a significant decline in airflow (cumulative effect). Increasing number of studies has shown that LBB, in the absence of infection, has a similar implication for graft dysfunction as ACR, in adult and pediatric series (10,13,48–50). Boehler et al. have shown that lymphocytic airway infiltration is a precursor of fibrous obliteration in an experimental model of BOS (12). Ross et al. showed that BOS developed approximately 8 months after detection of histologic LBB, and 2 years after transplantation (51). The ‘active’ form of constrictive bronchiolitis, with attendant lymphocytic inflammation of the airways, likely precedes the ‘inactive’ or scarred form of constrictive bronchiolitis (52). In other studies, LBB appeared to be significantly associated with BOS only by univariate analysis, but in a two-risk-factor model, it did not augment the influence of acute rejection (53). In the present study, LBB exhibited a significant association with BOS (even stronger with grade 2–3 BOS, relative risk = 9) both in uni- and multi-variate analysis.
The association between HLA-specific antibodies and ACR-PR or high-grade LBB may define a dual anti-allograft immune response, involving both cellular and humoral arms. Most immunosuppressive drugs have an anti-T-cell action, but little or no effect on B and plasma cells, therefore the mixed forms of rejection often lack a response to conventional immunossupression.
HLA-DR mismatches and BOS
In LTX, the influence of HLA-DR matching on BOS has been shown in large population studies (1,3,54,55). In our study, patients who were matched for at least one HLA-DR locus had significant lower prevalence of BOS when compared to patients with two DR loci mismatches. Although our observations are confirmatory, it was interesting to detect this association in a relatively small group of patients.
The limitations in the statistic power of our study are linked to the relatively small number of patients (51). However, hundreds of data points (samples analyzed by ELISA, transbronchial biopsies, spirometry tests, etc.) conferred a fair number for statistical purposes, even in the case of multi-factorial regression models. Furthermore, a single-center study has the advantage of homogeneity and consistency in clinical and pathological evaluations, pulmonary function tests and therapeutic protocols. It is the first study to assess the association of HLA-specific antibodies with lymphocytic bronchiolitis, and also the first prospective study to investigate the impact of HLA antibodies on BOS in a multi-factorial analysis with other immunologic risk factors.
Conclusions
The analysis of potential risk factors for chronic lung allograft dysfunction, in a single-center prospective study, showed that HLA-specific antibodies, ACR-PR, lymphocytic bronchiolitis and the number of HLA-DR mismatches had a high degree of association with BOS. A significant proportion (25%) of the cohort developed HLA antibodies post-transplantation, predominantly in the first year. The antibodies showed their impact both early (first 6 months post-LTX), by increased frequency of ACR-PR, and later (after 1 year), by higher prevalence of LBB and BOS. Furthermore, the antibodies were detected before the diagnosis of acute and chronic rejection, and exhibited a cumulative effect in the presence of ACR-PR and/or LBB. The combination of sensitive methods for pre- and post-transplant antibody screening with therapeutic strategies directed towards both cellular and humoral allo-sensitization should be considered in the clinical management of lung transplanted patients.
Acknowledgments
This work has been supported by NIH Grant HL-65189.




