Complete Inhibition of Acute Humoral Rejection Using Regulated Expression of Membrane-tethered Anticoagulants on Xenograft Endothelium
Abstract
Xenotransplantation promises an unlimited supply of organs for clinical transplantation. However, an aggressive humoral immune response continues to limit the survival of pig organs after transplantation into primates. Because intravascular thrombosis and systemic coagulopathy are prominent features of acute humoral xenograft rejection, we hypothesized that expression of anticoagulants on xenogeneic vascular endothelium might inhibit the process. Hearts from novel transgenic mice, expressing membrane-tethered fusion proteins based on human tissue factor pathway inhibitor and hirudin, respectively, were transplanted into rats. In contrast to control non-transgenic mouse hearts, which were all rejected within 3 days, 100% of the organs from both strains of transgenic mice were completely resistant to humoral rejection and survived for more than 100 days when T-cell-mediated rejection was inhibited by administration of ciclosporin A. These results demonstrate the critical role of coagulation in the pathophysiology of acute humoral rejection and the potential for inhibiting rejection by targeting the expression of anticoagulants to graft endothelial cells. This genetic strategy could be applied in a clinically relevant species such as the pig.
Introduction
Organs from transgenic pigs expressing human regulators of complement activation are resistant to hyperacute rejection after transplantation into primates (1,2). However, many are eventually rejected by acute humoral xenograft rejection (AHXR), which has proven difficult to prevent or treat. AHXR is associated with widespread fibrin deposition within the vasculature of the graft (3–5), the extent of which is often sufficient to cause systemic clotting abnormalities in the recipient, manifesting as acute thrombocytopenia and a consumptive coagulopathy(4–6).
In AHXR, as in humoral rejection affecting HLA-mismatched and blood group-incompatible allografts, the major stimulus to thrombosis is thought to be antibody deposition on graft endothelial cells (EC), which instigates a series of inflammatory changes (7), in turn activating circulating leukocytes and platelets. Coagulation is initiated by expression of tissue factor (TF) on activated monocytes (8) and the luminal aspect of the vessel wall (9). At the same time, antibody-activated-EC rapidly lose expression of several anticoagulants and platelet inhibitors, such as TF pathway inhibitor (TFPI), antithrombin III (ATIII), thrombomodulin (TM) and CD39 leading to unregulated intravascular clotting and platelet activation (10–12).
In AHXR, but not in allogeneic organs, three additional factors may operate to exacerbate intravascular thrombosis (13). Firstly, some coagulation inhibitors fail to inhibit xenogeneic clotting factors. Examples of this are porcine TFPI (14) and TM (11) that fail to regulate human factor Xa and thrombin respectively. Secondly, some proteins involved in the coagulation process are constitutively active across species barriers. For instance, porcine vWF spontaneously aggregates primate (including human) platelets through glycoprotein 1b (15). Finally, there is evidence from in vitro studies that xenogeneic EC may be constitutively procoagulant since primary cultures of porcine EC promote fibrin clot formation when added to recalcified human plasma (16).
Intravascular thrombosis has been identified as a reliable marker of early acute humoral rejection (3). However, its precise pathophysiological significance is uncertain, since both anti-graft antibody and complement are known to induce coagulation-independent pathology in vitro (17–19) and systemic anticoagulants or anti-platelet agents, although known to enhance graft performance, have failed to inhibit AHXR (20–24). We have previously described novel anticoagulant fusion proteins, based on TFPI or the leech anticoagulant protein hirudin, designed to be expressed on the EC of transplanted xenografts (25). The fusion proteins are tethered to the EC membrane through a covalent linkage to the membrane proximal domains of human CD4 (26,27). Additionally, expression is targeted to Weibel-Palade bodies in resting EC by incorporation of a cytoplasmic sequence from P-selectin (28). This ensures that they are only expressed on the luminal surface when EC are activated. Recently, we described the phenotype of transgenic mice expressing these novel anticoagulants under the control of the CD31 promoter and showed that this strategy is an effective way of inhibiting the widespread intravascular thrombosis associated with systemic endotoxaemia (29). In this study, we assessed whether organs from these mice are resistant to AHXR after transplantation into rats. The results indicate that effective control of intravascular thrombosis on activated graft endothelium can completely inhibit AHXR and the associated systemic coagulation defects and in combination with inhibition of T lymphocyte-mediated responses, promotes long-term xenograft survival without evidence of chronic rejection. These data indicate a critical role for activated coagulation factors in the pathophysiology of humoral rejection and have a direct applicability for clinical xenotransplantation, as this strategy could be applied to a clinically relevant species such as the pig.
Methods
Animals
Male inbred Lewis rats, weighing 190–210 g, were used as recipients and C57BL/6 (BL/6), TFPI-transgenic (TFPI-Tg) or Hirudin-transgenic (Hir-Tg) mice, weighing 25–30 g, were used as donors (29). All transgenic organs were from heterozygous mice that had been backcrossed onto the BL/6 background for >5 generations.
Cardiac transplantation
All experimental protocols conformed to the UK national and institutional guidelines. Mouse donors were anaesthetized using 60 μg/g of Sagatal intraperitoneally before thoracotomy. Hearts were flushed with cold heparin (100 U/mL), removed following ligation of caval and pulmonary veins and stored in ice-cold saline. Rat recipients were anaesthetized with halothane before midline laparotomy, at which donor aorta and pulmonary artery were sutured end-to-side to the recipient abdominal aorta and inferior vena cava, respectively, using 10–0 sutures (BEAR Medical Corporation, Tokyo, Japan). Haemostasis was achieved by compression and application of Surgicel™ (Ethicon Sarl, Neuchâtel, Switzerland). In some experiments, rats were injected with rabbit anti-human TFPI or sheep anti-hirudin, (both from Enzyme Research Lab., Swansea, UK) at the time of transplantation. Graft function was assessed by daily palpation; rejection was defined as loss of regular palpable contractions.
Administration of ciclosporin A
In some rats, ciclosporin A (Sandimmun®, Novartis Pharmaceuticals, Surrey, UK) was administered orally through a gastric feeding tube (CH 5, Popper & Sons, NY) at a dose of 20 mg/kg from day 1 to day 4, followed by 10 mg/kg daily until the end of the experiment.
Immunohistochemistry
Tissues were embedded in OCT (BDH, Lutterworth, UK) by freezing in dry ice, sectioned and fixed in methanol at –20°C for 1 h. Frozen sections were incubated with 0.2% Triton-X100/PBS for 1 h, washed and then immersed in 1% BSA-PBS for 30 min before overnight incubation at 4°C with one of the following antibodies; FITC-conjugated goat anti-rat CD3 (BD Bioscience, Oxford, UK); FITC-conjugated rabbit anti-human fibrinogen that also binds fibrin (DAKO, Cambridgeshire, UK); mouse anti-rat IgM (Sigma, Dorset, UK); mouse anti-rat IgG (BD Bioscience); goat anti-rat C3 (Autogen Bioclear, Wiltshire UK); rabbit anti-human vWF (DAKO), rabbit anti-human α-actinin or mouse anti-human α-actin (both from Sigma). Second layer staining, where appropriate, was with a sheep anti-mouse IgG-FITC, donkey anti-goat IgG-FITC (both from Sigma), goat anti-rabbit IgG-Texas red (DAKO) or horse anti-mouse IgG-Texas red (Vector Labs, Burlingame, CA). Sections were examined on a immunofluorescence microscope (Axiovert S100 TV, Zeiss, Welwyn Garden City, UK).
Flow cytometry analysis
A total of 1 × 105 freshly isolated cardiac microvascular EC were incubated with primary antibodies for 30 min on ice, followed by secondary antibodies for 30 min. The antibodies used were described above. Stained cells were analyzed on a cytofluorometer (Becton Dickinson, Franklin Lakes, NJ).
Fibrinogen assay
Fibrinogen levels were measured using the Clauss method with bovine thrombin (Sigma) as described previously (29). Assays were terminated at 20 min if no clot was observed. Serial dilutions of rat plasma in 0.05 M imidazole/0.1 M sodium chloride (pH 7.3) were used to determine the relationship between clotting times and plasma fibrinogen levels. Results are expressed as percentage fibrinogen relative to control, unmanipulated rats.
Platelet counts
Purified platelets were counted as described previously(29). Simply, platelet-rich plasma was obtained from blood by centrifugation at 80 × g for 10 min, followed by dilution 1 in 20 with 1% ammonium oxalate and 2.5 mM of Gly-Pro-Arg-Pro peptide (Sigma). Samples were placed in a counting chamber in a moist petri dish, allowed to settle and the platelets in 1 mm2 counted (=N). The number of platelets per litre of blood = 2N × 109. Platelet phenotype was confirmed by flow cytometric analysis with an anti-CD41 mAb (Pharmingen).
Statistical analyses
Graft survival data were analyzed by Log-rank test. Differences between platelets and fibrinogen levels were analyzed by unpaired t-test. Values were regarded as statistically significant if p < 0.05.
Results
Mouse hearts were transplanted heterotopically into the abdomen of Lewis rats. Primary haemostasis at the vascular anastomoses was achieved in all cases, with no difference between technical failure rates for BL/6 and transgenic hearts (data not shown). In the first series of experiments, no immunosuppression was administered. Kaplan-Meier survival curves are shown in Figure 1A. All BL/6 hearts were rejected by day 3 (mean survival 2.8 ± 0.2 days), whereas hearts from transgenic mice were rejected significantly later (TFPI-Tg, mean survival 6.7 ± 0.2 days, p < 0.05; Hir-Tg, mean survival 7.0 ± 0.3 days, p < 0.05).
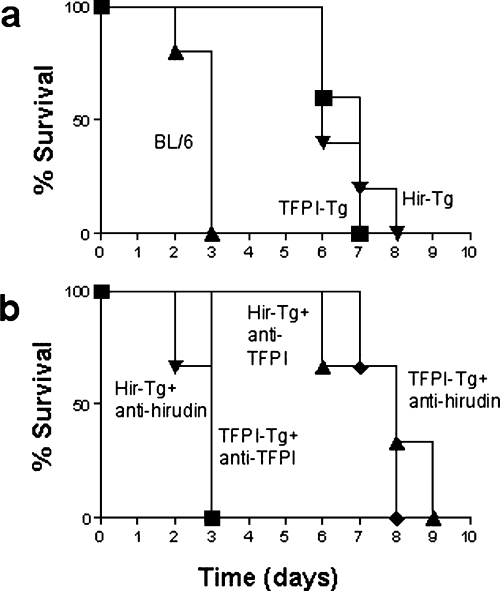
Kaplan-Meier survival curves following xenotransplantation of mouse hearts into rats. Comparison of hearts from BL/6 versus TFPI-Tg and Hir-Tg donors (n = 6). Differences in organ survival between BL/6 and either transgenic strain were statistically significant (p < 0.05). There was no significant differences between the survival of TFPI-Tg and Hir-Tg organs (A). Survival of hearts after injection of anti-TFPI or anti-hirudin antibody at the time of transplantation. Mean survival time of TFPI-Tg hearts after administration of anti-hirudin antibody was 7.2 ± 0.5 days, whereas after anti-TFPI, survival reduced to 2.8 ± 0.2 days (p < 0.05). Mean survival time of Hir-Tg hearts after administration of anti-TFPI antibody was 7.7 ± 0.2 days, whereas after anti-hirudin antibody, survival reduced to 2.7 ± 0.2 days (p < 0.05) (B).
Platelet counts and fibrinogen levels were measured in rat recipients on the day they rejected their cardiac xenografts. As shown in Figure 2, animals transplanted with BL/6 hearts developed significant thrombocytopenia and fibrinogen depletion at the time of rejection, consistent with the development of a consumptive coagulopathy, whereas animals transplanted with TFPI-Tg or Hir-Tg hearts maintained normal platelet counts and fibrinogen levels. These data establish that systemic, xenograft-induced intravascular coagulation was inhibited by expression of the anticoagulant fusion proteins.
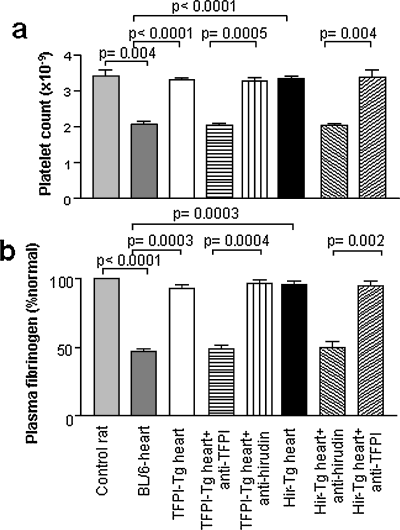
Systemic coagulopathy associated with AHXR. Mean platelet counts (±SEM) (A) and relative fibrinogen levels (±SEM) in rat recipients of mouse cardiac xenografts compared to control, untransplanted rats (n = 3). p-values calculated using unpaired t-test. Samples from transplanted rats were taken on the day of rejection. Animals receiving anti-TFPI or anti-hirudin antibodies on the day of transplantation are indicated (B).
Enhanced survival and maintenance of normal clotting parameters seen with transplantation of transgenic hearts was due entirely to expression of the anticoagulant fusion proteins, as injection of anti-TFPI and anti-hirudin antibodies at the time of transplantation completely inhibited the survival advantage, in the appropriate strains, compared to transplanted BL/6 hearts (Figure 1B) and led to recipients developing platelet and fibrinogen abnormalities indistinguishable from rats receiving BL/6 hearts (Figure 2). In these experiments, injection of antibody into the irrelevant strain (anti-TFPI into Hir-Tg or anti-hirudin into TFPI-Tg) served as controls to illustrate that the antibodies themselves had no inherent effect on graft survival or clotting parameters.
Figure 3A shows representative immunohistological sections from cardiac xenografts harvested at the time of rejection. BL/6 hearts showed widespread intravascular, perivascular and intracardiac deposition of fibrin. This pattern of fibrin distribution within the heart was similar to that seen in BL/6 mice during endotoxaemia (29). In contrast, hearts from neither transgenic strain showed detectable fibrin within vessels or in extravascular sites. However, both control and transgenic hearts showed comparable and extensive deposition of rat IgM, IgG and complement component C3 on graft vasculature (data not shown), suggesting there was no difference between the humoral response generated against BL/6 and either of the two transgenic cardiac xenografts. Rather, hearts from TFPI-Tg and Hir-Tg mice appeared resistant to the rejection that would normally result from this humoral response.
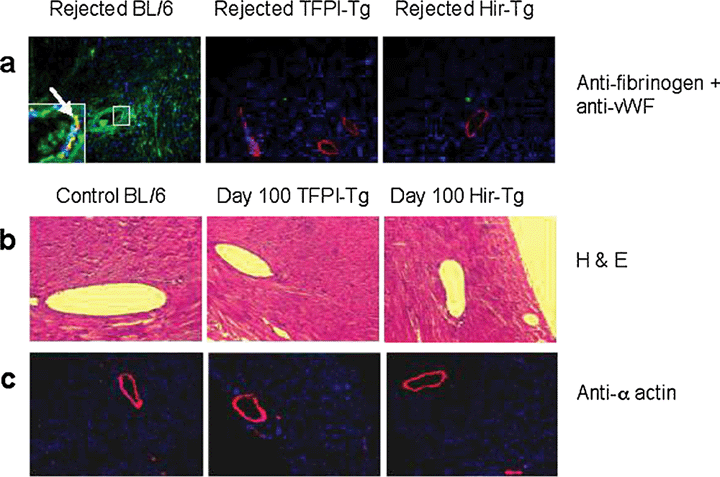
Immunohistology of rejected cardiac xenografts (×100). BL/6, TFPI-Tg and Hir-Tg hearts on day of rejection stained with DAPI nuclear stain (blue) and antibodies to human vWF (red) and human fibrinogen that also recognizes fibrin (green) (all ×100). Enlarged area in first section shows intravascular fibrin deposits; arrow indicates co-localization of vWF and fibrin (yellow) on the endothelium of a sub-epicardial vessel in a rejected BL/6 heart (A). H & E staining of control BL/6 (untransplanted) and day 100-sacrificed transgenic hearts removed from ciclosporin-treated rats (×200) (B). Control BL/6 (day 3-rejected) and day 100-sacrificed transgenic hearts, all removed from ciclosporin-treated rats stained with DAPI nuclear stain (blue) and anti-α actin (red) (all ×100) (C).
Further immunohistological analysis showed that hearts from both transgenic strains were heavily infiltrated with CD3+ T cells at the time of rejection (data not shown), in contrast to BL/6 hearts, which showed no T-cell infiltration. This suggested that rejection of transgenic xenografts was T-cell mediated. Therefore, cardiac xenografts were transplanted into rats given daily ciclosporin A at standard doses to inhibit the rat anti-mouse T-cell xenoresponse. All BL/6 hearts were rejected before day 3, whereas all TFPI-Tg and Hir-Tg xenografts (n = 6 in each group) showed long-term survival out to 100 days, at which stage the experiment was terminated (data not shown). Histological analysis of these long surviving hearts is shown in Figure 3B,C. By haematoxylin and eosin staining, the hearts appeared to have normal subepicardial vessels and showed no evidence of chronic ischaemia or fibrosis. Immunohistological analysis revealed that a pattern of α-actin staining in the subepicardial vessels that was indistinguishable from that seen in control hearts, with no evidence of thickening suggestive of chronic transplant arteriosclerosis.
Discussion
The mouse-heart-to-rat model is an established model of AHXR (30). Rats possess no natural xenoreactive antibodies against mouse endothelium, but mount a rapid humoral response resulting in antibody- and complement-dependent rejection within the first 3 days. Administration of ciclosporin A to rat recipients limits the T lymphocyte-dependent humoral response, as assessed by inhibition of IgG xenoreactive antibody production, but fails to inhibit IgM-dependent AHXR unless co-administered with agents such as cobra venom factor to prevent complement activation, after which long-term xenograft survival is often seen (31).
Because intravascular thrombosis and systemic clotting derangements are prominent features of AHXR, we investigated whether anticoagulants would have a significant impact on the humoral response. Although the potential of soluble agents has been shown in a variety of studies, no single agent or combination has been shown to inhibit AHXR completely. Additionally, the experimental and clinical use of systemic agents is associated with haemorrhagic complications, particularly after surgery, so they are likely to have only limited usefulness in any clinical xenotransplantation programme.
Our approach has been to target fusion proteins based on human TFPI and the leech anticoagulant hirudin to the endothelium of the transplanted xenograft. The fusion proteins were designed so that the anticoagulants were tethered to the EC surface through a covalent linkage to the membrane proximal domains of human CD4. Additionally, they were targeted to Weibel-Palade bodies in resting EC by incorporation of a cytoplasmic sequence from P-selectin (28). This ensured they were only expressed on the luminal surface when EC were activated. Our previous studies have demonstrated that EC expression is a particularly effective way to inhibit intravascular thrombosis after systemic endotoxaemia (29).
The data presented here demonstrate for the first time that the expression of single anticoagulant agents hirudin and TFPI on activated endothlium can achieve effective inhibition of thrombosis in transplanted cardiac xenografts, and offer complete protection against humoral rejection.
In non-ciclosporin A-treated recipients, prolonged survival of the transgenic hearts was achieved without specific manipulation of complement or antibody responses and accordingly, immunohistological analyses revealed patterns of antibody and complement deposition on intracardiac vessels similar to those seen in control BL/6 hearts. Before these experiments were performed, evidence from both in vitro and in vivo studies led us to predict that antibody and complement deposition within the transgenic grafts would, independently of any thrombosis and coagulation disturbances, promote significant pathology leading to rejection (17–19). However, transgenic organs appeared not to suffer any adverse consequence from the acute toxic effects of antibody and complement. By definition, these hearts have ‘accommodated’ to the conditions that would ordinarily mediate humoral rejection, although this work has not addressed the mechanisms by which the anticoagulant fusion proteins mediate this protection.
Importantly, hearts from the transgenic mice survived for >100 days in rats that received ciclosporin to inhibit T-cell-mediated rejection. These hearts appeared histologically normal, without evidence of arterisoclerosis, suggesting that expression of the anticoagulant fusion proteins may protect against the development of this lesion after xenotransplantation. We are currently investigating the hypothesis that activated coagulation factors play a significant role in the development of both the acute and chronic manisfestations of antibody- and complement-mediated rejection.
We propose that targeting anticoagulants to the EC of transplanted xenografts may have a significant impact on AHXR in pig-to-primate models and that the approach described here would be suitable for use in pre-clinical and clinical xenotransplantation, using pigs transgenic for fusion proteins based on TFPI and hirudin. An effective strategy to control intravascular thrombosis, used perhaps in conjunction with others to limit complement activation and antibody deposition, would represent a significant advance towards the goal of clinical xenotransplantation.
Acknowledgments
This work was supported by PPL Therapeutics plc and the Medical Research Council, UK. Imperial College London owns a patent on the use of membrane-targeted anticoagulants in xenotransplantation, which is currently licensed to Revivicor Inc., Pittsburgh.




