Minimization of Immunosuppressive Therapy After Renal Transplantation: Results of a Randomized Controlled Trial
Abstract
Modern immunosuppressive regimens reduce the acute rejection rate by combining a cornerstone immunosuppressant like tacrolimus or cyclosporine with adjunctive agents like corticosteroids, mycophenolate mofetil (MMF) or azathioprine, often associated with untoward side effects.
A 6-month randomized study was conducted in 47 European centers. Triple therapy with tacrolimus (trough levels 5–15 ng/mL), corticosteroids (dosage 10 mg/day) and MMF (1 g/day) was administered for 3 months. From day 92, patients either continued with triple therapy (control, n = 277), or stopped steroids (n = 279), or stopped MMF (n = 277). Surrogate markers for long-term benefits were changes in lipid profiles and occurrence of hematological, gastrointestinal and infectious complications.
The 6-month acute rejection incidence (biopsy-proven) was similar in all groups (17.0% vs. 15.1% vs. 14.8%, p = 0.744), although the incidence after month 3 was higher in the steroid stop group than in the two other groups. Mean reductions in total cholesterol (18.9 mg/dL [0.49 mmol/L]) and LDL-cholesterol (8.1 mg/dL [0.21 mmol/L]) between months 4 and 6 were greater in the steroid stop group (p < 0.001). Leukopenia (p = 0.0082), serious CMV infection (p = 0.024), anemia (p = NS) and diarrhea (p = NS) were less frequent in the MMF stop group.
In a study population of immunologically low-risk patients' withdrawal of corticosteroids or MMF from a tacrolimus-based therapy at 3 months was feasible. A longer follow-up will be needed to confirm the expected advantages for the long-term outcome and to assess the long-term safety of this minimization of immunosuppressive therapy.
Introduction
In recent years, renal transplantation has seen the advent of a number of new immunosuppressive agents. Including monoclonal antibodies, the transplant physician is currently able to choose among at least eight immunosuppressive agents to devise an efficacious and safe regimen and tailoring of immunosuppressive therapy to the individual patient becomes conceivable. However, there is also the danger to devise regimens that result in over-immunosuppression leading to an increased susceptibility to infections and malignancies, and higher rates of cardiovascular complications and hematological disorders (1,2).
In our study we investigated a controlled withdrawal of two adjunctive immunosuppressants from a tacrolimus-based therapy. Corticosteroids are associated with numerous post-transplant complications predominantly related to the cardiovascular system (3,4). Hyperlipidemia, which is increased in patients receiving steroids, is one of the most important risk factors for cardiovascular diseases (5). Although it has been shown that the addition of mycophenolate mofetil (MMF) to either tacrolimus or cyclosporine reduces the incidence of acute rejection, its use has been associated with the development of hematological and gastrointestinal side effects, and increased incidences of viral infections (6–8).
Since the advantages of minimizing the immunosuppressive medication may only become apparent in the long term, we used two surrogate markers to assess the benefits in our trial, namely the change in the lipid profile after steroid withdrawal and the occurrence of hematological, gastrointestinal and infectious side effects following stop of MMF.
Study Design
This was a 6-month, randomized, parallel-group, prospective study comparing continued triple therapy with tacrolimus, corticosteroids and MMF (control), with withdrawal of either corticosteroids or MMF at the end of month 3 (day 92). The investigators were blinded with respect to randomization until the month-3 visit (Figure 1). Randomization (1:1:1) was stratified by center and donor type (living or cadaveric).
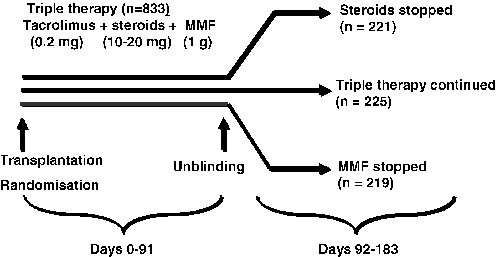
Randomisation was 1:1:1 and the investigators were blinded to the assignment of the treatment group until day 91. During the first 3 months, all patients received triple therapy of tacrolimus, MMF and steroids. After day 92 either patients remained on triple therapy (control) or steroids were stopped, or MMF was stopped and patients were observed for another 3 months.
The initial oral dose of tacrolimus was 0.2 mg/kg per day given in two divided doses. Target whole-blood trough levels were 10–20 ng/mL between days 0 and 14, and 5–15 ng/mL thereafter. Corticosteroids (methylprednisolone or equivalent) were administered as an intravenous bolus of 500 mg or less on day 0 and 125 mg on day 1, followed by oral prednisone starting at daily doses of 20 mg on days 2–14, 15 mg on days 15–28 and 10 mg from day 29 onwards. MMF was given at 1 g/day in two divided doses. At day 92, in the steroid stop group, dosage was tapered from 10–0 mg/day within 2 weeks. In the MMF stop group, MMF was discontinued on day 92. The introduction of lipid lowering therapy was not permitted after day 85.
Patients who suffered steroid-resistant acute rejection or graft loss, those who had their dose of steroids or MMF modified for more than 10 consecutive days or had stopped taking tacrolimus for more than 1 day were excluded from entering the second 3-month phase of the study. Patients who had failed to comply with the protocol during the first 3 months were also excluded.
The study was undertaken in accordance with the Declaration of Helsinki (1964) and all subsequent amendments, and was approved by the local ethics committees. Written informed consent was obtained from all participants.
Patients
The study was conducted in 47 centers in 11 European countries between September 1998 and December 2000. Adult candidates for primary renal transplantation or retransplantation were eligible. Donors (living or cadaveric) were between 5 and 65 years of age and had a compatible ABO blood type. Female patients of childbearing potential were using adequate contraception. Patients were excluded from the study if they had previously undergone organ transplantation other than a renal transplant, or if they had lost a previous kidney transplant due to early acute rejection. Those with panel reactive antibody grade ≥50% were ineligible, as were patients requiring immunosuppressive therapy for any reason other than kidney transplantation. Patients were also excluded if they were HIV positive, had familial hypercholesterolemia, significant liver disease, gastrointestinal disorders, a history of malignancy, ongoing infection or were allergic to any of the study drugs.
Efficacy and Safety Assessments
Blood samples for lipid analysis were collected from fasting patients and were analyzed by a central laboratory (Medical Research Laboratories International, Zaventem, Belgium). HDL was determined using the enzymatic endpoint method and LDL-cholesterol was calculated using the Friedewald formula. The mean absolute change in serum cholesterol, LDL-, HDL-cholesterol and triglycerides between days 91 and 183 was calculated.
The efficacy endpoints were the incidence of and time to first acute rejection, the overall frequency of acute rejection, severity of biopsy-proven acute rejection and the incidence of and time to first steroid-resistant acute rejection. All clinical or laboratory signs of rejection were confirmed histologically and graded for severity according to the Banff criteria (9,10). Biopsy-proven acute rejection was defined as any acute rejection episode for which a Banff score of I (mild), II (moderate) or III (severe) was recorded. The primary treatment for acute rejection was bolus steroid therapy. Rejection episodes not resolving following treatment with steroids (maximum 2000 mg, including taper to protocol doses) were classified as being steroid-resistant. Patients with steroid-resistant rejection were treated with mono- or polyclonal antibodies.
The safety evaluation was based on the incidence of adverse events, patient and graft survival, renal function (assessed by serum creatinine), change in laboratory parameters and vital signs. Graft loss was defined as retransplantation, transplantectomy, death or a return to long-term dialysis.
Statistical Analysis
The statistical analysis was performed by the contract research organization Quintiles, who also carried out all steps of data entry and validation.
The planned sample size of 540 patients (180 patients per treatment group) provided at least 80% power to reject the null hypothesis of equality of treatment groups in the absolute change in serum cholesterol between months 3 and 6 on a 5% significance level (analysis of variance), if in two of the three treatment groups this change differed by at least 20 mg/dL (0.52 mmol/L). This allowed for a patient dropout rate of approximately 30%.
Intention-to-treat (ITT) analysis was used for all variables and included all randomized patients who received at least one dose of study medication. The Kruskal-Wallis test (or analysis of variance depending on distribution of data) was used to test changes in serum total cholesterol, HDL- and LDL-cholesterol and triglycerides between months 4 and 6 for equality among all treatment groups. Subsequent pairwise treatment comparisons were performed using the Wilcoxon-Mann-Whitney test or the Student's t-test.
Acute rejection rates were analyzed using the Chi-square test, patient and graft survival rates were estimated using Kaplan-Meier analysis, with treatment comparisons made using the Wilcoxon-Gehan test. Fisher's exact test was used to analyze the incidence of adverse events.
RESULTS
Patients
A total of 838 patients were recruited into the study (Figure 2). Five patients did not undergo transplantation. The ITT analysis included 277 patients receiving triple therapy (control), 279 who stopped steroids and 277 who stopped MMF. A total of 14 patients died; 7 deaths occurred during the study (control n = 4; steroid stop n = 2; MMF stop n = 1) and 7 after discontinuation from the study (control n = 2; steroid stop n = 1; MMF stop n = 4). The pattern of study discontinuation was similar in all groups, until day 91, 52 (control), 58 (steroid stop) and 58 (MMF stop) patients discontinued the study; the reasons for discontinuation are summarized in Table 1.
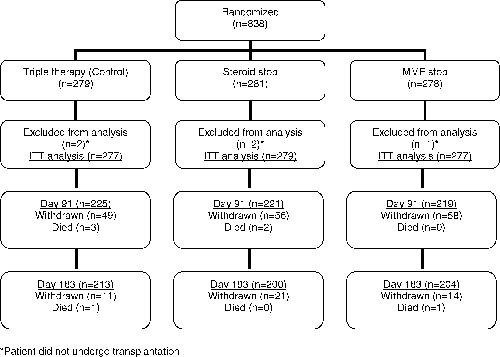
Patient flow. In the control group 225 patients (80.6%), in the steroid stop group 221 patients (79.2%) and in the MMF stop group 219 patients (78.8%) entered the second part of the study. The study was completed by 213 patients (76.3%) in the control group, 200 patients (71.2%) in the steroid stop group and 204 patients (73.4%) in the MMF stop group.
| Control (n = 277) n (%) | Steroid stop (n = 279) n (%) | MMF stop (n = 277) n (%) | |
|---|---|---|---|
| Between day 0 and 91 (total) | 49 (17.7) | 56 (20.1) | 58 (20.9) |
| Steroid-resistant acute rejection during first 3 months | 16 (5.8) | 11 (3.9) | 15 (5.4) |
| Graft loss | 6 (2.2) | 13 (4.7) | 15 (5.4) |
| Protocol violation with respect to treatment regimens1 | 13 (4.7) | 14 (5.0) | 12 (4.3) |
| Other2 | 14 (5.1) | 18 (6.5) | 16 (5.8) |
| After day 91 (total) | 11 (4.0) | 21 (7.5) | 46 (5.5) |
| Protocol violation with respect to treatment regimens1 | 6 (2.2) | 10 (3.6) | 8 (2.9) |
| Other2 | 5 (1.8) | 11 (3.9) | 6 (2.2) |
- 1Modification of steroids or MMF, tacrolimus stopped for more than 1 day, administration of prohibited medication, introduction or stop of statin therapy.
- 2Incorrect enrolment, withdrawal of consent, and lost to follow-up.
The three study groups were well balanced with respect to demographic and baseline characteristics (Table 2). Donor characteristics were also well matched except for mean age, which was lower in the steroid stop group (40.9 years; p = 0.044) than in MMF stop (43.8 years) or control groups (43.1 years).
| Characteristic | Control (n = 277) | Steroid stop (n = 279) | MMF stop (n = 277) |
|---|---|---|---|
| Mean age (years) | 47.1 | 45.5 | 46.4 |
| Sex (n; %) | |||
| Male | 172 (62.1) | 187 (67.0) | 189 (68.2) |
| Female | 105 (37.9) | 92 (33.0) | 88 (31.8) |
| Race or ethnic group (n; %) | |||
| Caucasian | 273 (98.6) | 274 (98.2) | 271 (97.8) |
| Most common primary diagnoses (n; %) | |||
| Glomerulonephritis | 90 (32.5) | 97 (34.8) | 87 (31.4) |
| Polycystic kidney disease | 43 (15.5) | 42 (15.1) | 41 (15.8) |
| Uropathy | 26 (9.4) | 25 (9.0) | 36 (13.0) |
| Recipient and Donor characteristics (n; %) | |||
| First transplant | 245 (88.8) | 249 (89.6) | 257 (92.8) |
| Cadaveric donor | 255 (92.0) | 257 (92.1) | 256 (92.4) |
| Non heart-beating donor | 13 (4.7) | 13 (4.6) | 11 (4.0) |
| CMV (R– and D+) | 48 (19.4) | 51 (20.6) | 41 (16.2) |
| Mean total HLA mismatch | 2.7 | 2.6 | 2.8 |
Blood lipids
Table 3 summarizes the mean change in blood lipids during the study. During the initial 3 months, mean serum levels of total, LDL- and HDL-cholesterol and triglycerides were comparable in each treatment group. During months 4 and 6, total serum cholesterol levels decreased in the steroid stop group (mean absolute change ± SD: 18.9 ± 31.3 mg/dL [−0.49 ± 0.81 mmol/L]), whereas levels increased minimally in the control group (4.6 ± 43.6 mg/dL [+0.12 ± 1.13 mmol/L]), and the MMF stop group (3.5 ± 34.4 mg/dL [+0.09 ± 0.89 mmol/L]). The reductions in mean total cholesterol and in LDL-cholesterol were significantly greater in the steroid stop group than in the control group and the MMF stop group, (p < 0.001). Mean HDL-cholesterol decreased in the steroid stop group (−7.3 mg/dL [−0.19 mmol/L]) and was not changed in the control group (+0.4 mg/dL [+0.01 mmol/L]) and the MMF stop group (+1.9 mg/dL [+0.05 mmol/L]) during months 4–6. Consequently, the LDL/HDL ratio in all three treatment groups changed only minimally between months 4 and 6. For the changes in mean serum triglycerides, no significant treatment differences were detected (Table 3).
| Blood lipid | Control (n = 277) mean ± SD | Steroid stop (n = 279) mean ± SD | MMF stop (n = 277) mean ± SD | p-value |
|---|---|---|---|---|
| Total cholesterol (mmol/L) | ||||
| Day 0 | 4.81 ± 1.20 | 4.81 ± 1.09 | 4.88 ± 1.11 | |
| Month 3 | 4.99 ± 1.12 | 5.08 ± 1.23 | 5.03 ± 1.07 | |
| Month 6 | 5.16 ± 1.34 | 4.58 ± 1.00 | 5.13 ± 1.05 | |
| Mean change between months 4 and 6 | 0.12 ± 1.13 | –0.49 ± 0.81 | 0.09 ± 0.89 | <0.001 |
| LDL-cholesterol (mmol/L) | ||||
| Day 0 | 2.73 ± 0.98 | 2.74 ± 0.88 | 2.78 ± 0.91 | |
| Month 3 | 2.87 ± 0.80 | 2.93 ± 0.99 | 2.90 ± 0.79 | |
| Month 6 | 2.99 ± 1.05 | 2.70 ± 0.83 | 2.94 ± 0.83 | |
| Mean change between months 4 and 6 | 0.13 ± 0.88 | –0.21 ± 0.71 | 0.05 ± 0.71 | <0.001 |
| HDL-cholesterol (mmol/L) | ||||
| Day 0 | 1.15 ± 0.37 | 1.15 ± 0.35 | 1.15 ± 0.41 | |
| Month 3 | 1.26 ± 0.44 | 1.26 ± 0.42 | 1.25 ± 0.44 | |
| Month 6 | 1.28 ± 0.43 | 1.11 ± 0.36 | 1.33 ± 0.41 | |
| Mean change between months 4 and 6 | 0.01 ± 0.38 | –0.19 ± 0.32 | 0.05 ± 0.36 | <0.001 |
| LDL/HDL ratio | ||||
| Day 0 | 2.58 ± 1.25 | 2.57 ± 1.21 | 2.66 ± 1.20 | |
| Month 3 | 2.53 ± 1.27 | 2.55 ± 1.18 | 2.55 ± 1.16 | |
| Month 6 | 2.59 ± 1.50 | 2.67 ± 1.20 | 2.39 ± 1.00 | |
| Mean change between months 4 and 6 | 0.12 ± 1.42 | 0.18 ± 1.05 | –0.09 ± 0.87 | 0.027 |
| Triglycerides (mmol/L) | ||||
| Day 0 | 2.07 ± 1.15 | 2.04 ± 1.14 | 2.12 ± 1.23 | |
| Month 3 | 1.96 ± 1.07 | 1.91 ± 0.98 | 1.99 ± 1.12 | |
| Month 6 | 1.98 ± 1.56 | 1.70 ± 0.89 | 1.87 ± 1.00 | |
| Mean change between months 4 and 6 | 0.03 ± 1.20 | –0.14 ± 0.81 | –0.06 ± 0.89 | 0.177 |
- All values are mean ± SD.
Rejection
All three regimens were comparable in terms of preventing acute rejection during the 6-month study period. The majority of rejection episodes were steroid-sensitive; there were no differences between the treatment groups with respect to severity of acute rejection, the majority of episodes being assessed as mild (Banff I) or moderate (Banff II) (Table 4). Between months 4 and 6, the incidence of biopsy-proven acute rejection was low in all groups, but was higher in the steroid stop group (n = 13; 5.9%) than in the MMF stop group (n = 4; 1.8%, p = 0.044) or the control group (n = 2; 0.9%; p = 0.004). In all but three cases the episodes were histologically mild (Banff I) and responsive to steroids.
| Control (n = 277) | Steroid stop (n = 279) | MMF stop (n = 277) | |
|---|---|---|---|
| Clinically apparent acute rejection (n; %) | |||
| Overall | 60 (21.7) | 67 (24.0) | 64 (23.1) |
| Corticosteroid-resistant | 20 (7.2) | 17 (6.1) | 19 (6.9) |
| Biopsy-proven acute rejection (n; %) | |||
| Overall | 47 (17.0) | 42 (15.1) | 41 (14.8) |
| Corticosteroid-resistant | 18 (6.5) | 13 (4.7) | 16 (5.8) |
| Histological grade (n; %) | |||
| Mild (Banff I) | 20 (7.2) | 23 (8.2) | 19 (6.9) |
| Moderate (Banff II) | 24 (8.7) | 13 (4.7) | 19 (6.9) |
| Severe (Banff III) | 3 (1.1) | 5 (1.8) | 2 (0.7) |
Patient and graft survival
There was no difference in patient survival at 6 months (control 98.2%, steroid stop 99.3% and MMF stop 98.2%; p = 0.467). Causes of death during the study (n = 7) were myocardial infarction, pneumonia, sepsis and Legionella infection (control), sepsis and pancreatitis (steroid stop), and sudden silent death (MMF stop). After withdrawal from the study, patients died from septicemia and cardiac arrhythmia (control), brain tumor (steroid stop); and multi-organ failure, sepsis (n = 2) and anoxia (MMF stop). Graft survival at 6 months was also similar with 94.2% (control), 92.8% (steroid stop) and 92.4% (MMF stop) (p = 0.669). Almost all graft losses occurred during the first 3 months post-transplant; only one graft loss in the control group, two in the steroid stop group and one in the MMF stop group occurred after day 91.
Adverse events
The overall safety profile was similar in all groups over the 6-month study period (Table 5). The most frequently reported adverse events were hypertension and anemia. Over 6 months, there was a significant difference over all treatment groups for hyperkalemia (p = 0.022) with the lowest value in the steroid stop group. Also for leukopenia (p = 0.008) and for serious CMV-infection (p = 0.024) the treatment groups differed significantly; the lowest values were observed in the MMF stop group.
| Incidence of adverse events over the 6-month treatment period | Control (n = 277) (n; %) | Steroid stop (n = 279) (n; %) | MMF stop (n = 277) (n; %) | p-value |
|---|---|---|---|---|
| Hypertension | 75 (27.1) | 76 (27.2) | 97 (35.0) | NS1 |
| Anemia | 72 (26.0) | 87 (31.2) | 73 (26.4) | NS1 |
| Tremor | 35 (12.6) | 30 (10.8) | 41 (14.8) | NS1 |
| Diarrhea | 33 (11.9) | 47 (16.8) | 44 (15.9) | NS1 |
| IDDM (>30 days insulin)4 | 12 (5.1) | 8 (3.2) | 13 (5.3) | NS1 |
| Hyperkalemia | 28 (10.1) | 21 (7.5) | 41 (14.8) | 0.02161 |
| Leukopenia | 23 (8.3) | 36 (12.9) | 15 (5.4) | 0.00821 |
| Serious CMV infection | 18 (6.5) | 24 (8.6) | 9 (3.2) | 0.0241 |
| New onset MMF-related adverse events during months 4–6 | (n = 225) | (n = 221) | (n = 219) | |
| Leukopenia | 9 (4.0) | 23 (10.4) | 3 (1.4) | p = 0.00992 |
| p < 0.00013 | ||||
| Anemia | 4 (1.8) | 11 (5.0) | 5 (2.3) | p = 0.06982 |
| p = 0.20183 | ||||
| Diarrhea | 8 (3.6) | 13 (5.9) | 5 (2.3) | p = 0.27112 |
| p = 0.08953 | ||||
| New onset infections during months 4–6 | (n = 225) | (n = 221) | (n = 219) | |
| All infections | 55 (24.4) | 68 (30.8) | 42 (19.2) | p = 0.13962 |
| p = 0.00583 | ||||
| Bacterial | 29 (12.8) | 32 (14.5) | 24 (11.0) | NS1 |
| Viral | 16 (7.1) | 22 (10.0) | 10 (4.6) | p = 0.31182 |
| p = 0.04213 | ||||
| Fungal | 3 (1.3) | 5 (2.3) | 3 (1.4) | NS1 |
| Other/unknown | 21 (9.3) | 25 (11.3) | 15 (6.8) | NS1 |
- 1p-value for the comparison of all three treatment groups.
- 2p-value for the comparison of steroid stop versus control.
- 3p-value for the comparison of steroid stop versus MMF stop.
- 4New onset insulin dependent diabetes mellitus.
Among patients without glucose metabolism disorders at baseline, insulin therapy for more than 30 days was required by 3.2% of patients in the steroid stop group compared with 5.3% in the MMF stop group and 5.1% in the control group.
During months 4–6, significantly fewer patients in the control group and the MMF stop group developed leukopenia. Also the incidences of anemia and diarrhea were lower in the MMF stop group than in the steroid stop group, although statistical significance could not be shown.
Similarly, for new onset (i.e. developing during months 4–6) infections the highest incidences were found in the steroid stop group while the lowest incidences were observed in the MMF stop group (p = 0.0058, steroid stop vs. MMF stop). This was particularly true for new onset viral infections, (p = 0.0421, steroid stop vs. MMF stop).
The median values for red blood cell count, hemoglobin, and white blood cell count remained in the normal range in all three groups during the entire study. However, at month 6, the lowest median values were observed for the steroid stop group, while the MMF stop group showed similar values to the control (Figure 3). This difference was significant for hemoglobin, red blood cell count and white blood cell count (p < 0.0001, steroid stop against control, as well as against MMF stop).
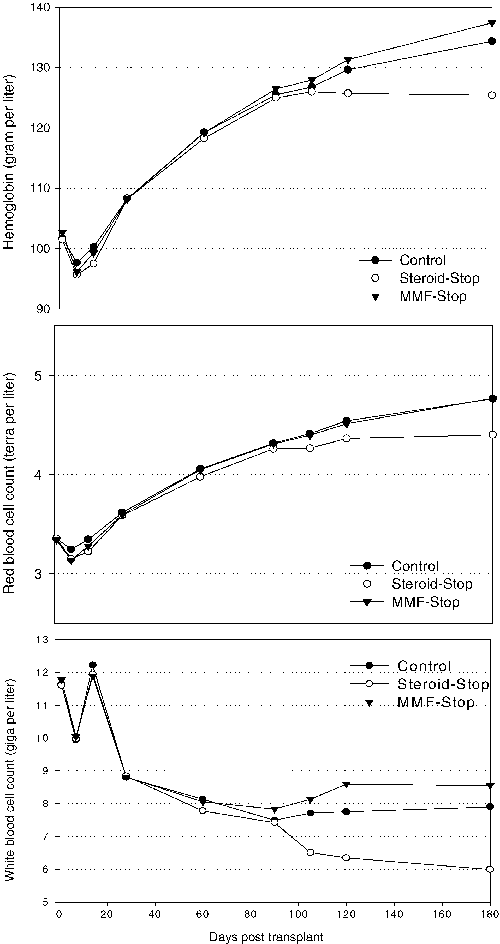
All three treatment groups remained within the normal ranges for the entire study. However, at month 6, the median values in the steroid stop group for hemoglobin, red blood count and white blood count were significantly (p < 0.0001) lower than in the other groups.
Tacrolimus dosing
The mean oral dose of tacrolimus was similar in all groups during the study. At month 6, the mean (±SD) dose was 0.09 ± 0.05 mg/kg in both the control group and the steroid stop group, and 0.10 ± 0.07 mg/kg in the MMF stop group. Whole-blood trough levels of tacrolimus steadily decreased in the control group and the MMF stop group whereas after month 3, the mean blood levels increased in the steroid stop group (Figure 4). At day 121, the mean trough levels were 12.3 ± 3.7 ng/mL (steroid stop), compared with 10.3 ± 3.7 ng/mL (MMF stop) and 10.3 ± 3.3 ng/mL (control).
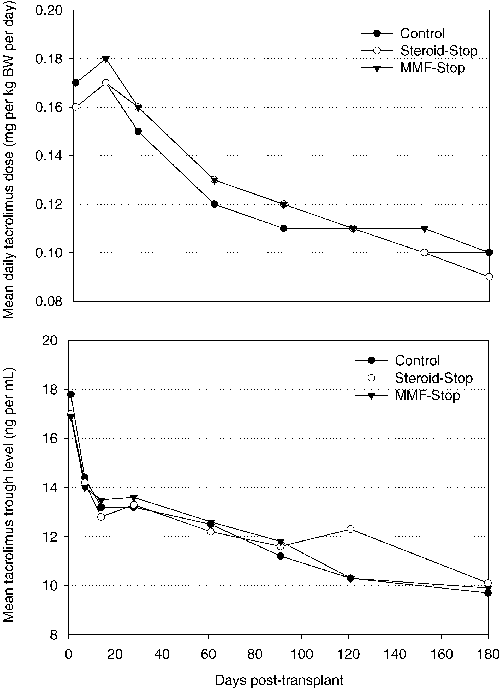
Mean tacrolimus doses were similar in all treatment groups for the entire duration of the study. After withdrawal of steroids, mean tacrolimus levels increased to 12.3 ng/mL while it decreased in the control group and the MMF withdrawal group. At study end trough levels were again similar in all treatment groups.
Renal function
Mean serum creatinine levels were comparable in all treatment groups throughout the study. Mean serum creatinine at month 6 was 1.49 ± 0.5 mg/dL (131.8 ± 46.6 μmol/L), 1.57 ± 0.49 mg/dL (138.8 ± 43.2 μmol/L) and 1.56 ± 0.47 mg/dL (138.3 ± 42.01 μmol/L) in the control, steroid stop and MMF stop groups, respectively.
Discussion
While modern immunosuppressive regimens employing a multitude of immunosuppressive drugs succeed in reducing the risk of acute rejection, the long-term dangers of cardiovascular disease and other post-transplant complications of immunosuppressive therapy persist (1). The minimization of immunosuppression therefore contributes to a reduction of long-term risk factors. As the long-term use of steroids is linked with a well-known pattern of side effects on skin, bone and the cardiovascular system in renal transplant patients (2–4), we withdrew adjunctive steroid therapy after 3 months of combination therapy with tacrolimus and MMF. By withdrawing MMF, we aimed to limit the hematological and gastrointestinal side effects of this compound (6–8).
This study was conducted in a European setting with almost all patients being of Caucasian origin and receiving fairly well-matched transplants. The protocol was developed together with the participating centers, and the MMF dose of 1 g/day, the steroid dose of 10 mg/day, and also the steroid taper over 2 weeks, in contrast to the sudden stop of MMF reflect the centers' practice at the time the study was planned.
The temporary increase of tacrolimus trough levels upon withdrawal of steroids is due to the reduced metabolism by cytochrome P450, an effect that has been described before (11). The higher exposure to tacrolimus may have caused the increased rate of viral infections in the steroid stop group after month 3.
Our results indicate that steroids or MMF can be successfully withdrawn from tacrolimus-based therapy 3 months after renal transplantation, in the group of low-risk patients as they were defined by the study protocol. The withdrawal of steroids resulted in a substantial reduction in total cholesterol and LDL-cholesterol levels, but also a decrease in HDL and no change in HDL/LDL ratio. In contrast, blood lipid levels increased slightly in the MMF stop group and the control group. The finding of a decrease in HDL-cholesterol levels after steroid withdrawal is in accordance with previous studies (12–14).
Our findings highlight the potential benefits of steroid withdrawal in terms of reducing the risk of hyperlipidemia; however, the risks of stopping steroids include a slightly increased incidence of acute rejection. Most episodes of rejection, however, were mild on histological inspection and reversible with steroid treatment; following rejection treatment the patients continued to receive steroids.
Also MMF withdrawal at 3 months post-transplantation did not increase the overall incidence of acute rejection. Its discontinuation was, however, associated with significant reductions in the incidence of leukopenia and viral infections. The observed reduction of diarrhea after MMF withdrawal is also of clinical importance because tacrolimus blood levels are increased in patients with diarrhea (15–16).
During months 4–6, leukopenia, anemia and diarrhea occurred with higher frequencies in the steroid stop group, that is, in patients who remained on tacrolimus and MMF therapy. This phenomenon is possibly linked to the pharmacokinetic interaction of steroids and MMF. A recent study has shown that discontinuation of steroids results in a higher MPA exposure (17). This may have lead to the higher incidence of MMF-related adverse events and infections in the steroid stop group compared with the control group.
In conclusion, our results demonstrate that steroids or MMF can be successfully withdrawn from tacrolimus-based triple therapy in low-risk patients after 3 months post-transplantation. Although, at a low level, more acute rejections occurred in the steroid stop group than in the two other groups, the overall 6-month incidence of acute rejection was similar in all groups. Substantially reduced blood lipid levels were seen after steroid withdrawal, and the stop of MMF resulted in fewer hematological, infectious and gastrointestinal side effects.
The withdrawal of immunosuppressive comedication employed in our study appeared to reduce long-term risk factors and may therefore improve the long-term prospects of the kidney transplant recipients. A longer follow-up will be necessary to confirm these expected advantages and to assess the long-term safety of the minimized immunosuppressive regimens. Finally, minimizing immunosuppressive therapy may also have a cost-sparing effect in the long term.
Acknowledgments
In addition to the 12 main authors the following investigators contributed to this study. Principal investigators were: M. Castagneto (Roma); L. Baeckman (Göteborg); J. Morales (Madrid); M.A. Gentil Govantes (Sevilla); R. Lauzurica (Badalona); D. Del Castillo (Cordoba); M. González Molina (Malaga); D. Garcia (Valencia); P. Lang (Créteil); H. Neumayer (Berlin); D. Durand, (Toulouse); S. Stefoni (Bologna); G. Rizzo (Pisa); H. Seiter (Rostock); D.J.M. Tabernero (Salamanca); A. Palmer (London); M. Meurisse (Liege); F. Valderrabano (Madrid); B. Ringe (Göttingen); D. Cantarovich (Nantes); G. Rifle (Dijon); P. Vialtel (Grenoble); F. Mignon (Paris); M. Kessler (Vandoeuvre les Nancy); U. Kunzendorf (Erlangen); D.M. Rivero (Cadiz); B. Charpentier (Le Kremlin Bicêtre); Y. Lebranchu (Tours); K. Claesson (Uppsala); G. Kirste (Freiburg); D.A. Barrientos (Madrid); P. Deteix (Clermont-Ferrand); F. Mühlbacher (Vienna); E. Hadjyannakis (Athens) and C. Legendre (Paris).
Coinvestigators in this study were: D. Chaib Eddour (Brussels); L. Kyllonen (Helsinki); M. Christiaans (Maastricht); B. Maes (Leuven); N. Baldan (Padva); S. Rodger (Glasgow); J. Ortuño Mirete (Madrid); A. Sanz-Guajardo (Madrid); S. Gil-Vernet (Barcelona); A. Kostakis (Athens); J.C. Ruiz (Santander); F. Cittero (Rome); A. Andrés (Madrid); G. García-Algarra (Sevilla); B. Bayes (Barcelona); R. Pérez-Calderón (Cordoba); M. Cabello (Malaga); D. Ramos (Valencia); M. Pastural (Creteil); K. Budde (Berlin); L. Rostaing (Toulouse); M.P. Scolari (Bologna); C. Tregnaghi (Pisa); D. Burmeister (Rostock); P. García Cosmes (Salamanca); A. de Roover (Liege); F. Anaya (Madrid); L. Moreno (Göttingen); Y. Tanter (Dijon); F. Bayle (Grenoble); B. Viron (Paris); E. Renoult (Vandoeuvre les Nancy); L. Renders (Erlangen); A. Mazuecos (Cadiz); F. Kriaa (Le Kremlin); A. Al'Najjar (Tours); J. Wahlberg (Uppsala); J. Geier (Freiburg); A. Sánchez-Fructuoso (Madrid); A.E. Heng (Clermont Ferrand) and S. Drakopoulos (Athens). We would like to thank G. Stiegler for expert editorial support.
This study was supported by Fujisawa GmbH, Munich, Germany.




