Pioglitazone in the Management of Diabetes Mellitus after Transplantation
Abstract
Type 2 diabetes mellitus is a common problem in patients after solid organ transplantation. We studied the safety and efficiacy of pioglitazone therapy in this setting. Ten patients with diabetes mellitus treated with insulin or glyburide after transplantation were studied after the addition of the thiazolidinedione pioglitazone. Serum creatinine, HBA1C, total daily insulin dose, tacrolimus dose, tacrolimus level and prednisone dose were followed for a mean of 242 days and compared to the corresponding values measured before the initiation of pioglitazone. The addition of pioglitazone caused no significant changes in serum creatinine or mean tacrolimus dose, and caused decreases in HBA1C (8.36%± 1.5% pre-pioglitazone, 7.08%± 1.5% post-pioglitazone, p = 0.018) and total daily insulin dose (125.1 ± 28.1 units pre-pioglitazone, 80.6 ± 22.8 units post-pioglitazone, p = 0.002). Our preliminary study suggests that pioglitazone is a safe and effective oral agent for the management of diabetes mellitus after transplantation.
Introduction
Type 2 diabetes mellitus is a common problem in patients after solid organ transplantation, and its management after transplantation is an important clinical challenge. Diabetes mellitus is associated with an increased incidence of cardiovascular events in the transplant population (1,2). The development of post-transplant diabetes mellitus (PTDM) is associated with decreased graft survival in patients (1,3,4), and also with an increased risk of infectious complications (1,2).
The immunosuppressive agents prednisone, tacrolimus and cyclosporine have been associated with worsening pre-existing diabetes mellitus and with the development of PTDM (1,2,5–10). Little is known about the safety and efficacy of the various oral diabetic agents after transplantation (1). The thiazolidinediones (TZDs) are oral PPAR gamma receptor agonists that are commonly used in the management of type 2 diabetes mellitus. They improve diabetic control by increasing insulin sensitivity. The first TZD, troglitazone, was withdrawn from the market due to liver toxicity. It is metabolized by the P-450 enzymes CYP3A4 and 2C8, and is known to induce CYP3A4 (11). This induction results in the increased metabolism of tacrolimus and cyclosporine. A newer TZD, pioglitazone is also metabolized by CYP3A4 and 2C8, but has been shown not to induce CYP3A4 (11). In order to assess the safety and efficacy of pioglitazone therapy therapy for type 2 diabetes mellitus after transplantation, we undertook a retrospective analysis of our preliminary experience.
Materials and Methods
Ten patients with poorly controlled type 2 diabetes mellitus (HBA1C >8%) after solid organ transplantation received pioglitazone in order to improve diabetic control. Patients with a clinical diagnosis of congestive heart failure were excluded. The diagnosis of diabetes was made on the basis of American Diabetes Association criteria of fasting plasma glucose greater than 126 mg/dL (1,8). Six patients had type 2 diabetes prior to transplantation, while 4 developed type 2 diabetes after transplantation (Table 1). Patients were followed for mean of 242 days (range: 104–431 days) after the initiation of pioglitazone. Serum creatinine, HBA1C, total daily insulin dose, tacrolimus and cyclosporine dose, 12 hour trough blood tacrolimus and cyclosporine levels, serum lipid levels and prednisone dose were monitored during the follow-up period. The data were retrospectively compared to data collected prior to the initiation of pioglitazone using paired t-test and Wilcoxon signed ranks test through SPSS software.
| Patient | Diabetes | BMI | Age/sex | Ethnicity | Transplant | Tacrolimus dose (mg BID) | CSA dose | Prednisone dose (mg/day) | Other anti-rejection | Diabetic Rx |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Post-transplant | 33 | 48 M | Cauc. | Kidney | 2 | 5 | Sirolimus | Insulin | |
| 2 | Pre-transplant | 34 | 68 F | AA | Kidney | 4 | 5 | None | Insulin | |
| 3 | Pre-transplant | 27 | 57 M | Hisp. | Kidney | 175 BID | 5 | Myfortec | Insulin | |
| 4 | Pre-transplant | 32 | 54 M | Asian | Kidney | 125 BID | 4 | Mycophenolate | Insulin | |
| 5 | Pre-transplant | 29 | 43 F | Cauc. | Liver | 5 | 20 | None | Insulin | |
| 6 | Post-transplant | 31 | 47 M | Asian | Kidney | 1 | 5 | Sirolimus | Insulin | |
| 7 | Post-transplant | 31 | 49 F | Hisp. | Kidney | 5 | 15 | None | Glyburide | |
| 8 | Pre-transplant | 34 | 49 M | Cauc. | Kidney | 3 | 5 | Mycophenolate | Insulin | |
| 9 | Pre-transplant | 29 | 55 M | AA | Liver | 5 | 5 | Mycophenolate | Insulin | |
| 10 | Post-transplant | 32 | 57 M | Cauc. | Kidney | 2 | 0 | Sirolimus | Insulin |
Results
No significant difference was found in serum creatinine levels before and after pioglitazone therapy: mean serum creatinine: 1.57 ± 0.67 mg/dL pre-pioglitazone, 1.6 ± 0.64 mg/dL post-pioglitazone, p = 0.636. Mean blood tacrolimus level was found to be significantly lower after pioglitazone initiation: 8.4 ± 4.4 ng/mL pre-pioglitazone, 7.3 ± 3.8 ng/mL post-pioglitazone, p = 0.005, however, variability existed within each patient's levels and comparison of each patient's tacrolimus levels before and after pioglitazone initiation revealed little absolute difference (Figure 1). Analysis of dose-normalized blood tacrolimus levels revealed no significant difference in levels before and after pioglitazone initiation: 1.05 ± 0.72 ng/mL/mg dose pre-pioglitazone, 1.36 ± 1.49 ng/mL/mg dose post-pioglitazone, p = 0.889. No difference was found in mean daily tacrolimus dose before and after pioglitazone initiation: 7.1 ± 2.8 mg pre-pioglitazone, 6.5 ± 3.5 mg post-pioglitazone, p = 0.562. No changes occurred in the two CSA-treated patients. Mean daily prednisone dose did decrease during the period of pioglitazone therapy, but was not significant: 9.4 ± 6.0 mg pre-pioglitazone, 7.7 ± 5.3 mg post-pioglitazone, p = 0.083. Mean body-mass index (BMI) increased after pioglitazone: 31.5 ± 2.4 kg/m2 pre-pioglitazone, 33.5 ± 3.6 kg/m2 post-pioglitazone, p = 0.032.
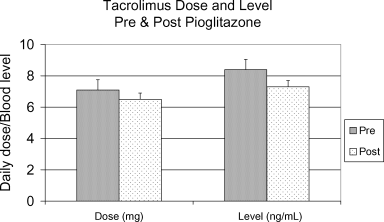
Tacrolimus dose and level pre- and post-pioglitazone.
Two patients who had not had HBA1C measured prior to the initiation of pioglitazone were excluded from analysis of the effect of pioglitazone on HBA1C. Mean HBA1C was found to be significantly lower after pioglitazone initiation: HBA1C 8.36%± 1.5% pre-pioglitazone, 7.08%± 1.5% post-pioglitazone, p = 0.018 (Figure 2). HBA1C decreased after the initiation of pioglitazone in six of the eight patients analyzed and was unchanged in the remaining two patients. This benefit was equally present in patients with preexisting diabetes mellitus and in those with PTDM. Mean total daily dose of insulin was significantly lower after pioglitazone initiation: total daily insulin dose 125.1 ± 28.1 units pre-pioglitazone, 80.6 ± 22.8 units post-pioglitazone, p = 0.002 (Figure 3). No patient developed significant fluid retention.
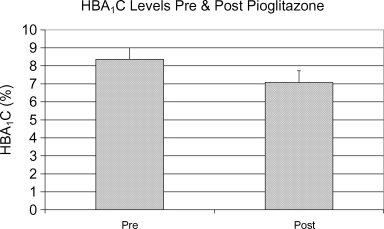
HBA1C levels pre- and post-pioglitazone.
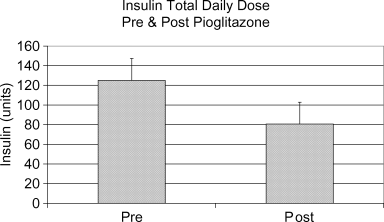
Insulin total daily dose pre- and post-pioglitazone.
Four patients who did not have serum lipid profiles measured after pioglitazone initiation were excluded from the analysis of the effect of pioglitazone on lipid profiles. There were no significant differences in mean serum lipid values after the initiation of pioglitazone: total cholesterol 187.5 ± 34.94 mg/dL pre-pioglitazone, 198.89 ± 49.98 mg/dL post-pioglitazone, p = 0.463; triglycerides 188.33 ± 102.26 mg/dL pre-pioglitazone, 214.33 ± 139.86 mg/dL post-pioglitazone, p = 0.753; HDL 48.0 ± 8.81 mg/dL pre-pioglitazone, 49.67 ± 7.07 mg/dL post-pioglitazone, p = 0.462; LDL 101.83 ± 20.7 mg/dL pre-pioglitazone, 98.5 ± 25.6 mg/dL post-pioglitazone, p = 0.600. However, 8/10 patients were treated with statins, which likely would have a more dominant effect on lipid levels than pioglitazone.
Discussion
Type 2 diabetes mellitus is a common clinical problem after transplantation, but little is known about the safety and efficacy of the various oral diabetic agents. Our study assessed the use of pioglitazone, a TZD commonly used in the treatment of type 2 diabetes. Our preliminary experience suggests that pioglitazone has no significant impact on kidney function in the post-transplant setting. We found that pioglitazone therapy did not have a relevant impact on tacrolimus levels or doses, while its impact on cyclosporine was difficult to assess in only two patients. The first TZD, troglitazone, induces the hepatic microsomal enzyme CYP3A4, decreasing cyclosporine and tacrolimus levels, making its use after transplant problematic. However, pioglitazone does not have inductive properties on CYP3A4, and appears in our study to have no effect on tacrolimus metabolism. The other available TZD, rosiglitazone, is metabolized solely via 2C8 and we have also found no significant impact of rosiglitazone on cyclosporine or tacrolimus metabolism (12,13).
Additionally, pioglitazone is an effective agent for the treatment of diabetes in this population, as evidenced by the significant decrease in HgbA1C. This occurred despite an overall decrease in total daily insulin requirements. The only negative aspect of therapy is the weight gain commonly associated with TZDs.
Pioglitazone and rosiglitazone are PPAR gamma receptor agonists and have been shown to be effective agents for the treatment of type 2 diabetes mellitus. By reducing insulin resistance, they are particularly potent in combination with insulin or agents with stimulate insulin secretion. Type 2 diabetes mellitus in transplant patients is typically due to a combination of insulin resistance and decreased insulin secretion. Glucocorticoids increase insulin resistance, but this effect can be ameliorated by the TZDs (14,15). In our experience, most patients with type 2 diabetes mellitus after transplantation have required insulin therapy since monotherapy with a sulfonylurea is rarely effective. We have always been reluctant to use metformin after transplantation given the risk of lactic acidosis associated with diminished renal function. Our recent study of the treatment of PTDM with a rosiglitazone/sulfonylurea combination has shown good diabetic control without insulin in 92% of patients (13). Since we have found that rosiglitazone or pioglitazone may be safely included with immunosuppressive agents, we propose that a TZD alone or in combination with a sulfonylurea or with insulin may be the approach of choice in treating PTDM.
The TZDs have many other beneficial non-glycemic properties: decreased blood pressure, increased HDL-cholesterol, decreased small dense LDL-cholesterol, decreased triglycerides, decreased vascular resistance and inflammation and improved endothelial cell function (16,17). Our study did not find improvement in lipid profiles with the initiation of pioglitazone, however, this may be secondary to our small patient number and concomitant statin therapy. It is hoped that these beneficial characteristics in addition to improved glycemic control will diminish the risk of post-transplant cardiovascular disease and to increase patient survival.
While we find our preliminary data encouraging, our study is limited both by its small number of subjects and by their heterogeneity. Future multi-center studies of therapy of diabetes after various organ transplants will be important to establish a consensus approach to the problem.




