FTY720 Attenuates Hepatic Ischemia-Reperfusion Injury in Normal and Cirrhotic Livers
Abstract
Hepatic ischemia-reperfusion injury is an inevitable consequence during liver surgery. The outcome is particularly poor in cirrhotic livers, which are more prone to hepatic ischemia-reperfusion injury. We aim to study whether FTY720 could attenuate hepatic ischemia-reperfusion injury both in normal and in cirrhotic livers. We applied a 70% liver-ischemia (60 min) model in rats with normal or cirrhotic livers. FTY720 was given 20 min before ischemia and 10 min before reperfusion (1 mg/kg, i.v.). Liver tissues and blood were sampled at 20 min, 60 min, 90 min, 6 h and 24 h after reperfusion for detection of MAPK-Egr-1, Akt pathways and caspase cascade. Hepatic ultrastructure and apoptosis were also compared. FTY720 significantly improved liver function in the rats with normal and cirrhotic livers. Akt pathway was activated at 6 and 24 h after reperfusion. FTY720 significantly down-regulated Egr-1, ET-1, iNOS and MIP-2 accompanied with up-regulation of A20, IL-10, HO-1 and Hsp70. MAPK (Raf-MEK-Erk) pathway was down-regulated. Hepatic ultrastructure was well maintained and fewer apoptotic liver cells were found in the FTY720 groups. In conclusion, FTY720 attenuates ischemia-reperfusion injury in both normal and cirrhotic livers by activation of cell survival Akt signaling and down-regulation of Egr-1 via Raf-MEK-Erk pathway.
Introduction
As liver injury related to warm ischemia and subsequently reperfusion is an inevitable consequence during liver surgery, development of new pharmaceutical strategies to attenuate hepatic ischemia-reperfusion (I/R) injury is always important for achieving a better clinical outcome. Hepatic I/R injury is a complex patho-physiological process with a number of contributing factors. The major events of hepatic I/R injury involve acute phase inflammatory response, microcirculatory disturbance related to sinusoidal injury and loss of intracellular homeostasis (1). Therefore, it is difficult to achieve effective protection by targeting only at an individual mediator or mechanism.
FTY720(2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) is synthetically derived from myriocin (ISP-1), a metabolite isolated from ascomycete, Isaria sinclarii (2). FTY720 was demonstrated to prolong allograft survival in several solid organ transplant animal models (3,4). The possible mechanism of its immuno-modulation was related to sequestration of peripheral blood lymphocytes to secondary lymphoid organs and selective induction of infiltrated lymphocytes apoptosis (5–7). Recently, FTY720 has been found to be able to prevent I/R injury in liver and kidney models under a similar mechanism (8,9). However, these previous studies mainly focused on the effect of FTY720 on lymphocytes. The effect of FTY720 on hepatocytes has not been studied, especially on diseased livers, which are prone to hepatic I/R injury. Investigation of the direct effect of FTY720 on hepatocytes will provide some novel insights for additional protective mechanism of FTY720 on hepatic I/R injury.
Previous animal studies demonstrated that early growth response-1 (Egr-1) was a master switch under I/R injury (10) and played an important role in liver graft damage after liver transplantation (11). Up-regulation of acute phase inflammatory pathways related to Egr-1 was a key mechanism of injury during hepatic I/R. On the other hand, cell survival (Akt) signaling was important for intracellular homeostasis. Akt plays a critical role in controlling the balance between survival and apoptosis (12). Akt activation has been demonstrated to be a key mediator in antiapoptotic signaling during liver regeneration (13) and to prevent cardiac I/R injury (14,15).
To understand the protective mechanism of FTY720 in hepatic I/R injury thoroughly in both normal and diseased livers, we investigated the acute phase inflammatory pathways related to Egr-1, Akt and apoptosis (caspase) signaling, together with molecular markers related to intracellular homeostasis in a rat hepatic I/R injury model with normal or cirrhotic liver.
Materials and Methods
Animals
Male Sprague-Dawley rats (body weight: 220–280 g) were used to isolate hepatocytes for primary culture and in the animal experiments. Liver cirrhosis was induced in the rats (5–6 weeks, body weight: 160–180 g) by subcutaneous injection of 50% carbon tetrachloride diluted with olive oil at a dose of 0.2 mL/100 g of body weight twice a week for 9 weeks. Rats were housed in a standard animal laboratory with free activity and access to water and chow. They were kept under constant environment conditions with a 12-h light–dark cycle. All operations were performed under clean conditions.
Experimental procedure
Hepatic I/R model and treatment regimen The experiment was conducted in two groups of rats: normal liver group (n = 60) and cirrhotic liver group (n = 60). A model of segmental (70%) hepatic ischemia was used. Under pentobarbital anesthesia (intraperitoneal injection, 40 mg/kg) and after midline laparotomy, all structures in the portal triad to the left and median liver lobes were clamped for 60 min followed by different periods of reperfusion.
FTY720 (molecular weight 343.94 Da) was kindly provided by Novartis Pharmaceuticals Ltd (Basel, Switzerland). FTY720 was dissolved in injection water at a concentration of 1 mg/mL. Treated rats received FTY720 by intravenous injection 20 min before ischemia and 10 min before reperfusion at a dose of 1 mg/kg. The rats in the control groups were given the same amount of normal saline.
Primary hepatocyte culture Primary hepatocytes were isolated by a modified two-step collagenase perfusion procedure (16). The cells were seeded into six-well plates. After seeding for 24 h, FTY720 was added at the concentration of 15 μM. The cells were harvested after FTY720 administration for 60 min, 90 min, 6 h and 24 h, respectively, for protein extraction.
Sample collection
Liver tissues and blood were sampled at 20 min, 60 min, 90 min, 6 h and 24 h after reperfusion for hepatic gene detection, morphologic examination, systemic cytokine expression and liver function tests. Six rats were included at each time point for the treatment group and control group, respectively. The normal liver tissues were sampled immediately after abdominal wall incision for normal control. To compare the expression levels with or without treatment accurately in the cirrhotic liver, liver biopsies were taken from the right inferior lobe before FTY720 treatment and liver ischemia for basal level in the cirrhotic liver group. As the liver biopsy was a small piece of liver tissue, it would not affect the results.
Liver function by biochemical test
Blood samples (0.5 mL) collected at different time points were used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin levels (Hitachi 747 Automatic Analyzer, Boehringer Mannheim Gmbh, Mannheim, Germany).
Hepatic gene expression profiles by real-time quantitative reverse-transcriptase-polymerase chain reaction (RT-PCR)
Liver biopsies were stored at −80°C until total RNA extraction. The total RNA was extracted using Rneasy Midi Kit (Qiagen, GmbH, Hilden, Germany). About 0.5 μg total RNA from each sample was used to perform reverse transcription (RT) reaction using TaqMan Reverse Transcription Reagents (17) (Applied Biosystems, Foster City, CA). RT product (1 μL) was used to perform real-time quantitative RT-PCR using TaqMan Core Reagent Kit (Applied Biosystems) by the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) (17). The probes and primers of Egr-1, ET-1, inducible nitric oxide synthase (iNOS), macrophage inflammatory protein-2 (MIP-2), heme oxygenase-1 (HO-1) and A20 were designed under the Primer Express software according to the criteria for real-time PCR (Applied Biosystems) (Table 1). The probes and primers of Hsp70 and interleukin-10 (IL-10) were commercially available from Applied Biosystems Limited. The TaqMan Ribosomal RNA Control Reagent (18S RNA probe and primer pair, Applied Biosystems) was used for internal control in the same PCR plate well to normalize the target gene amplification copies. All samples were detected in triplicate, and the readings from each sample and its internal control were used to calculate the gene expression level. After normalization with the internal control, the gene expression levels after I/R injury were calculated as the percentage of the basal levels in sham operation in the normal liver groups. In the cirrhotic liver group, the gene expression levels were expressed as the ratios to basal levels before hepatic ischemia in the same rat biopsy.
| Gene | Probe (FAM) | Primer pairs |
|---|---|---|
| Egr-1 | TGTGACACACCTTGCCGATGG | Sense: |
| AGTTTCACGTCTTGGTGCCTTT | ||
| Anti-sense: | ||
| CCCTCACGATTGCACATGTC | ||
| ET-1 | AGACCCCGCAGGTCCAA | Sense: |
| TGATGTCCAGGTGGCAGAAGT | ||
| Anti-sense: | ||
| TGCTCCTGCTCCTCCTTGATGGACAAG | ||
| HO-1 | TGCCCCGCTCTACTTCCCTGAGG | Sense: |
| CGAAACAAGCAGAACCCAGTCT | ||
| Anti-sense: | ||
| AGCCCTTCGGTGCAGCT | ||
| A20 | TTTAAAACCATGCACCGATACACGCTGG | Sense: |
| AACCTACCAATGGGATCATCTATCA | ||
| Anti-sense: | ||
| GGCAAAACTGGCATGTTCTGA | ||
| MIP2 | CCCAGACAGAAGTCA | Sense: |
| AGAACATCCAGAGCTTGAGTGTGA | ||
| Anti-sense: | ||
| TTTTGACCGCCCTTGAGAGT | ||
Cell signaling pathways related to cell survival, apoptosis and inflammatory response by Western blot
Western-blot assay was modified from the method described previously (11). The immunoreactive signals were visualized by ECL Plus Western blotting detection reagents (Amersham, Little Chalfont, UK), and quantified by scanning densitometry (Syngene, Cambridge, UK). Anti-cleaved caspase-3 Ab, anti-cleaved caspase-9 Ab, anti-Akt Ab, anti-phospho-Akt (ser473) Ab, anti-phospho-Akt (Thr308) Ab, anti-phospho-glycogen synthase kinase-3β (GSK)-3β Ab, anti-phospho-Bad Ab, anti-phospho-Forkhead transcription factor (FKHR) Ab, anti-phospho-Raf Ab, anti-phospho-MEK1/2 Ab, anti-p44/42 MAP kinase Ab and anti-phospho-p44/42 MAPK Ab were purchased from Cell Signaling Technology, Inc (Beverly, MA). Anti-actin Ab was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The protein expression levels after reperfusion in the cirrhotic liver group were demonstrated together with their paired basal levels.
Morphological study by light and electron microscopy
Liver biopsies were taken at different time points after reperfusion for light microscopy for hematoxylin-eosin staining. The specimens for electron microscopy were immediately cut into 1 mm cubes and fixed in 2.5% glutaraldehyde in sodium carcodylate-hydrochloride buffer overnight at 4°C for electron microscopy section. The sections would be examined under a transmission electron microscope (Philips EM208, Eindhoven, Holland).
Apoptotic cell detection by Terminal Deoxynucleotide Transferase-Mediated dUTP Nick-End Labeling (TUNEL)
The paraffin sections of the liver biopsies at different time points were detected for apoptotic cells by the TUNEL method (11) (In Situ Cell Death Detection Kit, Roche Biochemicals, Mannheim, Germany).
Statistical analysis
Continuous variables were expressed as median and range. Mann-Whitney U test was used for statistical comparison. Significance was defined as p < 0.05. Calculations were made with the help of SPSS computer software (SPSS, Chicago, IL).
Results
FTY720 significantly improved liver function in both normal and cirrhotic livers
FTY720 treatment significantly decreased the serum levels of ALT and AST in both normal and cirrhotic livers at 6 h and/or 24 h after reperfusion (Figure 1). Although the serum total bilirubin level was relatively higher in the cirrhotic liver group receiving FTY720 at 60 min after reperfusion, the median value of total bilirubin was 5 μmol/L (within normal range) and it declined to the same levels as the control group subsequently (Figure 1).

Liver function at different time points after reperfusion in normal (A) and cirrhotic (B) livers. a: Alanine aminotransferase (ALT); b: aspartate aminotransferase (AST); c: total bilirubin. *p < 0.05.
FTY720 down-regulated Egr-1 expression through MAPK pathway in both normal and cirrhotic livers
In the normal liver group, the protein expressions of phospho-c-Raf, MEK and Erk were down-regulated by FTY720 starting at 60 min after reperfusion (Figure 2A). The hepatic mRNA level of Egr-1 was decreased in the FTY720 treatment group during 60 min to 6 h after reperfusion compared to the control group (60 min: 156% (115–390%) vs. 1147 (634–1727%)) relative to basal level, p = 0.048; 90 min: 865% (788–1088%) vs. 1700% (1483–1890%) relative to basal level, p = 0.048; 6 h: 228% (152–288%) vs. 629% (300–1428%) relative to basal level, p = 0.021). Several down-stream genes, such as ET-1 (vasoconstrictor), iNOS and MIP-2 (inflammatory cytokine and chemokine), were also down-regulated by FTY720 treatment, especially at 6 h and 24 h after reperfusion (Figure 3A(b), A(c), A(d)) (ET-1: 6 h—769%[337–2118%] vs. 4679%[3334–6907%] relative to basal level, p = 0.021; iNOS: 24 h—134.5%[56–307%] vs. 297.3%[203.5–429.6%] relative to basal level, p = 0.037; MIP-2: 6 h—96%[21–372%] vs. 1948%[1638–2258%] relative to basal level, p = 0.019, 24 h—89%[60–239%] vs. 814%[357–2577%] relative to basal level, p = 0.014).

Hepatic protein expression of MAPK pathway in normal (A) and cirrhotic (B) livers after reperfusion. N: Normal; Ctr: control group; FTY: FTY720 treatment group; b: basal level from liver biopsy before ischemia; a: expression level from liver biopsy after reperfusion.
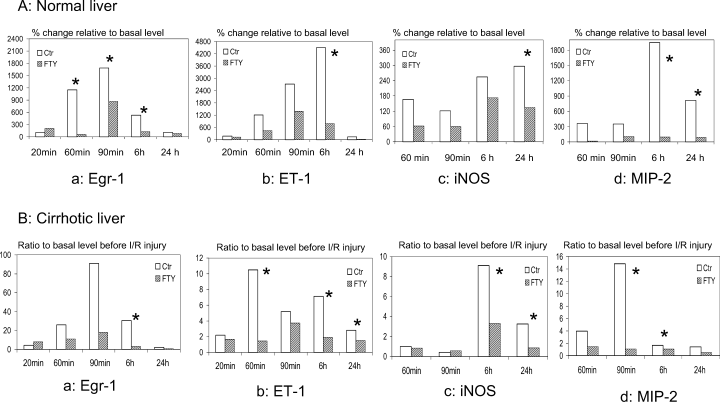
Hepatic gene expression (mRNA levels) related to acute phase inflammatory responses by real-time RT-PCR in normal (A) and cirrhotic (B) livers after reperfusion. a: Early growth response-1 (Egr-1); b: endothelin-1 (ET-1); c: inducible nitric oxide synthase (iNOS); d: macrophage inflammatory protein-2 (MIP-2). *p < 0.05.
In the cirrhotic liver group, up-regulation of Phospho-c-Raf, MEK and Erk after I/R injury compared to the basal level was attenuated by FTY720 treatment during the first 24 h after reperfusion (Figure 2B). Consistent with the results of normal livers, the mRNA levels of Egr-1, ET-1, iNOS and MIP-2 were also down-regulated by FTY720 during the first 24 h after reperfusion compared to those of the control groups (Figure 3B).
FTY720-activated Akt pathway together with heat shock proteins, anti-inflammatory cytokine in both normal and cirrhotic livers
Up-regulation of Phospho-Akt-ser473 was found after FTY720 treatment in primary hepatocytes isolated from normal rats. On the other hand, cleaved-caspase-3 was down-regulated by FTY720 (Figure 4A).
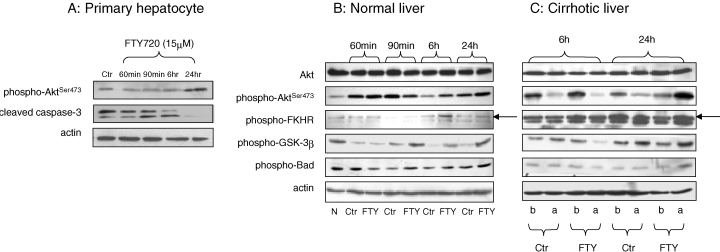
Hepatic protein expression of Akt pathway in primary hepatocyte culture (A) after FTY treatment, normal (B) and cirrhotic (C) livers after reperfusion. N: Normal; Ctr: control group; FTY: FTY720 treatment group; b: basal level from liver biopsy before ischemia; a: expression level from liver biopsy after reperfusion.
The Akt signaling pathway was compared between the rats with or without FTY720 treatment (Figure 4B, C). The phosphorylation of Akt was mainly found at ser473. The activation of Akt phosphorylation by FTY720 in normal liver was mainly found at 6 h and 24 h after reperfusion. Three targets of Akt, FKHR, GSK-3β and Bad, were phosphorylated and inactivated by phospho-Akt mainly in the FTY720 treatment group at 6 h and 24 h after reperfusion (Figure 4B).
In the cirrhotic liver, expression of phospho-Akt and its targets were only found at 6 h and 24 h after reperfusion (Figure 4C). Up-regulation of phospho-Akt and its targets after I/R injury compared to the basal level was mainly found in the FTY720 treatment group at 24 h after reperfusion.
Anti-inflammatory cytokine IL-10 was found with relatively higher mRNA (Figure 5A) and protein (213 [133–277] pg/mL vs. 151.6 [124–171] pg/mL, p = 0.025) levels in normal liver after FTY720 treatment at 24 h after reperfusion. Two heat shock proteins, HO-1 and Hsp70, were found with higher mRNA levels after reperfusion mainly in the cirrhotic liver group with FTY720 treatment (Figure 5B).
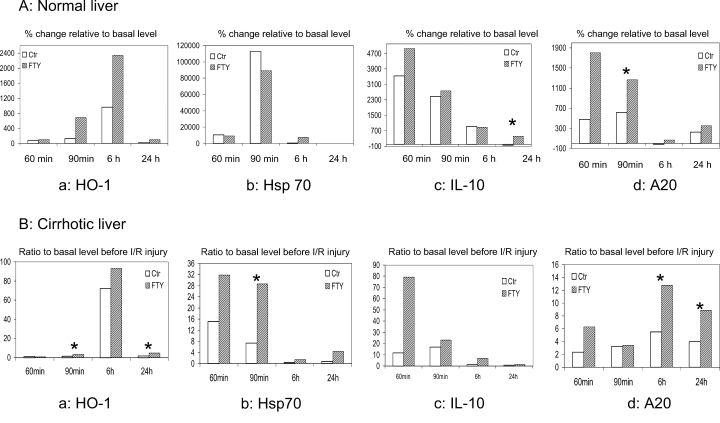
Hepatic gene expression (mRNA levels) related to intracellular homeostasis by real-time RT-PCR in normal (A) and cirrhotic (B) livers after reperfusion. a: Heme oxygenase-1 (HO-1); b: heat shock protein 70 (Hsp70); c: interleukin-10 (IL-10); (D) A20. *p < 0.05.
FTY720 up-regulated anti-apoptotic genes together with partial down-regulation of apoptotic genes
Anti-apoptotic protein-A20 was found with relatively higher mRNA levels in both normal and cirrhotic livers with FTY720 treatment (Figure 5A(d), B(d)). Over-expression of A20 occurred earlier (90 min after reperfusion) in normal livers than in cirrhotic livers (6 and 24 h after reperfusion).
There was no significant difference of cleaved caspase-3, -7, -9 between the rats with or without FTY720 treatment in normal liver during the first 24 h after reperfusion (Figure 6A). Down-regulation of cleaved caspase-7 was found in cirrhotic livers with FTY720 treatment, especially at 90 min and 6 h after reperfusion (Figure 6B). However, the changes of cleaved caspase-3, -9 after I/R injury were comparable between the rats with or without FTY720 treatment in the cirrhotic groups (Figure 6B).
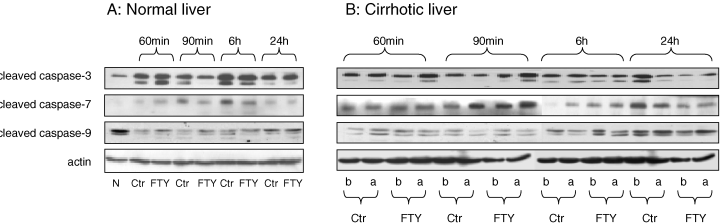
Hepatic protein expression of apoptotic genes in normal (A) and cirrhotic (B) livers after reperfusion. N: Normal; Ctr: control group; FTY: FTY720 treatment group; b: basal level from liver biopsy before ischemia; a: expression level from liver biopsy after reperfusion.
FTY720 maintained liver architecture in both normal and cirrhotic livers
In the normal liver, hepatic lobular architecture was well preserved after FTY720 treatment at 6 h and 24 h after reperfusion (Figure 7A(a), A(b)). The hepatocytes and portal tracts showed normal morphological features. On the contrary, patchy necrosis was found around the portal tract in the control group at 6 h after reperfusion (Figure 7A(c)). Twenty-four hours after reperfusion, ballooning change of hepatocytes accompanied with focal necrosis was also found in the control group (Figure 7A(d)).
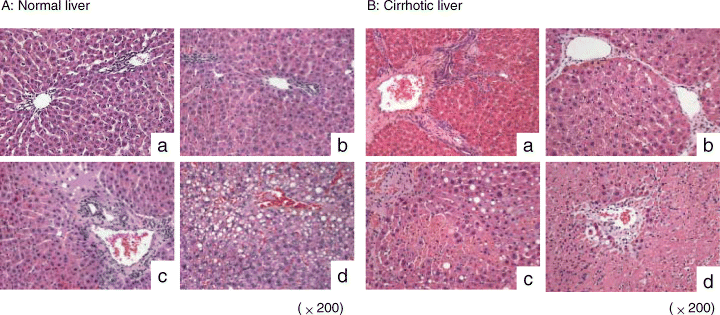
Hepatic architecture after reperfusion in normal (A) and cirrhotic (B) livers. a: FTY720 treatment group at 6 h after reperfusion; b: FTY720 treatment group at 24 h after reperfusion; c: control group at 6 h after reperfusion; d: control group at 24 h after reperfusion.
As for the cirrhotic liver, apart from the perivenular fibrosis induced by CCl4, there was no obvious necrosis of hepatocytes at 6 h and 24 h after reperfusion in the FTY720 treatment group (Figure 7B(a), B(b)). Hepatic necrosis developed focally at 6 h after reperfusion in the control group (Figure 7B(c)) and deteriorated at 24 h after reperfusion (Figure 7B(d)).
The liver architecture was well maintained by FTY720 pre-treatment in the normal liver group. The hepatic ultrastructural features were significantly different between the cirrhotic livers with or without FTY720 treatment (Figure 8). The hepatic sinusoid was well maintained after FTY720 treatment (Figure 8a, b) compared to the total disruption of sinusoidal lining cells in the control group (Figure 8c). Degeneration of cytoplasm (Figure 8c) and tremendous mitochondria swelling (Figure 8d) were also found in the control group.
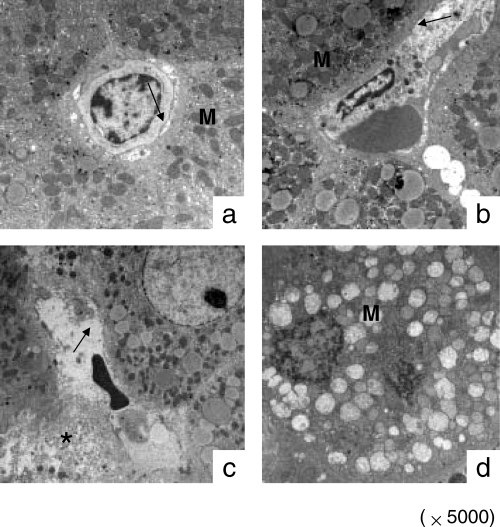
Hepatic ultrastructure in cirrhotic liver after reperfusion. a: FTY720 treatment group at 6 h after reperfusion; b: FTY720 treatment group at 24 h after reperfusion; c: control group at 6 h after reperfusion; d: control group at 24 h after reperfusion. M: Mitochondria; arrow: sinusoidal lining cells; *degeneration of cytoplasm.
FTY720 attenuated apoptosis in both normal and cirrhotic livers
FTY720 prevented liver apoptosis remarkably in both normal and cirrhotic livers (Figure 9). The peak of apoptosis occurred at 6–24 h after reperfusion in the normal and cirrhotic liver groups. Only a few apoptotic liver cells were found in the FTY720 treatment group (Figure 9A(a), A(b); 9B(a), B(b)). Most of the apoptotic liver cells were found around the perivenular area in the control groups (Figure 9B(c), B(d)). Patchy necrosis was also found together with apoptotic nuclei at 24 h after reperfusion in normal livers without FTY720 treatment. Both massive apoptotic nuclei and focal necrosis were present in the control group with cirrhotic livers (Figure 9B(d)).

Apoptosis after reperfusion in normal (A) and cirrhotic (B) livers. a: FTY720 treatment group at 6 h after reperfusion; b: FTY720 treatment group at 24 h after reperfusion; c: control group at 6 h after reperfusion; d: control group at 24 h after reperfusion. Arrow: apoptotic nucleus; arrow head: patchy or focal necrosis.
Discussion
To our knowledge, this is the first report on marked prevention of hepatocyte apoptosis by FTY720 and maintenance of hepatic sinusoidal integrity in both normal and cirrhotic rat livers after I/R injury. Previous animal studies showed that attenuation of I/R injury by FTY720 was resulted from remarkable reduction of lymphocytes infiltration (8). However, the effect of FTY720 on hepatocytes has not been studied. Furthermore, no study has investigated the pharmaceutical treatment for hepatic I/R injury in a rat cirrhotic liver model. The investigation of the effect of FTY720 on hepatocytes might provide novel insight for the additionally protective mechanism of FTY720 on hepatic I/R injury. Therefore, we studied the dual effect of FTY720 on acute phase inflammatory response, as well as cell survival and apoptosis pathways, in both normal and cirrhotic liver models.
The current study clearly illustrated that FTY720 down-regulated Raf-MEK-Erk pathway in normal livers from 60 min after reperfusion. Although hepatic I/R injury was exacerbated in cirrhotic liver (18,19), up-regulation of the above pathway in cirrhotic livers after I/R was also attenuated by FTY720 treatment during the first 24 h after reperfusion. The contribution of activation of MEK pathway to the induction of Egr-1 expression was demonstrated previously (20,21). Consistently, significant down-regulation of Egr-1 was found in both normal and cirrhotic livers after FTY720 treatment. Egr-1 is a master switch in I/R liver injury and plays an essential role in the pathogenesis of ischemic tissue damage through triggering the expression of pivotal regulators of inflammation, coagulation and vascular hyperpermeability (10,11). Early and over-expression of Egr-1 was found to be related to severe graft injury by cDNA microarray screening and real-time RT-PCR detection in our liver transplantation animal models (22). Down-regulation of Egr-1 pathway was beneficial for the improvement of graft function (23). Several down-stream genes such as vasoconstrictive gene ET-1, which was considered to be related to hepatic sinusoidal injury (17,22–26), were also down-regulated after reperfusion accompanied with the inflammatory markers iNOS(26) and MIP-2 (27).
In addition to the suppression of acute phase inflammatory response via down-regulation of Egr-1 through Raf-MEK-Erk pathway by FTY720 in hepatic I/R models, the present study also first demonstrated that FTY720 directly activated Akt pathway in hepatocytes. Akt was activated by phosphorylation within the C-terminus at ser473 and promoted cell survival by inhibiting apoptosis by means of its ability to phosphorylate and inactivate several targets, including FKHR, GSK-3β and Bad. Up-regulation of Akt pathway was found in both normal and cirrhotic livers. Therefore, FTY720 was not only effective on normal liver but also beneficial for cirrhotic liver. Together with the up-regulation of phospho-Akt expression, several molecular markers related to intracellular homeostasis including anti-inflammatory cytokine IL-10 (28) and two heat shock proteins, HO-1 (29) and Hsp70 (17,30), were also found up-regulated in normal and/or cirrhotic livers during the first 24 h after reperfusion and FTY720 treatment. The activation of these heat shock protein genes was probably directly resulted from FTY720 rather than from inflammatory stress. Although FTY720 evoked apoptosis in lymphocytes through targeting G-protein-coupled receptors (7), the same effect on hepatocyte and endothelial cells was not found in the current study. By our in vitro study, down-regulation of cleaved caspase-3 was found after FTY720 treatment. Although the significant down-regulation of cleaved caspase-7 (31) was mainly present in cirrhotic liver, another anti-apoptotic gene A20 (32,33) was found up-regulated in both normal and cirrhotic livers. Therefore, the liver cell survival was promoted by activation of Akt pathway together with up-regulation of several protective genes. This pivotal role in cell survival has made Akt an important therapeutic target in hepatic I/R injury.
In conclusion, our study first demonstrated the dual effect of FTY720 on attenuation of hepatic I/R injury in both normal and cirrhotic livers. FTY720 not only ameliorated acute phase inflammatory response through down-regulation of Egr-1 involving Raf-MEK-Erk pathway, but also activated cell survival signaling Akt pathway and up-regulated several protective genes including heat shock proteins and anti-apoptotic genes. The protective mechanism of FTY720 on hepatic I/R injury in both normal and cirrhotic livers might extend its potential clinical applications in liver surgery.
Acknowledgments
The authors thank Dr. Joseph Lee and his staff in the Division of Clinical Biochemistry, the University of Hong Kong in performing the liver enzyme assay; and Ms. Amy Wong, Mr. Bosco Yau and Mr. W.S. Lee of the Electron Microscope Unit for their assistance in performing electron microscopy sections. The authors also thank Novartis Pharmaceuticals Ltd (Basel, Switzerland) for their kind gift—FTY720. This study was supported by the Distinguished Research Achievement Award, and Sun C.Y. Research Foundation for Hepatobiliary and Pancreatic Surgery of the University of Hong Kong.




